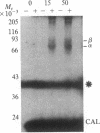
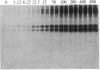
Abstract
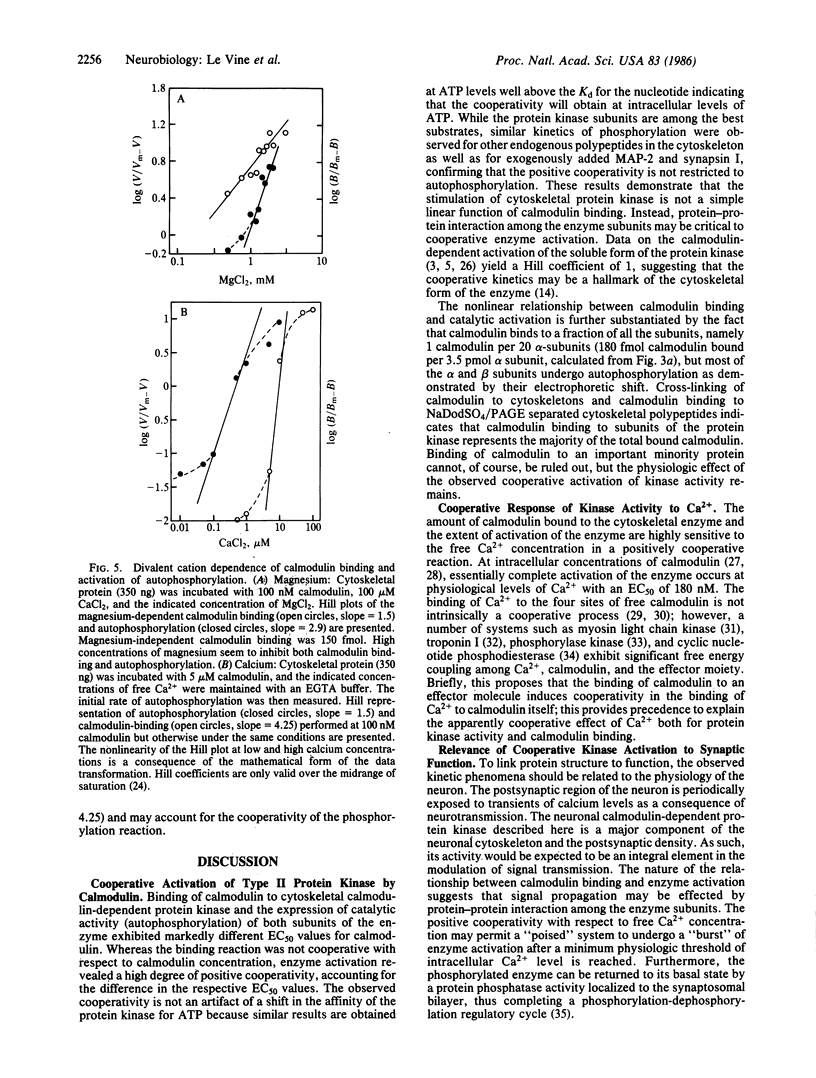
The kinetics of autophosphorylation of the cytoskeletal form of the neuronal calmodulin-dependent protein kinase type II were studied as a function of calmodulin binding under the same conditions. Whereas calmodulin binding was noncooperative with respect to calmodulin concentration (Hill coefficient = 1), the activation of autophosphorylation and the phosphorylation of exogenous substrates showed marked positive cooperativity (Hill coefficient greater than or equal to 1.6). Reduction of the active calmodulin concentration by the addition of the calmodulin antagonist trifluoperazine confirmed the cooperative nature of enzyme activation, because autophosphorylation was more sensitive to the drug than was binding at high concentrations of calmodulin. At intracellular levels of calmodulin the binding and activation of autophosphorylation were cooperative functions of magnesium and calcium concentration. The calmodulin-dependent cooperative activation seems to be a unique feature of the cytoskeletal, but not the soluble, form of the protein kinase and may result from the supramolecular organization of the cytoskeletal enzyme. These observations suggest that interactions among the subunits of the oligomeric cytoskeletal calmodulin-dependent protein kinase regulate enzyme activation, enhancing the sensitivity of the enzyme to small changes in the intracellular calcium levels that may be particularly relevant to signaling at the synapse.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., DePaoli-Roach A. A., Roach P. J. Purification and characterization of a rabbit liver calmodulin-dependent protein kinase able to phosphorylate glycogen synthase. J Biol Chem. 1982 Jul 25;257(14):8348–8355. [PubMed] [Google Scholar]
- Bader M. F., Hikita T., Trifaró J. M. Calcium-dependent calmodulin binding to chromaffin granule membranes: presence of a 65-kilodalton calmodulin-binding protein. J Neurochem. 1985 Feb;44(2):526–539. doi: 10.1111/j.1471-4159.1985.tb05445.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burger D., Stein E. A., Cox J. A. Free energy coupling in the interactions between Ca2+, calmodulin, and phosphorylase kinase. J Biol Chem. 1983 Dec 10;258(23):14733–14739. [PubMed] [Google Scholar]
- Caceres A., Bender P., Snavely L., Rebhun L. I., Steward O. Distribution and subcellular localization of calmodulin in adult and developing brain tissue. Neuroscience. 1983 Oct;10(2):449–461. doi: 10.1016/0306-4522(83)90145-8. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Goldenring J. R., McGuire J. S., Jr, DeLorenzo R. J. Identification of the major postsynaptic density protein as homologous with the major calmodulin-binding subunit of a calmodulin-dependent protein kinase. J Neurochem. 1984 Apr;42(4):1077–1084. doi: 10.1111/j.1471-4159.1984.tb12713.x. [DOI] [PubMed] [Google Scholar]
- Haiech J., Klee C. B., Demaille J. G. Effects of cations on affinity of calmodulin for calcium: ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry. 1981 Jun 23;20(13):3890–3897. doi: 10.1021/bi00516a035. [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Chau V., Chock P. B., Wang J. H., Sharma R. K. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C. H., Olwin B. B., LaPorte D. C., Storm D. R. Determination of the free-energy coupling for binding of calcium ions and troponin I to calmodulin. Biochemistry. 1982 Jan 5;21(1):156–162. doi: 10.1021/bi00530a027. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Identification of glycoproteins and proteins at synapses in the central nervous system. J Biol Chem. 1977 Jan 25;252(2):786–793. [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- Kuret J., Schulman H. Mechanism of autophosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1985 May 25;260(10):6427–6433. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd, Sahyoun N. E., Cuatrecasas P. Calmodulin binding to the cytoskeletal neuronal calmodulin-dependent protein kinase is regulated by autophosphorylation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):287–291. doi: 10.1073/pnas.82.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H., 3rd, Sahyoun N., Cuatrecasas P. Endogenous dephosphorylation of synaptosomal calmodulin-dependent protein kinase type II. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1212–1218. doi: 10.1016/0006-291x(85)90220-7. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Lisman J. E. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985 May;82(9):3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin B. B., Edelman A. M., Krebs E. G., Storm D. R. Quantitation of energy coupling between Ca2+, calmodulin, skeletal muscle myosin light chain kinase, and kinase substrates. J Biol Chem. 1984 Sep 10;259(17):10949–10955. [PubMed] [Google Scholar]
- Ouimet C. C., McGuinness T. L., Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. Methodology for in vitro studies of Ca-2+ transport. Anal Biochem. 1975 Jul;67(1):44–54. doi: 10.1016/0003-2697(75)90270-5. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., LeVine H., 3rd, Bronson D., Cuatrecasas P. Ca2+-calmodulin-dependent protein kinase in neuronal nuclei. J Biol Chem. 1984 Aug 10;259(15):9341–9344. [PubMed] [Google Scholar]
- Sahyoun N., LeVine H., 3rd, Bronson D., Siegel-Greenstein F., Cuatrecasas P. Cytoskeletal calmodulin-dependent protein kinase. Characterization, solubilization, and purification from rat brain. J Biol Chem. 1985 Jan 25;260(2):1230–1237. [PubMed] [Google Scholar]
- Sahyoun N., LeVine H., 3rd, Cuatrecasas P. Ca2+/calmodulin-dependent protein kinases from the neuronal nuclear matrix and post-synaptic density are structurally related. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4311–4315. doi: 10.1073/pnas.81.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol. 1984 Jul;99(1 Pt 1):11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Calmodulin-dependent protein phosphatase: a developmental study. Biochemistry. 1983 Jul 19;22(15):3630–3635. doi: 10.1021/bi00284a014. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Purification and characterization of the brain calmodulin-dependent protein kinase (kinase II), which is involved in the activation of tryptophan 5-monooxygenase. Eur J Biochem. 1983 Apr 15;132(1):15–21. doi: 10.1111/j.1432-1033.1983.tb07319.x. [DOI] [PubMed] [Google Scholar]