Abstract
The objectives of this study were to evaluate the kinetics of antibody decline through childhood in a longitudinal study of a single cohort following serogroup C meningococcal (MenC) vaccine immunization in early childhood and to calculate the proportion of 11 to 13 year olds with protective levels of bactericidal antibody 10 years after immunization. United Kingdom children aged 11 to 13 years in 2010 who had previously taken part in a longitudinal study at the Oxford Vaccine Group had blood samples drawn between 2001 and 2010. Sera from each time point were analyzed for the MenC serum bactericidal antibody titer using a baby rabbit complement (rSBA) assay. The median age at MenC immunization was 21 months (range, 1 year 3 months to 3 years 9 months). The MenC rSBA geometric mean titer (GMT) at age 3.5 to 5 years was 8.0 (95% confidence interval, 6.5 to 9.9; n = 287). By age 11.5 to 13.5 years, the rSBA GMT had declined to 3.3 (2.5 to 4.4; n = 98). The percentage of children with rSBA titers of ≥1:8 (the threshold for protection) also declined from 38% (35% to 41%) to 15% (12% to 19%). We concluded that MenC rSBA titers wane rapidly following vaccination in early childhood and continue to decline into the second decade of life. Since nasopharyngeal colonization in adolescents probably provides the major reservoir for MenC in the population, declining immunity in this cohort is of concern. Sustaining high levels of antibody through booster vaccination in this cohort is likely necessary to avoid a resurgence of disease in the decade ahead.
INTRODUCTION
Neisseria meningitidis causes invasive disease globally with rates that are highly age dependent. The greatest burden of disease occurs in infancy, with a second, slightly smaller peak in late adolescence (18), and despite sensitivity of the organism to antibiotics used empirically, the case-fatality rate of invasive disease is around 10% in treated cases (7) with significant long-term sequelae in up to 20% of survivors (12). Thus, prevention of meningococcal disease relies on effective immunization programs.
In 1999, serogroup C meningococcal (MenC) conjugate vaccines were introduced into the routine infant immunization schedule at 2, 3, and 4 months in the United Kingdom. A catch-up campaign was launched over the subsequent 11 months that offered a single dose of vaccine to those aged between 1 and 18 years (later extended to 25 years of age). Previous studies have demonstrated rapid waning of MenC-specific antibody concentrations and serum bactericidal antibody (SBA) titers following immunization in young children (3, 13, 17, 20) but much better persistence following immunization of older children and adolescents (19, 21, 22) Furthermore, the effectiveness of MenC vaccines is known to decline in populations whose SBA titers have waned over time (6). Thus, persistence of adequate levels of bactericidal antibody is important to ensure immunity for each individual. In 2006, a combined Haemophilus influenzae type b (Hib)-MenC vaccine given as a booster dose at 12 months of age was introduced into the routine schedule in an attempt to sustain protection, but recent data show that antibody also wanes rapidly after this booster dose (3, 13).
Population immunity (herd immunity) has been maintained over the past decade by high levels of antibody among teenagers and young adults who were immunized during the catch-up campaign of 1999 to 2000, since asymptomatic colonization in the upper airways of individuals in this age group was the major reservoir of MenC in the population prior to vaccine introduction. However, children immunized in early childhood in the 1999 to 2000 campaign whose antibody titers are waning are now entering adolescence (8). There is a risk of increased transmission of the organism when these nonimmune individuals reach this high-risk age, and a resurgence of meningococcal disease in children who do not have protective levels of antibody could ensue. The aim of this study was to establish the changes in baby rabbit complement (rSBA) titers over time in a cohort of children who received a single dose of MenC vaccine as toddlers 10 years previously.
MATERIALS AND METHODS
In a previous study conducted by the Oxford Vaccine Group, 300 preschool children who had received routine infant immunizations had 4 blood samples taken over 6 years between 2001 and 2007 (10). At the time of taking informed consent for this study, permission was obtained to store the remaining serum for use in further vaccine-related research.
All of these children received a single dose of a licensed MenC conjugate vaccine at age 1 to 4 years in the catch-up campaign of 1999 to 2000. The stored serum samples from these children were analyzed to evaluate MenC rSBA titers. In addition, all children from the “preschool” study who were still available were invited to take part in the current study. Healthy children who agreed to take part (and whose parents gave informed consent) had a blood sample taken in 2010. Exclusion criteria included a history of invasive MenC disease, additional doses of a MenC-containing vaccine (beyond the single dose administered as part of the national campaign), and any immunodeficient condition or other major congenital defect or serious chronic disease. The type of MenC conjugate vaccine received was not an inclusion or exclusion criterion. All children who participated in the final visit of this study were offered an optional booster dose of a MenC conjugate vaccine to be administered after the blood sample was drawn.
A MenC SBA titer of ≥1:8 using rSBA has been validated in population studies in the United Kingdom as a correlate of protection against invasive MenC disease (1, 4). The primary objective was to calculate the percentage of 11- to 13-year-old children with MenC rSBA titers of ≥1:8 from a cohort of children who received a single dose of a MenC conjugate vaccine as preschoolers. Secondary objectives included calculating the percentage of children at each time point with rSBA titers of ≥1:8. The percentages are presented with their 95% confidence intervals (CI), calculated using the normal approximation to the binomial distribution. In addition, MenC rSBA geometric mean titers (GMTs) were calculated for each time point as the exponential of the mean of the log of these titers. Negative results were given a value of half the lowest level of detection (1:4). Every available serum sample was used in the analysis at each relevant time point. Random-effects regression analysis of repeated measurements of MenC rSBA GMTs and the percentage of protected children over time was performed to determine their rates of change. Calculations were made using STATA version 11.1.
SBA analysis was undertaken at the Health Protection Agency reference laboratory in Manchester, United Kingdom. SBA assays were performed against the group C target strain, C11 (phenotype C:16:P1.7-1,1) as previously described by Maslanka et al. (15). The complement source used in the SBA was pooled sera from 3- to 4-week-old rabbits (Pel Freez Biologicals, WI). Titers were expressed as the reciprocal serum dilutions yielding ≥50% killing after 60 min. The lower limit of detection was a titer of 4. Ethical approval was gained from the Berkshire Research Ethics Committee, and the study was registered at http://www.ClinicalTrials.gov (NCT01126996).
RESULTS
All participants received a single dose of a MenC conjugate vaccine between 1999 and 2002. The majority of these were given as part of the national campaign in 1999 to 2000, while 4 children were vaccinated between February 2001 and March 2002. The age at vaccination was between 1 year and 1 month and 3 years and 9 months (median, 21 months). Two hundred fifty-one children had received the licensed CRM197 conjugated vaccine Meningitec (Wyeth Vaccines, Pearl River, NY). For the remaining 36 children, the specific vaccine received could not be determined.
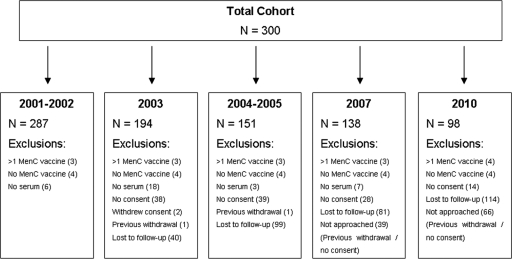
The number of children included in the analysis at each time point is given in Fig. 1 and Table 1, along with the percentages that had rSBA titers of ≥1:8 and the rSBA GMTs for each sampling time point (including 95% CI). The main reason for not including enrolled children in the analysis at any time point was lack of a serum sample. Four children were excluded from the study due to receipt of more than one dose of a MenC vaccine, and a further 4 were excluded for not having any record of MenC vaccine administration. The MenC rSBA GMT at age 3.5 to 5 years (approximately 1 to 2 years after vaccination) was 8.0 (95% CI, 6.5 to 9.9; n = 287). By age 11.5 to 13.5 years, the rSBA GMT had declined to 3.3 (95% CI, 2.5 to 4.4; n = 98). The percentage of children with rSBA titers of ≥1:8 (the threshold for protection) also declined from 38% (95% CI, 35% to 41%) to 15% (95% CI, 12% to 19%).
Fig. 1.
Number of children included in the final analysis.
Table 1.
Percentages of children with rSBA titers of >1:8 and rSBA GMTs for each time point
| Yr | Age (range) (yr) | No. | No. [% (95% CI)] with rSBA titer of >1:8 | rSBA GMT (95% CI) |
|---|---|---|---|---|
| 2001-2002 | 3.9 (3.5-5) | 287 | 109 [38.0 (35.1-40.8)] | 8.0 (6.5-9.9) |
| 2003 | 5.0 (4.5-6.1) | 194 | 56 [28.9 (25.6-32.1)] | 5.4 (4.2-6.8) |
| 2004-2005 | 7.1 (6.0-8.3) | 151 | 46 [30.5 (26.7-34.2)] | 5.8 (4.4-7.7) |
| 2007 | 9.0 (8.6-10.1) | 138 | 31 [22.5 (18.9-26.0)] | 4.3 (3.4-5.5) |
| 2010 | 12.2 (11.5-13.5) | 98 | 15 [15.3 (11.7-18.9)] | 3.3 (2.5-4.3) |
The data (log10 of the rSBA titers) were analyzed using a random-effects regression analysis (random-intercept) model to determine the rate of change over time since vaccination (Table 2). The first model includes only time since vaccination (in years), and the second also includes the age of the child at the time of vaccination. In Table 3, the proportion of participants protected at each time point (the percentage with an rSBA titer of ≥1:8) was analyzed using a logistic random-intercept model. The coefficient for time since vaccination in model 1 indicates that for each 1-year increase in time since vaccination, the log10 rSBA titer decreases by 0.036 (95% CI, 0.025 to 0.047), which corresponds to a decrease in the rSBA GMT of 7.9% (95% CI, 5.6% to 10.2%). Including age at vaccination in the model has no effect on the relationship between outcome and time since vaccination (model 2).
Table 2.
Random-effects regression analysis for rate of change in log10 SBA titers
| Model | Parameter | Coefficient | 95% CI | P value |
|---|---|---|---|---|
| 1 | Time (yr) since vaccination | −0.036 | −0.047 to −0.025 | <0.0001 |
| 2 | Time (yr) since vaccination | −0.036 | −0.047 to −0.025 | <0.0001 |
| Age (yr) at vaccination | −0.042 | −0.18 to 0.098 | 0.56 |
Table 3.
Random-effects regression analysis for proportion of protected children
| Model | Parameter | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
| 1 | Time (yr) since vaccination | 0.77 | 0.70 to 0.85 | <0.0001 |
| 2 | Time (yr) since vaccination | 0.77 | 0.69 to 0.85 | <0.0001 |
| Age (yr) at vaccination | 0.62 | 0.27 to 1.44 | 0.27 |
The odds ratio from model 1 indicates that the odds of protection (rSBA titer ≥ 1:8) are multiplied by 0.77 for each 1-year increase in the time since vaccination. This is highly statistically significant, with a confidence interval ranging from 0.70 to 0.85. This translates into a 23% reduction in the odds of protection each year following vaccination (95% CI, 15% to 30%). Including the age at vaccination in the model has no effect on the relationship between rates of protection and time since vaccination (model 2).
DISCUSSION
This is the first study to describe, within a single cohort of children, the kinetics of the decline in bactericidal antibody until early adolescence following immunization with a MenC conjugate vaccine at 1 to 3 years of age. In our cohort, only 15% of 11- to 13-year-old children had protective levels of antibody, slightly higher than the 6.9% of 10 to 14 year olds with rSBA titers of ≥1:8 in the prevaccine era (samples taken between 1996 and 1999) (23), and the rate of decline in antibody titers was independent of the age at vaccination. Since the children in our study had been immunized between 1 and 3 years of age, these results may underestimate the decline in bactericidal antibody in children immunized in infancy. Borrow et al. found that doubling the time from vaccination resulted in a two-thirds reduction in MenC SBA titers. This decay was similar following 3 doses of MenC vaccine at 2, 3, and 4 months of age compared to after a two-dose course of MenC followed by a 12-month booster dose of a Hib-MenC vaccine (3), similar to the current United Kingdom immunization schedule. Importantly, the current study shows that this pattern of antibody waning is maintained over at least 10 years.
A rapid decline in the incidence of MenC disease was noted in the United Kingdom following institution of routine MenC vaccination of children in 1999, and between 1999 and 2009 there was a 99% reduction in the number of confirmed cases (6). This was probably mostly due to the immunization of adolescents and young adults in the nationwide campaign of 1999 to 2000 (2). Carriage of N. meningitidis is low in the first few years of life, rising sharply during adolescence to a peak of 10 to 35% in 20 to 24 year olds (8). Cross-sectional studies have demonstrated that a small proportion of meningococcal isolates are serogroup C (14); however, this may underestimate the proportion of young people who are exposed to the organism due to rapid transmission, short duration of carriage, and reacquisition over time. Adequate immunity in adolescents and young adults prevents nasopharyngeal carriage of MenC and transmission of the organism within the population, establishing herd immunity (14). Interpretation of mathematical models of herd immunity effects following the national immunization campaign in the United Kingdom has suggested that this could last for several years (6, 25). However, in the absence of continued vaccination of adolescents, persistence of antibody following infant or early childhood immunization may not be sufficient to maintain this in the long term. Our results demonstrate that the current cohorts of children entering adolescence are unlikely to have protective levels of antibody, and thus, herd immunity may also start to wane. Furthermore, children in this study mainly received a MenC-CRM197 conjugated vaccine, which was found to be the least immunogenic of the three licensed MenC conjugate vaccines (3, 5). It was in wide use during the national immunization campaign, so these results could be generalized to a large group of United Kingdom adolescents.
Waning effectiveness has been demonstrated beyond 1 year after infant immunization with MenC conjugate vaccines and is associated with a fall in SBA titers over the same period (6, 24). Decreases in SBA titers over time have also been demonstrated in other age groups, with more marked waning in children immunized at younger ages (3, 13, 17, 19, 20) and much better persistence demonstrated following immunization of older children and adolescents (19, 21, 22). A study evaluating MenC SBA titers in 11 to 20 year olds in the United Kingdom, approximately 5 years after immunization with a conjugate MenC vaccine, demonstrated that higher SBA titers were observed in children who had been vaccinated at age 10 years or older than in children who had been vaccinated at a younger age (21). Similar findings were observed in Greece, where 5 years after vaccination with a MenC conjugate vaccine, SBA titers in most children immunized at less than 6 years of age were below the threshold for protection, whereas protective levels were seen in the majority of children immunized at over 10 years of age (19).
Our results demonstrate an overall waning of antibody levels throughout childhood and into early adolescence. It is thus unlikely that exposure to cross-reactive antigens (such as Escherichia coli K92 [11]) could boost levels of MenC bactericidal antibody in older children and adolescents. The evidence suggests that age-dependent differences noted between young children and adolescents are probably due to a better immune response with less rapid decline in antibody levels caused by immunological maturity in older children and adolescents. Our GMT results (Table 1) suggest that the greatest decline in antibody titers occurs in the first year after vaccination. No clear decline is noted between 2003 (5 years of age) and 2004 to 2005 (7 years of age); however, there is then a persistent decline in antibody titers over the subsequent 5 years of the study. The same results viewed on a log10 scale (data not presented) show a more linear decay over time, and the regression analysis to determine the rate of change over time was performed with this assumption.
In the United States, young adolescents have been targeted for immunization with the quadrivalent serogroup A, C, W-135, and Y meningococcal (MenACWY) conjugate vaccine since 2005. However, even in this age group, waning of effectiveness has been observed (preliminary data), and the Advisory Committee on Immunization Practices in the United States now recommends that a booster dose of vaccine be administered to adolescents at 16 years of age, following the first dose given at around 12 years (9). The routine childhood immunization schedule in Canada has also recently been modified to include a booster dose of vaccine (either a MenC or a MenACWY conjugate vaccine) at 12 years of age, following infant immunization (16). The results of this study provide evidence to support these changes and suggest that similar changes may be necessary in the United Kingdom. However, these results should be considered in conjunction with those obtained from nasopharyngeal carriage studies and vaccine effectiveness data.
Conclusions.
MenC bactericidal antibody titers wane rapidly following vaccination in early childhood, without evidence of natural boosting of antibody levels through exposure to cross-reactive antigens. In the United Kingdom, consideration should be given to a routine adolescent booster of MenC conjugate vaccine to protect this cohort of children who are entering the potentially high-risk period of adolescence. Such a booster will help to maintain herd immunity through ongoing prevention of nasopharyngeal carriage.
ACKNOWLEDGMENTS
We acknowledge the contributions of the staff members and nurses who were involved in this study, in particular, Jeremy Taylor, who assisted with gathering vaccination data. We thank colleagues at Sanofi Pasteur MSD and Sanofi Pasteur for making sera available for this study. We also thank the participants in this study and their family members.
The study was sponsored by the University of Oxford, and funding was received from the NIHR Oxford Biomedical Research Centre (including salary support for M.D.S. and A.K.) and the Oxfordshire Health Services Committee.
All the authors have had full access to the study data and take responsibility for the integrity and accuracy of the data analysis. A.K. has received funding from the James Martin Vaccine Design Institute (James Martin Fellowship) and has received assistance from vaccine manufacturers to attend scientific meetings. A.J.P. and M.D.S. act as chief and principal investigators for clinical trials conducted on behalf of Oxford University sponsored by vaccine manufacturers (Novartis Vaccines, GlaxoSmithKline, Sanofi-Aventis, Sanofi-Pasteur MSD, and Pfizer Vaccines). M.D.S. is a consultant pediatrician within the NHS and also receives assistance from vaccine manufacturers to attend scientific meetings. A.J.P. is a Jenner Investigator and James Martin Senior Fellow. A.J.P. does not receive any financial support from vaccine manufacturers. H.F. has performed contract research on behalf of the Health Protection Agency funded by Pfizer, Novartis Vaccines, Baxter Bioscience, GlaxoSmithKline, Sanofi Pasteur, Alexion Pharmaceuticals Inc., and Merck.
Footnotes
Published ahead of print on 28 October 2011.
REFERENCES
- 1. Andrews N., Borrow R., Miller E. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bille E., et al. 2008. Association of a bacteriophage with meningococcal disease in young adults. PLoS One 3:e3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borrow R., et al. 2010. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin. Vaccine Immunol. 17:154–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borrow R., Balmer P., Miller E. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 23:2222–2227 [DOI] [PubMed] [Google Scholar]
- 5. Burrage M., et al. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell H., Andrews N., Borrow R., Trotter C., Miller E. 2010. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin. Vaccine Immunol. 17:840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartwright K., Strang J., Reilly S., White D. 1992. Mortality in meningococcal disease. BMJ 304:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caugant D. A., Tzanakaki G., Kriz P. 2007. Lessons from meningococcal carriage studies. FEMS Microbiol. Rev. 31:52–63 [DOI] [PubMed] [Google Scholar]
- 9. CDC 2011. Updated recommendations for use of meningococcal conjugate vaccines. Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 60:72–76 [PubMed] [Google Scholar]
- 10. Collins C. L., et al. 2004. Immunogenicity and safety of a low-dose diphtheria, tetanus and acellular pertussis combination vaccine with either inactivated or oral polio vaccine as a pre-school booster in UK children. Vaccine 22:4262–4269 [DOI] [PubMed] [Google Scholar]
- 11. Glode M. P., et al. 1977. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J. Infect. Dis. 135:94–104 [DOI] [PubMed] [Google Scholar]
- 12. Healy C. M., et al. 2002. Influence of serogroup on the presentation, course, and outcome of invasive meningococcal disease in children in the Republic of Ireland, 1995-2000. Clin. Infect. Dis. 34:1323–1330 [DOI] [PubMed] [Google Scholar]
- 13. Khatami A., et al. 2011. Persistence of immunity following a booster dose of Haemophilus influenzae type B-meningococcal serogroup C glycoconjugate vaccine: follow-up of a randomized controlled trial. Pediatr. Infect. Dis. J. 30:197–202 [DOI] [PubMed] [Google Scholar]
- 14. Maiden M. C., et al. 2008. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maslanka S. E., et al. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin. Diagn. Lab. Immunol. 4:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Advisory Committee on Immunization 2009. An update on the invasive meningococcal disease and meningococcal vaccine conjugate recommendations. An Advisory Committee Statement (ACS). Can. Commun. Dis. Rep. 35:1–40 [PubMed] [Google Scholar]
- 17. Perrett K. P., et al. 2010. Antibody persistence after serogroup C meningococcal conjugate immunization of United Kingdom primary-school children in 1999-2000 and response to a booster: a phase 4 clinical trial. Clin. Infect. Dis. 50:1601–1610 [DOI] [PubMed] [Google Scholar]
- 18. Rosenstein N. E., Perkins B. A., Stephens D. S., Popovic T., Hughes J. M. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378–1388 [DOI] [PubMed] [Google Scholar]
- 19. Sakou I. I., et al. 2009. Investigation of serum bactericidal activity in childhood and adolescence 3-6 years after vaccination with a single dose of serogroup C meningococcal conjugate vaccine. Vaccine 27:4408–4411 [DOI] [PubMed] [Google Scholar]
- 20. Snape M. D., et al. 2005. Lack of serum bactericidal activity in preschool children two years after a single dose of serogroup C meningococcal polysaccharide-protein conjugate vaccine. Pediatr. Infect. Dis. J. 24:128–131 [DOI] [PubMed] [Google Scholar]
- 21. Snape M. D., et al. 2008. Seroprotection against serogroup C meningococcal disease in adolescents in the United Kingdom: observational study. BMJ 336:1487–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snape M. D., et al. 2006. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin. Infect. Dis. 43:1387–1394 [DOI] [PubMed] [Google Scholar]
- 23. Trotter C., Borrow R., Andrews N., Miller E. 2003. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 21:1094–1098 [DOI] [PubMed] [Google Scholar]
- 24. Trotter C. L., Andrews N. J., Kaczmarski E. B., Miller E., Ramsay M. E. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365–367 [DOI] [PubMed] [Google Scholar]
- 25. Trotter C. L., Gay N. J., Edmunds W. J. 2005. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am. J. Epidemiol. 162:89–100 [DOI] [PubMed] [Google Scholar]