Abstract
We previously identified Mannheimia haemolytica outer membrane proteins (OMPs) that may be important immunogens by using immunoproteomic analyses. Genes for serotype 1-specific antigen (SSA-1), OmpA, OmpP2, and OmpD15 were cloned and expressed, and recombinant proteins were purified. Objective 1 of this study was to demonstrate immunogenicity of the four recombinant OMPs in mice and cattle. Objective 2 was to determine if the addition of individual recombinant OMPs or combinations of them would modify immune responsiveness of mice to the recombinant chimeric protein SAC89, containing the main epitope from M. haemolytica outer membrane lipoprotein PlpE and the neutralizing epitope of M. haemolytica leukotoxin. Mice vaccinated with recombinant OmpA (rOmpA), rSSA-1, rOmpD15, and rOmpP2 developed significant antibody responses to M. haemolytica outer membranes and to the homologous recombinant OMP. Cattle vaccinated with rOmpA and rSSA-1 developed significant antibodies to M. haemolytica outer membranes by day 28, whereas cattle vaccinated with rOmpD15 and rOmpP2 developed only minimal responses. Sera from cattle vaccinated with each of the recombinant proteins stimulated complement-mediated killing of the bacterium. Concurrent vaccination with SAC89 plus any of the four rOMPs singly resulted in increased endpoint anti-SAC89 titers, and for the SAC89/rSSA-1 vaccinees, the response was increased significantly. In contrast, the SAC89/P2/SSA-1 and SAC89/OmpA/P2/D15/SSA-1 combination vaccines resulted in significant decreases in anti-SAC89 antibodies compared to SAC89 vaccination alone. In conclusion, under the conditions of these experiments, vaccination of mice and cattle with rOmpA and rSSA-1 stimulated high antibody responses and may have protective vaccine potential.
INTRODUCTION
The major cause of severe bacterial pneumonia in cattle is Mannheimia haemolytica serotype 1 (S1), and current vaccines against M. haemolytica are only moderately efficacious (9, 36). Shewen and Wilkie (43) demonstrated that immunity against M. haemolytica requires antibodies against leukotoxin (LKT), which causes necrosis, apoptosis, or activation of ruminant leukocytes, as well as antibodies against bacterial cell surface antigens. The important surface immunogens needed to stimulate protective immunity against M. haemolytica appear to be outer membrane proteins (OMPs) (13, 33, 34, 37, 38).
Our laboratory demonstrated that high antibody responses to a major 45-kDa outer membrane lipoprotein, PlpE, correlated with resistance against experimental challenge, and PlpE proteins were nearly identical among serotype 1 and serotype 6 isolates (2). Cattle vaccinated with commercial M. haemolytica vaccines to which 100 μg of recombinant M. haemolytica S1 PlpE (rPlpE) was added had significantly greater resistance against experimental challenge with either S1 or S6 than did cattle vaccinated with the commercial vaccine alone (11, 12). The major epitope region of M. haemolytica S1 PlpE, designated region 2 (R2), consists of 8 imperfect hexapeptide repeats of QAQNAP located near the N-terminal region (3, 37). We therefore developed several chimeric constructs containing the R2 epitope of PlpE and the neutralizing epitope of LKT (NLKT), which is localized in a 32-amino-acid region near the C terminus (5, 28). Vaccination of mice with these R2-NLKT chimeric proteins stimulated anti-PlpE antibodies that caused complement-mediated bacteriolysis of M. haemolytica, as well as LKT-neutralizing antibodies. Vaccination of cattle with an R2-NLKT-R2-NLKT chimera (SAC89) plus a formalin-killed bacterin resulted in approximately 75% lesion reduction in transthoracically challenged cattle compared to controls (10).
Although LKT and PlpE are important antigens for enhancing immunity against M. haemolytica, it would be naïve to consider that immunity against these two antigens alone would be protective against a complex Gram-negative bacterium. We therefore undertook the process of identifying other OMPs that may be important immunogens. Through immunoproteomic analyses using two-dimensional (2D) gel electrophoresis and Western blots of M. haemolytica outer membrane preparations probed with convalescent-phase cattle sera, we identified several additional OMPs of interest for further study (4). We cloned and expressed the genes for five of these proteins, namely PlpF, serotype 1-specific antigen (SSA-1), OmpA, OmpP2, and OmpD15, and then purified the proteins. We previously immunologically characterized PlpF (6). OmpA and SSA-1 have been characterized partially (19, 30, 31). OmpP2 and OmpD15, however, have only been recognized in the published M. haemolytica sequence (23).
The first objective of these studies was to demonstrate immunogenicity of each recombinant protein in mice and cattle. The second objective was designed because we are seeking to add epitopes from additional OMPs to R2-NLKT chimeras; we thus immunized mice with various combinations of SSA-1, OmpA, OmpP2, and OmpD15 in conjunction with SAC89 (R2-NLKT-R2-NLKT) to determine if there are enhancing or depressing effects of combinations of these proteins on immunity to LKT or PlpE.
MATERIALS AND METHODS
Bacterial cultures.
The 89010807N strain of M. haemolytica S1 (3, 37) was used as a source of genomic DNA. The strain was routinely streaked on brain heart infusion (BHI) agar supplemented with 5% defibrinated sheep blood (Hardy Diagnostics, Santa Maria, CA) to obtain isolated colonies and then transferred into BHI broth and grown to mid-log phase in a 37°C shaker incubator. Escherichia coli DH5α (Invitrogen, Carlsbad, CA) was used for cloning purposes. Recombinant proteins were expressed in either BL21(DE3)pLysS or BLR(DE3)pLysS (Novagen, Madison, WI). All E. coli strains were grown in Luria-Bertani (LB) agar supplemented with appropriate selection at 37°C in an incubator with 5% CO2, and broth cultures were incubated in a shaker incubator.
Construction of recombinant plasmids and expression and purification of chimeric proteins.
A complete and detailed account of the construction, expression, and purification of the chimeric SAC89 protein was published previously (5). A brief description of the production of recombinant forms of OmpA, OmpD15, OmpP2, and SSA-1 is as follows. DNA fragments carrying each of the genes were amplified from M. haemolytica genomic DNA by PCR using the primer pairs shown in Table 1. Amplimers were cleaned with a QIAquick PCR purification kit (Qiagen, Germantown, MD), cut with pairs of restriction endonucleases whose recognition sequences were engineered into the primers (shown in lowercase letters in Table 1), and gel purified. Each processed DNA fragment was ligated to pET28a or pET28b cut with the same restriction endonucleases as the amplimers. Aliquots of ligated vector and insert were used to transform chemically competent E. coli DH5α cells (Invitrogen, Carlsbad, CA), and transformants were seeded on LB agar plates supplemented with 30 μg/ml of kanamycin. After overnight incubation at 37°C, colonies were transferred into Falcon tubes (Fisher, Becton Dickinson, Franklin Lakes, NJ) containing 5 ml LB with 30 μg/ml kanamycin and incubated in a 37°C shaker incubator. Plasmid DNA was extracted from 1.5 to 3 ml of the overnight growth, cut with restriction endonucleases, and resolved in 1% agarose. Candidates with the appropriate migration patterns were sequenced, and constructs were identified. Confirmed constructs were introduced into E. coli expression hosts, such as BL21(DE3), BL21(DE3)pLysS, BLR(DE3), BLR(DE3)pLysS, and Rosetta II(DE3) (EMD Millipore, San Diego, CA), by transformation. Selection of expression hosts was dictated by the codon bias of each gene.
Table 1.
Oligonucleotides used in this study
| Antigen | Primer sequence | Calculated molecular mass (kDa) |
|---|---|---|
| OmpA | AAgaattcGCTAACACTTTCTACGCAGGTGCTA | 41.7 |
| GCctcgagTTACATAGTTACTTCTTTTGAACCTTG | ||
| OmpD15 | TATAccatggGCCCTTTCGTAGTAAAAGAC | 88 |
| GTGctcgagGAATGAACTACCAATGTT | ||
| OmpP2 | CGCggatccGTTTACGATGCAGAAGGT | 42.8 |
| GTGctcgagTTACCAGTATACACGCATACC | ||
| SSA-1 | CTAGggatccGAATCAATAGAGAATCCACAAC | 104 |
| CGATctcgagTTAGAAACTAAAGCCAACATT |
Well-isolated colonies of expression hosts carrying the constructs, grown on LB agar plates supplemented with an appropriate selection factor, were transferred into larger volumes of LB with selection and incubated in a 37°C shaker incubator. Expression was induced at an optical density at 600 nm (OD600) of 0.8 to 1.0 by adding 1 mM isopropyl-β-d-thiogalactopyranoside, and incubation was continued for 3 h. Cells were harvested by centrifugation at 10,000 × g for 20 min at 4°C. Pellets were processed with BugBuster master mix (Novagen, EMD Chemicals, San Diego, CA) to isolate inclusion bodies, which were then solubilized in binding buffer (50 mM Tris-Cl, pH 8.0, 500 mM NaCl) with 6 M urea. Recombinant proteins were purified on HisTrap FF columns with Äkta purifier (GE Healthcare, Piscataway, NJ). Excess urea was reduced by stepwise dialysis. The integrity of recombinant proteins was confirmed by SDS-PAGE. Each recombinant protein was quantified by bicinchoninic acid (Pierce, Rockford, IL) protein assay.
Animal vaccination studies.
All animal studies were approved by the Oklahoma State University Institutional Animal Care and Use Committee (protocol VM1045). Female BALB/c mice weighing 20 to 24 g (Charles River Laboratories, Wilmington, MA) were used in these studies and housed in the animal resource facilities of Oklahoma State University, an AAALAC-accredited facility. Weaned, female, 4- to 5-month-old Angus crossbred beef calves were used and housed in the Wendal Wallace Bovine Research Park at Oklahoma State University. Calves had been screened by enzyme-linked immunosorbent assay (ELISA) for anti-M. haemolytica (whole cell) antibodies and found to have antibody values of <0.5 OD490 unit, which was found previously to be a normal background concentration for calves susceptible to challenge with M. haemolytica S1 (A. W. Confer, unpublished data). Upon arrival at the research facility, all calves were vaccinated with a 7-way clostridial and leptospiral vaccine and treated with an anthelmintic. The calves received free-choice native grass hay supplemented with grain rations throughout the study.
For mouse experiment 1, 84 mice were divided among 8 groups. On day 0, 12 mice each were vaccinated subcutaneously with either 10, 50, or 100 μg of OmpA, OmpD15, OmpP2, or SSA-1 plus Freund's incomplete adjuvant (FIA) (Sigma, St. Louis, MO), whereas 12 control mice were vaccinated with FIA plus saline. On day 0, 6 mice were anesthetized and their blood obtained from cardiac puncture, followed by euthanasia. On day 14, 6 mice per recombinant protein vaccine group were anesthetized, bled, and euthanized, and the remaining mice per group were revaccinated. On day 28, the remaining vaccinated and control mice were anesthetized, bled, and euthanized. Additionally, 5 naïve mice were kept in a separate cage for the duration of the study. Sera from all vaccinated, negative-control, and naïve mice were collected and analyzed quantitatively individually to determine antibody responses to M. haemolytica whole cells and OMPs via single dilution and to the homologous recombinant protein via endpoint ELISA.
Mouse experiment 2 was designed to determine the interactions among the four recombinant proteins and their effects on the immune response to LKT/PlpE chimeric protein SAC89 (R2-NLKT-R2-NLKT). One hundred fifty mice were divided equally among 15 groups (Table 2). Mice were vaccinated on days 0 and 14 and bled on day 28. Control mice were bled on days 0 and 28.
Table 2.
Experimental design for mouse experiment 2 for determination of interactions of four M. haemolytica recombinant OMPs and their influences on antibody responses to the M. haemolytica PlpE/LKT chimeric protein (SAC89) and to each individual rOMP a
| Group | Vaccineb | No. of mice | Sample day |
|---|---|---|---|
| 1 | Day 0 control | 10 | 0 |
| 2 | SAC89 | 10 | 28 |
| 3 | SAC89/P2 | 10 | 28 |
| 4 | SAC89/D15 | 10 | 28 |
| 5 | SAC89/SSA-1 | 10 | 28 |
| 6 | SAC89/OmpA | 10 | 28 |
| 7 | SAC89/OmpA/P2 | 10 | 28 |
| 8 | SAC89/OmpA/D15 | 10 | 28 |
| 9 | SAC89/OmpA/SSA-1 | 10 | 28 |
| 10 | SAC89/P2/D15 | 10 | 28 |
| 11 | SAC89/P2/SSA-1 | 10 | 28 |
| 12 | SAC89/D15/SSA-1 | 10 | 28 |
| 13 | SAC89/OmpA/P2/D15 | 10 | 28 |
| 14 | SAC89/OmpA/P2/D15/SSA-1 | 10 | 28 |
| 15 | Day 28 control | 10 | 28 |
| Total | 150 |
Control groups were vaccinated with PBS.
FIA was used in all vaccines.
Seventeen calves were used in two studies (A and B). In cattle experiment A, three calves each were vaccinated subcutaneously on days 0 and 14 with 100 μg of OmpP2 or OmpD15 plus FIA. Three calves were likewise vaccinated with buffer plus FIA. In cattle experiment B, three calves each were vaccinated subcutaneously on days 0 and 14 with 100 μg of OmpA or SSA-1 plus FIA. Two calves were likewise vaccinated with buffer plus FIA. Serum samples were obtained from each calf in both experiments on days 0, 7, 14, 28, and 35.
SDS-PAGE and Western blots.
Recombinant proteins were resolved in 8 to 16% SDS-polyacrylamide preparative gels and transferred to nitrocellulose membranes as previously described (3). Following transfer, the nitrocellulose membranes were blocked with 1% casein in Tris-buffered saline (TBS) and cut into strips for use in immunoblots. Anti-His-tag monoclonal antibodies and sera from recombinant protein-vaccinated mice were used as primary antibodies at a dilution of 1:500 in TBS with 1% casein. Alkaline phosphatase-conjugated goat anti-mouse IgG(H+L) (Kirkegaard and Perry Labs, Gaithersburg, MD) was used as the secondary antibody, at a dilution of 1:2,500. The substrate for alkaline phosphatase was 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (BCIP/NBT) (Kirkegaard and Perry Labs, Gaithersburg, MD).
Enzyme-linked immunosorbent assay.
Antibody endpoint titers to recombinant proteins were determined using 2-fold serial dilutions of murine sera ranging from 1:400 to 1:819,200 (6). Purified recombinant proteins were used as ligands to coat ELISA plates at a concentration of 50 ng per well. Sera collected from recombinant protein-vaccinated or control mice and calves were used as primary antibodies. One-hundred-microliter aliquots of each serum dilution were dispensed into wells of microtiter plates in triplicate. Affinity-purified horseradish peroxidase-conjugated goat anti-mouse or goat anti-bovine IgG(H+L) (Kirkegaard and Perry Labs, Gaithersburg, MD) was used as the secondary antibody, and o-phenylenediamine (Amresco, Solon, OH) was used as the substrate, as described previously (3). Plates were read at 490 nm to determine the optical density on a Vmax Kinetic microtiter plate reader (Molecular Devices, Sunnyvale, CA). Statistically defined endpoint titers were calculated by the method of Frey et al. (17), a practical method for establishing a statistically valid cutoff. Sera were also assayed for anti-M. haemolytica IgG antibodies to OMPs by single-dilution ELISA as previously described (3, 11).
Complement-mediated serum bactericidal assay.
Complement-mediated killing assays were performed as previously described (5). Briefly, M. haemolytica cells were grown in BHI broth to an OD600 of 1.0. The cells were harvested by centrifugation at 10,000 × g for 10 min, resuspended in 1× phosphate-buffered saline (PBS), and decapsulated at 41°C for 1 h (7). The decapsulated cells were washed with 1× PBS, resuspended to an OD600 of 0.500, and diluted 1:1,000 in PBS (∼7.4 × 105 CFU/ml) for use in the assay. Prior to use, bovine sera were incubated at 56°C for 30 min to inactivate resident complement. Serum obtained from a colostrum-deprived neonatal calf was used as the source of complement for the assay. The assay was performed by mixing 25 μl each of bovine anti-rOMP serum, complement source, and decapsulated M. haemolytica cells (∼1.8 × 104 CFU) with PBS. At the beginning of the experiment (T0) and after 30 min of incubation at 37°C (T30), six replicates were plated on BHI blood agar plates. Viability was determined by counting the number of colonies after 15 to 16 h of incubation at 37°C and 5% CO2. Percent killing was calculated as follows: % killing = [(T0 growth − T30 growth)/T0 growth] × 100. Bovine hyperimmune serum from a calf vaccinated with rPlpE and naïve bovine serum were used as positive and negative controls, respectively (11). The assay was repeated at least three times.
Statistical analysis.
Log2 transformations were done for endpoint antibody titer analyses against individual rOMPs. Q-Q plots of transformed data were linear, and tests for kurtosis and skewness indicated that the transformed data approached normal distributions (39). Q-Q plots of OD values for anti-OMP antibodies for mouse and cattle studies were linear, and those data were not transformed. Log2 endpoint antibody responses against rOMPs and OD490 values for anti-OMP antibodies were analyzed by one-way analysis of variance with Tukey's honestly significant difference (HSD) post hoc test (39). Differences were considered significant when P values were <0.05.
RESULTS
Antigens.
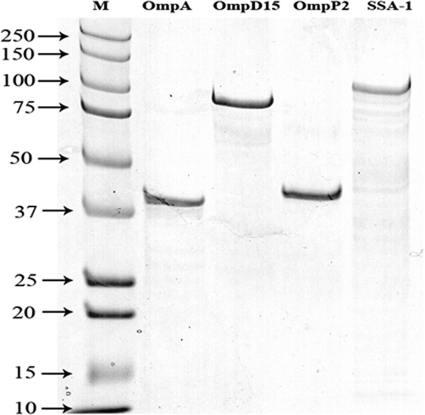
On SDS-PAGE, purified recombinant OmpA, OmpD15, OmpP2, and SSA-1 were visible as single bands, with molecular masses of approximately 41, 88, 42, and 104 kDa, respectively (Fig. 1).
Fig. 1.
SDS-PAGE analysis of recombinant outer membrane proteins OmpA, OmpD15, OmpP2, and SSA-1 stained with Coomassie brilliant blue. “M” designates Kaleidoscope Precision Plus protein standards, and the numbers indicate sizes in kDa.
Murine serologic responses.
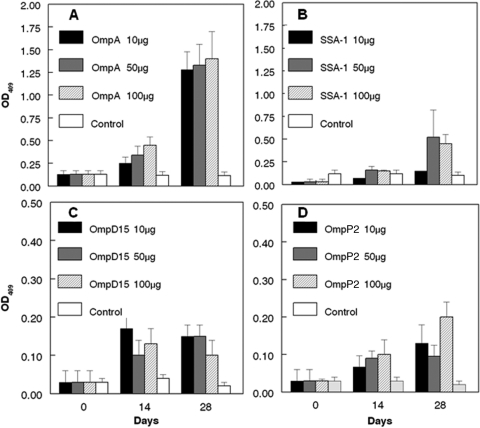
Mice vaccinated with rOmpA, rSSA-1, rOmpD15, and rOmpP2 developed significant antibody responses (P < 0.05) to M. haemolytica OMPs, in a generally dose-responsive manner (Fig. 2A to D). By day 14, significant responses (P < 0.05) to OMPs were seen for mice vaccinated with the following doses: 50 and 100 μg rOmpA, 50 μg rSSA-1, and 10 μg rOmpD15. By day 28, significant responses (P < 0.05) to OMPs were seen for mice vaccinated with the following doses: all rOmpA doses, 10 and 100 μg rSSA-1, 10 and 50 μg rOmpD15, and 100 μg rOmpP2. Endpoint titers against the respective individual rOMPs used for vaccination were determined for the mouse groups, and those responses were significantly increased (P < 0.05) for each antigen at each dose (Table 3). No significant differences (P > 0.05) were seen among endpoint titers for the vaccine doses used within each vaccine group.
Fig. 2.
Anti-M. haemolytica OMP antibodies for mice vaccinated with one of three different doses of rOmpA (A), rSSA-1 (B), rOmpD15 (C), or rOmpP2 (D). Antibody responses are expressed as mean OD490 values ± standard deviations (SD). Note that the y axis scales are the same for panels A and B and the same for panels C and D.
Table 3.
Geometric mean endpoint titers determined on day 28 for mice vaccinated with one of three doses of rOmpP2, rOmpD15, rSSA-1, or rOmpA or with PBSa
| Vaccine | Antigen dose (μg) | Geometric mean endpoint titer ± SD |
|||
|---|---|---|---|---|---|
| OmpP2 ELISA | OmpD15 ELISA | SSA-1 ELISA | OmpA ELISA | ||
| Control | 0 | 0 | 0 | 0 | 0 |
| OmpP2 | 10 | 6,400 ± 2.72 | |||
| 50 | 2,785.6 ± 1.2 | ||||
| 100 | 2,218.1 ± 4.8 | ||||
| OmpD15 | 10 | 819,200 ± 0.0 | |||
| 50 | 819,200 ± 0.0 | ||||
| 100 | 819,200 ± 0.0 | ||||
| SSA-1 | 10 | 310,428.8 ± 1.1 | |||
| 50 | 620,837.5 ± 0.4 | ||||
| 100 | 819,200 ± 0.0 | ||||
| OmpA | 10 | 713,155 ± 3.0 | |||
| 50 | 819,200 ± 0.0 | ||||
| 100 | 819,200 ± 0.0 | ||||
Endpoint titers were determined by the method of Frey et al. (17). All vaccines contained FIA.
Bovine antibody responses.
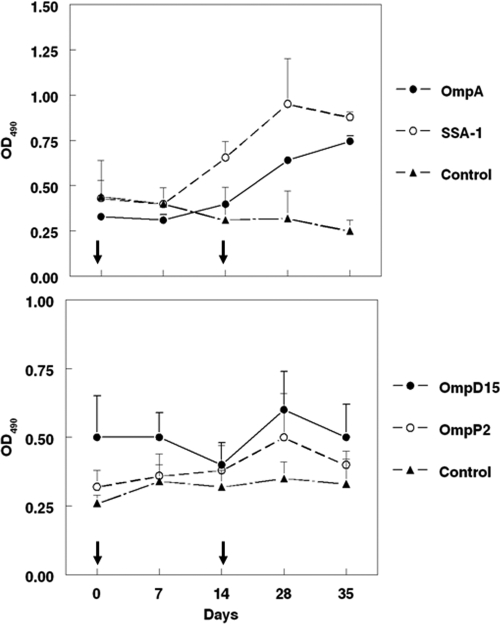
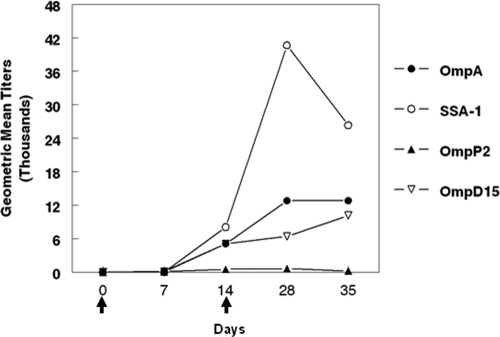
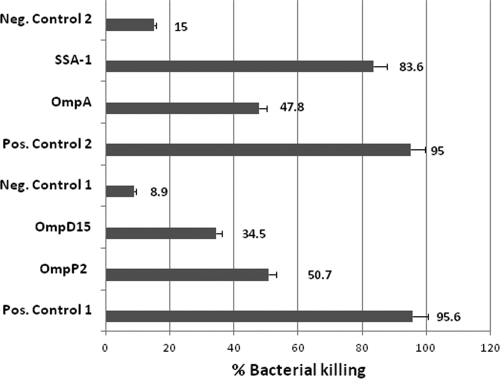
Cattle vaccinated with rOmpA and rSSA-1 developed significant antibodies (P < 0.05) to M. haemolytica OMPs by day 28, whereas cattle vaccinated with rOmpD15 and rOmpP2 developed only minimal responses to OMPs (P > 0.05) (Fig. 3). Endpoint antibody titers to rOmpA, rSSA-1, rOmpD15, and rOmpP2 were determined for cattle vaccinated with the respective antigens. Vaccination with rOmpA, rSSA-1, or rOmpD15 resulted in significant antibody responses to the respective antigens by day 28 (P < 0.05) (Fig. 4). Sera from cattle vaccinated with each of the recombinant proteins stimulated complement-mediated killing of the bacterium (Fig. 5).
Fig. 3.
Anti-OMP antibodies in cattle vaccinated with 100 μg of rOmpA, rSSA-1, rOmpD15, or rOmpP2. Controls were vaccinated with PBS. All vaccines contained FIA. Antibody responses are expressed as mean OD490 ± SD. Arrows indicate vaccination days.
Fig. 4.
Geometric mean endpoint antibody titers for cattle vaccinated with M. haemolytica rOMPs. The endpoint antibody titer for each vaccinee group against its respective rOMP is shown. Negative-control sera had values of <2 for each antigen and are not shown. Standard deviations calculated for geometric mean titers were too low to be demonstrated on the graph. Arrows indicate vaccination days.
Fig. 5.
Complement-mediated killing of M. haemolytica with sera from rOMP-vaccinated cattle. Results from two separate experiments were combined and reported. Positive and negative controls 1 were used in the experiments with sera from rOmpP2 and rOmpD15 vaccinees, and positive and negative controls 2 were used in experiments with sera from rOmpA and rSSA-1 vaccinees.
Vaccination of mice with multiple rOMPs.
Vaccination of mice with the chimeric protein SAC89 (R2-NLKT-R2-NLKT) alone stimulated a significant antibody response (P < 0.05) to SAC89 (Table 4). Concurrent vaccination with SAC89 plus any of the four rOMPs singly resulted in increased endpoint anti-SAC89 titers, and for the SAC89/rSSA-1 vaccinees, the response was increased significantly (P < 0.05). In contrast, the SAC89/P2/SSA-1 and SAC89/OmpA/P2/D15/SSA-1 combination vaccines resulted in significant decreases (P < 0.05) in anti-SAC89 antibodies compared to SAC89 vaccination alone.
Table 4.
Geometric mean antibody titers for mice vaccinated with SAC89, with or without additional recombinant OMPs (SSA-1, OmpD15, OmpA, or OmpP2)a
| Group | Vaccine | Geometric mean antibody titer ± SD |
|||||
|---|---|---|---|---|---|---|---|
| SAC89 ELISA | SSA-1 ELISA | OmpD15 ELISA | OmpA ELISA | OmpP2 ELISA | |||
| 1 | Control | 0 | 0 | 0 | 0 | 0 | |
| 2 | SAC89 | 58,813 ± 2.5a | |||||
| 3 | SAC89/OmpP2 | 155,209 ± 1.5a | 919.0 ± 0.3a | ||||
| 4 | SAC89/OmpD15 | 102,400 ± 0.5a | 540,470.4 ± 0.7a | ||||
| 5 | SAC89/SSA-1 | 270,235 ± 2.2b | 25,600 ± 1.1a | ||||
| 6 | SAC89/OmpA | 77,604 ± 3.5a | 38,802.3 ± 0.4a | ||||
| 7 | SAC89/OmpA/OmpP2 | 44,572 ± 2.5a | 9,700.6 ± 0.4b | 459.5 ± 0.3a | |||
| 8 | SAC89/OmpA/OmpD15 | 19,401 ± 1.8* | 356,577.5 ± 0.6a | 9,700.6 ± 0.8b | |||
| 9 | SAC89/OmpA/SSA-1 | 25,600 ± 0.0a | 5,571.5 ± 1.4b | 38,802.3 ± 0.6a | |||
| 10 | SAC89/OmpP2/OmpD15 | 44,572 ± 1.8a | 819,200 ± 0.0a | 800 ± 0.8a | |||
| 11 | SAC89/OmpP2/SSA-1 | 9,700 ± 2.9b | 9,700.6 ± 1.3a | 1,212.6 ± 0.8a | |||
| 12 | SAC89/OmpD15/SSA-1 | 51,200 ± 2.0a | 4,850.3 ± 1.2b | 470,506.8 ± 0.3a | |||
| 13 | SAC89/OmpA/OmpP2/OmpD15 | 33,778 ± 3.5a | 540,470.4 ± 0.7a | 11,143.1 ± 1.3b | 919.0 ± 0.8a | ||
| 14 | SAC89/OmpA/OmpP2/OmpD15/SSA-1 | 16,889 ± 1.5b | 1,392.9 ± 0.6b | 713,155 ± 0.3a | 44,572.2 ± 0.6a | 4,222.4 ± 1.3b | |
The control group was vaccinated with PBS. All vaccines contained FIA. Endpoint titers were determined by the method of Frey et al. (17). Different superscript letters (a and b) indicate significant differences (P < 0.05). The asterisk indicates that the value approaches significance (P = 0.056).
Endpoint antibody titers to the rOMPs with which the groups were vaccinated were determined (Table 3). Addition of OmpA, OmpD15, or OmpA/P2/D15 to SAC89/SSA-1 resulted in significant decreases in anti-SSA-1 antibodies. Anti-OmpD15 titers were not significantly different (P > 0.05) when the other rOMPs were added singly or in combination to the SAC89/D15 vaccine. Anti-OmpP2 titers were the lowest of all of the anti-rOMP titers. There were no significant differences (P > 0.05) among anti-OmpP2 titers for groups receiving the SAC89/OmpP2 vaccine with various combinations of other rOMPs, except for significantly increased (P < 0.05) responses induced by the SAC89/OmpA/OmpP2/OmpD15/SSA-1-vaccinated group. Finally, anti-OmpA titers were reduced significantly (P < 0.05) when OmpP2 and OmpD15 were added either singly or collectively to the SAC89/OmpA vaccine. Addition of other rOMPs failed to significantly (P > 0.05) reduce the anti-OmpA titers.
DISCUSSION
Vaccines that stimulate high levels of antibodies to LKT and to surface antigens enhance resistance to M. haemolytica challenge (43). We previously found that the outer membrane lipoprotein PlpE is an important surface antigen for stimulating immunity against M. haemolytica (10–12, 43). However, other outer membrane proteins most likely contribute to immunity against this complex Gram-negative bacterium. We therefore undertook the goal of immunologically studying other OMPs identified through immunoproteomic analyses of M. haemolytica outer membrane preparations (4). These include SSA-1, OmpP2, OmpD15, and OmpA (6, 19, 22, 31). M. haemolytica SSA-1 is an approximately 104-kDa OMP, and its gene was previously cloned and sequenced (19–21). Sequence comparisons of that antigen demonstrated it to be an autotransporter serine protease belonging to the peptidase S8 or subtilase family (18, 44). M. haemolytica OmpA, previously called PomA, is a heat-modifiable porin (28 to 35 kDa) that shares characteristics with other members of the Gram-negative OmpA family of proteins (31). M. haemolytica OmpP2 (41.4 kDa) is a homologue of the major outer membrane protein P2 of Haemophilus influenzae, which is known to have antigenic variation among H. influenzae isolates (1, 16). OmpD15 (also referred to as Omp85 or Oma87) is a high-molecular-mass OMP (∼88.8 kDa) that is a member of the envelope translocon complex with homologues in various Gram-negative bacteria, including Haemophilus ducreyi, Pasteurella multocida, H. influenzae, Shigella dysenteriae, Shigella flexneri, and Neisseria spp., as well as in mitochondria and chloroplasts of eukaryotes (32, 40–42).
In the studies reported here, as done previously with other M. haemolytica recombinant proteins, mice were used initially to determine the potential immunogenicity of rOMPs and to examine the dose response following vaccination. Although such studies would be more appropriate with cattle, the number of cattle needed and the expense of the study would be substantial and cost prohibitive. Therefore, our approach has been to conduct preliminary studies with mice and then, if a rOMP(s) appears to be immunogenic, to follow up with vaccination trials in cattle. In our previous vaccination studies using M. haemolytica rPlpE and the R2-NLKT-R2-NLKT chimera (SAC89), we demonstrated the induction of functional antibodies in mice, and subsequent cattle vaccination studies have corroborated those findings as well as demonstrating enhanced resistance against challenge (5, 10–12).
In the current study, vaccination of mice with each rOMP stimulated antibody responses to M. haemolytica outer membrane preparations, with rOmpA stimulating the highest responses, rSSA-1 stimulating intermediate responses, and rOmpD15 and rOmpP2 stimulating the lowest responses. When serologic responses were determined for the rOMP with which each group was vaccinated, rOmpP2 vaccination stimulated only low responses to that OMP. Although vaccination with rOmpD15 stimulated low antibody responses to OMP preparations, all three doses stimulated high responses to the OMP itself, which indicates that rOmpD15 is highly immunogenic. However, native OmpD15 may be present at low concentrations in the M. haemolytica outer membrane; therefore, a boost of antibodies to OmpD15 would not necessarily be reflected in a substantially higher response to the whole bacterium or its outer membrane. Low antibody responses to rOmpP2 and to M. haemolytica outer membrane preparations following rOmpP2 vaccination most likely indicate a low immunogenicity of rOmpP2. In contrast, the high antibody responses stimulated by vaccination with rOmpA indicate both high immunogenicity and a likely high copy number of the protein in the outer membrane. E. coli OmpA has been shown to be an abundant protein and a predominant antigen, occurring at a copy number of approximately 100,000 copies per cell (27, 45). With the demonstrated pathogenic functions of OmpA, such as epithelial adhesion and binding to fibronectin, and its immunogenicity, addition of rOmpA to M. haemolytica vaccines should be examined further (26, 29, 31).
Similarly, vaccination of cattle with rOmpA or rSSA-1 stimulated significant responses to M. haemolytica outer membrane preparations, whereas vaccination with rOmpD15 and rOmpP2 did not. Vaccination with rOmpP2 not only failed to stimulate antibodies to the outer membrane but also failed to induce antibodies to rOmpP2 itself. This further supports the findings obtained with mice and indicates that rOmpP2 is not highly immunogenic. In nontypeable H. influenzae, native OmpP2 has been shown to be highly immunogenic for rabbits and mice (8). Because immunoproteomic analyses of the M. haemolytica outer membrane demonstrated intense binding of convalescent-phase calf serum to native OmpP2, immunogenicity of the protein during natural infection can be assumed. The low immunogenicity following vaccination with rOmpP2 most likely indicates either conformational differences compared to the native protein or a lack of posttranscriptional modifications such as glycosylation or acylation, which could influence the host immune response.
SSA-1 colony and 2D gel immunoblots with bovine convalescent-phase anti-M. haemolytica sera previously demonstrated high antibody responses to the native protein (4, 30). Southern blotting demonstrated genomic fragments homologous to ssa1 in numerous M. haemolytica serotypes, and sequence homology between the ssa1 genes from serotypes 1 and 2 is >99% (19, 21). Vaccination of mice and cattle with rSSA-1 stimulated high antibody responses to M. haemolytica OMPs and to the protein itself. Addition of rSSA-1 also increased antibody responses of mice when it was given concurrently with the PlpE/LKT chimeric protein, SAC89. Given those results and previous findings of shared sequence homologies among SSA-1 proteins from multiple M. haemolytica serotypes, SSA-1 is a candidate for further study as a potential vaccine component.
Addition of the four M. haemolytica rOMPs individually to SAC89 caused approximately 1.5- to 5-fold increases in anti-SAC89 antibodies. Only rSSA-1 induced a significant increase. The cause of that phenomenon is unknown and may indicate that rSSA-1 had an adjuvant effect. However, with SSA-1 most likely being a serine protease, the recombinant protein could have modified SAC89, resulting in increased immunogenicity, perhaps by release of the two copies of each antigen and by increased efficacy of antigen-processing and antibody-producing cells (5). Vaccination with most other combinations of rOMPs and SAC89 resulted in reduced anti-SAC89 responses. Antigenic competition or interference is when “the immune response to an antigen may be reduced if an unrelated antigen is administered simultaneously…” (http://medical-dictionary.thefreedictionary.com/antigenic+competition) and has been documented with several polyvalent bacterial antigens (24, 35). Similarly, addition of a native OMP, PalA, to Actinobacillus pleuropneumoniae toxoid vaccines reduced the protective immunity of pigs challenged with this bacterium (46). In addition, suppression of the immune responses to multiple capsular polysaccharide conjugate vaccines was seen when vaccines were combined into a single multivalent injection compared to monovalent vaccines (15). Insel (25) listed several possible causes of reduced immunogenicity associated with multiple vaccines. First, physical or chemical interactions among proteins and polysaccharides may occur that affect the stability of antigens. Second, different solubilities of the combined antigens in a single buffer could result in precipitation of one or more antigens. Third, use of a single adjuvant may affect individual antigen immunogenicity. In the current study, antigenic competition was demonstrated when these antigens were combined at a single dose. Combining various recombinant proteins into a functional and licensable vaccine requires optimization of each antigen dose, buffer solubility, and adjuvant compatibility. Demonstration of a lack of antigenic competition or interference is required for licensing multivalent vaccines (14).
In conclusion, under the conditions of these experiments, vaccination of mice and cattle with rOmpA and rSSA-1 stimulated high antibody responses, whereas rOmpD15 and rOmpP2 were less immunogenic. Epitope mapping and future vaccination and challenge studies are needed to determine if rOmpA and rSSA-1 have potential in vaccines to enhance protection of cattle against M. haemolytica infection.
ACKNOWLEDGMENTS
This project was supported by Agriculture and Food Research Initiative competitive grant 2009-01626 from the USDA National Institute of Food and Agriculture.
Footnotes
Published ahead of print on 5 October 2011.
REFERENCES
- 1. Andersen C., et al. 2003. Porin OmpP2 of Haemophilus influenzae shows specificity for nicotinamide-derived nucleotide substrates. J. Biol. Chem. 278:24269–24276 [DOI] [PubMed] [Google Scholar]
- 2. Ayalew S., Blackwood E. R., Confer A. W. 2006. Sequence diversity of the immunogenic outer membrane lipoprotein PlpE from Mannheimia haemolytica serotypes 1, 2, and 6. Vet. Microbiol. 114:260–268 [DOI] [PubMed] [Google Scholar]
- 3. Ayalew S., Confer A. W., Blackwood E. R. 2004. Characterization of immunodominant and potentially protective epitopes of Mannheimia haemolytica serotype 1 outer membrane lipoprotein PlpE. Infect. Immun. 72:7265–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayalew S., Confer A. W., Hartson S. D., Shrestha B. 2010. Immunoproteomic analyses of outer membrane proteins of Mannheimia haemolytica and identification of potential vaccine candidates. Proteomics 10:2151–2164 [DOI] [PubMed] [Google Scholar]
- 5. Ayalew S., et al. 2008. Mannheimia haemolytica chimeric protein vaccine composed of the major surface-exposed epitope of outer membrane lipoprotein PlpE and the neutralizing epitope of leukotoxin. Vaccine 26:4955–4961 [DOI] [PubMed] [Google Scholar]
- 6. Ayalew S., Shrestha B., Montelongo M., Wilson A., Confer A. W. 2011. Identification and immunogenicity of Mannheimia haemolytica S1 outer membrane lipoprotein PlpF. Vaccine 29:8712–8718 [DOI] [PubMed] [Google Scholar]
- 7. Chae C. H., Gentry M. J., Confer A. W., Anderson G. A. 1990. Resistance to host immune defense mechanisms afforded by capsular material of Pasteurella haemolytica, serotype 1. Vet. Microbiol. 25:241–251 [DOI] [PubMed] [Google Scholar]
- 8. Chong P., et al. 1993. Immunogenicity of overlapping synthetic peptides covering the entire sequence of Haemophilus influenzae type b outer membrane protein P2. Infect. Immun. 61:2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Confer A. W. 2009. Update on bacterial pathogenesis in BRD. Anim. Health Res. Rev. 10:145–148 [DOI] [PubMed] [Google Scholar]
- 10. Confer A. W., et al. 2009. Immunity of cattle following vaccination with a Mannheimia haemolytica chimeric PlpE-LKT (SAC89) protein. Vaccine 27:1771–1776 [DOI] [PubMed] [Google Scholar]
- 11. Confer A. W., et al. 2003. Immunogenicity of recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE and augmentation of a commercial vaccine. Vaccine 21:2821–2829 [DOI] [PubMed] [Google Scholar]
- 12. Confer A. W., Ayalew S., Panciera R. J., Montelongo M., Wray J. H. 2006. Recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE enhances commercial M. haemolytica vaccine-induced resistance against serotype 6 challenge. Vaccine 24:2248–2255 [DOI] [PubMed] [Google Scholar]
- 13. Confer A. W., McCraw R. D., Durham J. A., Morton R. J., Panciera R. J. 1995. Serum antibody responses of cattle to iron-regulated outer membrane proteins of Pasteurella haemolytica A1. Vet. Immunol. Immunopathol. 47:101–110 [DOI] [PubMed] [Google Scholar]
- 14. Confer A. W., Montelongo M., Brown M. J., Fergen B. J., Clement J. C. 2001. Onset of serum antibodies to Pasteurella (Mannheimia) haemolytica following vaccination with five commercial vaccines. Bovine Pract. 35:141–148 [Google Scholar]
- 15. Fattom A., et al. 1999. Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine 17:126–133 [DOI] [PubMed] [Google Scholar]
- 16. Forbes K. J., Bruce K. D., Ball A., Pennington T. H. 1992. Variation in length and sequence of porin (ompP2) alleles of non-capsulate Haemophilus influenzae. Mol. Microbiol. 6:2107–2112 [DOI] [PubMed] [Google Scholar]
- 17. Frey A., Di Canzio J., Zurakowski D. 1998. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 221:35–41 [DOI] [PubMed] [Google Scholar]
- 18. Gioia J., et al. 2006. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. J. Bacteriol. 188:7257–7266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez C., Murtaugh M. P., Maheswaran S. K. 1991. Genomic distribution of a serotype 1-specific antigen-coding DNA fragment of Pasteurella haemolytica. Zentralbl. Veterinarmed. B 38:599–609 [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez C. T., Maheswaran S. K. 1993. The role of induced virulence factors produced by Pasteurella haemolytica in the pathogenesis of bovine pneumonic pasteurellosis: review and hypotheses. Br. Vet. J. 149:183–193 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez C. T., Maheswaran S. K., Murtaugh M. P. 1995. Pasteurella haemolytica serotype 2 contains the gene for a noncapsular serotype 1-specific antigen. Infect. Immun. 63:1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez-Rayos C., Lo R. Y., Shewen P. E., Beveridge T. J. 1986. Cloning of a serotype-specific antigen from Pasteurella haemolytica A1. Infect. Immun. 53:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Highlander S. K., Weissenberger S., Alvarez L. E., Weinstock G. M., Berget P. B. 2006. Complete nucleotide sequence of a P2 family lysogenic bacteriophage, varphiMhaA1-PHL101, from Mannheimia haemolytica serotype A1. Virology 350:79–89 [DOI] [PubMed] [Google Scholar]
- 24. Hunt J. D., Jackson D. C., Brown L. E., Wood P. R., Stewart D. J. 1994. Antigenic competition in a multivalent foot rot vaccine. Vaccine 12:457–464 [DOI] [PubMed] [Google Scholar]
- 25. Insel R. A. 1995. Potential alterations in immunogenicity by combining or simultaneously administering vaccine components. Ann. N. Y. Acad. Sci. 754:35–47 [DOI] [PubMed] [Google Scholar]
- 26. Kisiela D. I., Czuprynski C. J. 2009. Identification of Mannheimia haemolytica adhesins involved in binding to bovine bronchial epithelial cells. Infect. Immun. 77:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koebnik R., Locher K. P., Van Gelder P. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239–253 [DOI] [PubMed] [Google Scholar]
- 28. Lainson F. A., Murray J., Davies R. C., Donachie W. 1996. Characterization of epitopes involved in the neutralization of Pasteurella haemolytica serotype A1 leukotoxin. Microbiology 142:2499–2507 [DOI] [PubMed] [Google Scholar]
- 29. Lo R. Y., Sorensen L. S. 2007. The outer membrane protein OmpA of Mannheimia haemolytica A1 is involved in the binding of fibronectin. FEMS Microbiol. Lett. 274:226–231 [DOI] [PubMed] [Google Scholar]
- 30. Lo R. Y., Strathdee C. A., Shewen P. E., Cooney B. J. 1991. Molecular studies of Ssa1, a serotype-specific antigen of Pasteurella haemolytica A1. Infect. Immun. 59:3398–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahasreshti P. J., et al. 1997. Purification and partial characterization of the OmpA family of proteins of Pasteurella haemolytica. Infect. Immun. 65:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manning D. S., Reschke D. K., Judd R. C. 1998. Omp85 proteins of Neisseria gonorrhoeae and Neisseria meningitidis are similar to Haemophilus influenzae D-15-Ag and Pasteurella multocida Oma87. Microb. Pathog. 25:11–21 [DOI] [PubMed] [Google Scholar]
- 33. Morton R. J., et al. 1995. Vaccination of cattle with outer membrane protein-enriched fractions of Pasteurella haemolytica and resistance against experimental challenge exposure. Am. J. Vet. Res. 56:875–879 [PubMed] [Google Scholar]
- 34. Mosier D. A., Simons K. R., Confer A. W., Panciera R. J., Clinkenbeard K. D. 1989. Pasteurella haemolytica antigens associated with resistance to pneumonic pasteurellosis. Infect. Immun. 57:711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nikoskelainen S., et al. 2007. Multiple whole bacterial antigens in polyvalent vaccine may result in inhibition of specific responses in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 22:206–217 [DOI] [PubMed] [Google Scholar]
- 36. Panciera R. J., Confer A. W. 2010. Pathogenesis and pathology of bovine pneumonia. Vet. Clin. North Am. Food Anim. Pract. 26:191–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandher K., Confer A. W., Murphy G. L. 1998. Genetic and immunologic analyses of PlpE, a lipoprotein important in complement-mediated killing of Pasteurella haemolytica serotype 1. Infect. Immun. 66:5613–5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pandher K., Murphy G. L., Confer A. W. 1999. Identification of immunogenic, surface-exposed outer membrane proteins of Pasteurella haemolytica serotype 1. Vet. Microbiol. 65:215–226 [DOI] [PubMed] [Google Scholar]
- 39. Petrie A., Watson P. 1999. Statistics for veterinary and animal science, 1st ed. Blackwell Science, Ltd., Oxford, United Kingdom [Google Scholar]
- 40. Robb C. W., Orihuela C. J., Ekkelenkamp M. B., Niesel D. W. 2001. Identification and characterization of an in vivo regulated D15/Oma87 homologue in Shigella flexneri using differential display polymerase chain reaction. Gene 262:169–177 [DOI] [PubMed] [Google Scholar]
- 41. Ruffolo C. G., Adler B. 1996. Cloning, sequencing, expression, and protective capacity of the oma87 gene encoding the Pasteurella multocida 87-kilodalton outer membrane antigen. Infect. Immun. 64:3161–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schleiff E., Maier U. G., Becker T. 2011. Omp85 in eukaryotic systems: one protein family with distinct functions. Biol. Chem. 392:21–27 [DOI] [PubMed] [Google Scholar]
- 43. Shewen P. E., Wilkie B. N. 1988. Vaccination of calves with leukotoxic culture supernatant from Pasteurella haemolytica. Can. J. Vet. Res. 52:30–36 [PMC free article] [PubMed] [Google Scholar]
- 44. Siezen R. J., Leunissen J. A. 1997. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6:501–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith S. G., Mahon V., Lambert M. A., Fagan R. P. 2007. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol. Lett. 273:1–11 [DOI] [PubMed] [Google Scholar]
- 46. van den Bosch H., Frey J. 2003. Interference of outer membrane protein PalA with protective immunity against Actinobacillus pleuropneumoniae infections in vaccinated pigs. Vaccine 21:3601–3607 [DOI] [PubMed] [Google Scholar]