Abstract
Immunization of the world population before an influenza pandemic such as the 2009 H1N1 virus spreads globally is not possible with current vaccine production platforms. New influenza vaccine technologies, such as virus-like-particles (VLPs), offer a promising alternative. Here, we tested the immunogenicity and protective efficacy of a VLP vaccine containing hemagglutinin (HA) and M1 from the 2009 pandemic H1N1 influenza virus (H1N1pdm) in ferrets and compared intramuscular (i.m.) and intranasal (i.n.) routes of immunization. Vaccination of ferrets with VLPs containing the M1 and HA proteins from A/California/04/2009 (H1N1pdm) induced high antibody titers and conferred significant protection against virus challenge. VLP-vaccinated animals lost less weight, shed less virus in nasal washes, and had markedly lower virus titers in all organs tested than naïve controls. A single dose of VLPs, either i.m. or i.n., induced higher levels of antibody than did two doses of commercial split vaccine. Ferrets vaccinated with split vaccine were incompletely protected against challenge; these animals had lower virus titers in olfactory bulbs, tonsils, and intestines, but lost weight and shed virus in nasal washes to a similar extent as naïve controls. Challenge with heterologous A/Brisbane/59/07 (H1N1) virus revealed that the VLPs conferred minimal cross-protection to heterologous infection, as revealed by the lack of reduction in nasal wash and lung virus titers and slightly higher weight loss relative to controls. In summary, these experiments demonstrate the strong immunogenicity and protective efficacy of VLPs compared to the split vaccine and show that i.n. vaccination with VLPs has the potential for highly efficacious vaccination against influenza.
INTRODUCTION
Influenza viruses infect hundreds of millions of people each year, causing significant morbidity as well as hundreds of thousands of deaths worldwide (1, 50). In addition, novel influenza viruses can unpredictably enter the human population, leading to global pandemics in the naïve population. Vaccination is the cornerstone of public health programs to reduce seasonal and pandemic influenza morbidity and mortality. Inactivated influenza vaccines (IIVs) are highly effective in preventing disease caused by circulating viruses carrying the neutralization epitopes present in the vaccine. However, circulating viruses can rapidly escape host immunity by undergoing antigenic change. To maintain their efficacy, the antigen composition of IIVs has to be updated frequently to include newly emerged antigenic variants. Most recently, this was illustrated by the dramatic emergence and global spread of swine-origin 2009 pandemic H1N1 (H1N1pdm) influenza virus.
Several approaches have been proposed as alternatives to expand vaccine protection against antigenic variant viruses through vaccination. Live attenuated influenza viruses (LAIVs) are given intranasally (i.n.) and are thought to elicit protective immunologic memory against heterologous viruses by eliciting mucosal as well as cellular immunity, both of which are in general weakly induced by IIVs. Similarly, adjuvanted vaccines are thought to elicit protection against antigenically divergent viruses (29, 33, 43, 49). More recently, virus-like particle (VLP) vaccines against 1918 H1N1 influenza have elicited heterosubtypic anti-H5N1 immunity in mice and ferrets after intranasal, but not intramuscular (i.m.), administration (31).
VLPs mimic the influenza virus in size and structure, but are produced in insect cells by recombinant baculoviruses (reviewed in references 11, 16, 17, and 40). The advantages of this system over others used for vaccine production include its capacity for industrial-scale synthesis of multiple large proteins, while eliminating the requirement for embryonated eggs, thereby reducing the manufacturing time for VLPs in comparison with conventional IIVs. As well, even though the VLPs closely mimic the natural enveloped viruses, VLPs are noninfectious, reducing safety concerns related to pandemic and potentially pandemic influenza viruses, which also reduces costs and time of manufacture.
VLPs are more immunogenic than purified soluble viral proteins because their particulate structure mediates more efficient uptake into antigen-presenting cells and thus elicits effector and memory immune responses without adjuvants (11, 44). Accumulated evidence on VLP vaccines suggests that they are efficient at stimulating both cellular and humoral immune responses. Previous studies of influenza VLP vaccines against both seasonal (5, 9, 36, 42) and potential pandemic viruses (7, 12, 18, 25, 26, 32, 44–46, 48), delivered either intranasally or via conventional intramuscular immunization in mice, have demonstrated excellent immunogenicity and protection against viral challenge. As well as single-dose protection against the homologous (vaccine) viruses, in some cases influenza VLPs have also induced strong cross-protection against heterologous viruses (4, 5, 12, 34), especially after intranasal delivery (31). Several intranasal influenza VLP vaccines have been evaluated in humans with very promising results (reviewed in reference 40). The intranasal route for delivery of influenza vaccines offers a dual advantage by eliciting mucosal immunity and providing broader protection at the sites of virus entry. In addition, an intranasal vaccine may facilitate mass vaccination in cases of imminent pandemic threat.
In this study, we tested the efficacy of intranasal H1N1pdm VLPs in inducing immunity to homologous and heterologous influenza viruses, using the ferret model, which most closely reflects the human infection (3).
MATERIALS AND METHODS
Virus, vaccine, and cells.
An H1N1 influenza virus (A/California/04/2009) (CA/04) MDCK isolate was used to produce H1N1pdm VLPs (35). A/Texas/5/09 (H1N1) IDCDC-RG15 (TX/5) (39) was used as the antigen for H1N1pdm-specific antibodies in hemagglutination inhibition (HI) and enzyme-linked immunosorbent assays (ELISAs). A/California/07/2009 (CA/07), highly homologous to CA/04, was used in single radial immunodiffusion (SRID) assays. A/Brisbane/59/2007 (H1N1) (BR/59) was used as the ELISA antigen for seasonal H1N1-specific antibodies. Viruses used for ELISA antigens were grown in eggs, inactivated with β-propiolactone (BPL), and purified before use. A/New York/18/2009 (H1N1) (NY/18) was used as the homologous challenge virus (H1N1pdm), and BR/59 was used as the heterologous (seasonal influenza) challenge. Both viruses were generated from plasmids by a reverse genetics approach (14). Viruses derived by plasmid transfection of HK293 cells were propagated in MDCK cells. The genomes of the resulting viruses were sequenced to confirm the lack of mutations during propagation.
MDCK cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. A plaque assay was performed to determine virus titers using monolayers of MDKC cells with standard procedures (47).
Commercial monovalent H1N1pdm split vaccine containing CA/07 (Sanofi-Pasteur, Swiftwater, PA) was handled and stored according to the manufacturer's recommendations. Vaccine was used before the manufacturer's recommended expiration date.
VLP production and characterization.
cDNAs encoding hemagglutinin (HA) and M1 (to facilitate formation of VLPs) from the human pandemic H1N1 virus CA/04 MDCK isolate were subcloned into a baculovirus shuttle vector, and recombinant baculoviruses expressing HA and M1 were generated as previously described (35, 37). VLPs containing HA and M1 from CA/04 virus were produced in Sf9 insect cells, purified and characterized as previously described (34, 36, 37). Briefly, VLPs released into culture supernatants were harvested, concentrated using a hollow fiber filtration system, and subsequently purified by sucrose gradient ultracentrifugation. The amount of HA incorporated into VLPs was initially determined by Western blot analysis and by hemagglutination assays, as previously described (37, 38), and subsequently by single radial immunodiffusion (SRID) assays.
SRID assay.
The potency of the VLP vaccine was tested by single radial immunodiffusion (SRID) as described previously (51). Briefly, a reference antigen and sheep HA-specific antisera specific for CA/07 (H1N1) were obtained from the National Institute for Biological Standards and Control (NIBSC). VLPs and reference antigen were treated with 10% Zwittergent for 30 min at room temperature and then applied to the appropriate hole prepared on 1% agar gel containing antisera, according to the NIBSC instructions. Following 20 h of incubation at 22°C, the gel was stained with Coomassie brilliant blue to visualize the zone of antigen-antibody precipitation. After destaining, the gel was dried and the diameter of the zone produced by VLPs and reference antigen was measured. The concentration of HA in the VLP was calculated from the values obtained from the reference antigen (51).
Ferret immunization and viral challenge.
Male Fitch ferrets (Mustela putorius furo), 6 to 10 months of age (Triple F Farms, Sayre, PA) were used in the study. All animals were serologically negative based on the results of the HI test for currently circulating influenza viruses. Two groups of 5 ferrets received 3 μg HA of H1pdm VLPs (0.2 ml per injection) or 3 μg HA of commercial monovalent H1N1pdm split inactivated virus vaccine (0.1 ml per injection), via intramuscular (i.m.) injection. Another two groups of 5 animals were immunized with 15 μg HA of H1pdm VLPs via intranasal (i.n.) inoculation. For i.n. inoculation, ferrets were sedated by i.m. injection of ketamine/xylazine and laid on their backs, and 0.25 ml VLPs was instilled dropwise into the nares (half into each nostril), using a 1-ml syringe with a plastic catheter attached. Booster immunizations were given at 21 days postimmunization. Blood was collected from each ferret before the first immunization, on day 21 after the immunization, and on day 35 after the first immunization (day 14 after the boost). Sera were treated with receptor-destroying enzyme (RDE II “Seiken”) (Denka Seiken Co., Ltd., Tokyo, Japan), heat inactivated, and tested in a hemagglutination inhibition (HI) assay. Sera were also tested for total virus-binding IgG using an enzyme-linked immunosorbent assay (ELISA).
On day 40 after the first immunization (day 19 after the boost), animals were challenged intranasally with 0.5 ml of phosphate-buffered saline (PBS) containing 106 PFU of homologous virus (NY/18) or heterologous virus (BR/59), as described previously (27). Control groups of 5 naïve ferrets were also infected with NY/18 and BR/59. Morbidity was assessed by daily measurements of body weight and temperature. Body temperature was monitored using an electronic transponder chip implanted subcutaneously between the shoulder blades and read with an electronic probe (BioMedic Data Systems, Inc., Seaford, DE). Nasal washes were collected as described previously (27) on days 1, 3, 5, 7, and 9 after infection. In addition, two ferrets from each experimental and control group were euthanized on day 3 after infection to determine viral titers in tissues and organs. Olfactory bulb, brain, nasal turbinate, tonsils, trachea, lung, and small and large intestine were harvested, weighed, and homogenized in a volume of cold phosphate-buffered saline (PBS) to yield a 10% (wt/vol) suspension (23, 54). The material was clarified by centrifugation (2,200 × g) at 4°C, and the supernatant was used for virus quantification. Virus titers were determined by plaque assay in MDCK cells and expressed as log10 PFU in 1 ml of nasal washes and in 1 g of tissue sample. The limit of virus detection was 100 PFU per 1 ml of nasal washes or 200 PFU per g of organ tissues. Animal experiments were conducted in the enhanced animal biosafety level 3 facilities at the Centers for Disease Control and Prevention, and protocols were approved by the IACUC.
HI assay.
HI assays were performed as previously described (18). Briefly, receptor-destroying enzyme-treated serum samples (25 μl) were incubated with equal volumes of inactivated virus (4 hemagglutinating units [HAU] of TX/5 or BR/59 for homologous or heterologous assays, respectively) at room temperature for 30 min. After incubation, an equal volume of 0.5% turkey erythrocytes was added and incubated at 22°C for 1 h. The HI titer was expressed as the reciprocal of the highest dilution of the samples preventing hemagglutination.
ELISA.
Influenza virus-specific antibody titers were determined by ELISAs. Influenza viruses (TX/5 or BR/59) were grown in 10 to 11 days in embryonated eggs. Allantoic fluid was harvested and BPL inactivated. Viruses were pelleted by high-speed ultracentrifugation and then purified by sucrose gradient centrifugation. BPL-inactivated influenza viruses at a concentration of 5 μg/ml were used as a coating antigen (in 0.05 M sodium bicarbonate buffer, pH 9.6) on 96-well microtiter plates for 2 h at 37°C followed by 12 h at 4°C. Plates were washed once with PBS and then incubated at 37°C for 1 h in 0.1% bovine serum albumin (BSA)–PBS to block the remaining binding sites. Two-fold serial dilutions of 1:10-diluted ferret serum were added to each well and incubated for 1 h at 37°C. After removal of the test samples and subsequent washing (3 times with PBS plus 0.05% Tween 20 [PBST]), a 1:1,000 dilution of 1 mg/ml monoclonal mouse anti-mustelid IgG (AbD Serotec, Oxford, United Kingdom) or 1:10,000 goat anti-ferret IgA (Bethyl Laboratories, Montgomery, TX) was added to each well, and the plates were incubated at 37°C for 1 h. The plates were washed again (3 times with PBST), a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Alpha Diagnostic International, San Antonio, TX) or 1:20,000 rabbit anti-goat IgG (Bethyl Laboratories, Montgomery, TX) was added to each well, and the plates were incubated at 37°C for 1 h. This was followed by removal of the conjugate, washing and addition of O-phenylenediamine (OPD) and H2O2 as a substrate. Finally, absorbance was read at 450 nm.
RESULTS
VLP characterization.
Influenza vaccines are standardized based on the amount of HA contained in the vaccines. For comparative studies, it is important to quantify and normalize HA contents in different vaccines. As previously described (35), HA, predominantly in the precursor form, was the major component in VLP vaccines. VLPs were roughly spherical and were approximately 120 nm in diameter, as previously described (35). The concentration of HA in the VLP, as measured by an SRID test using a reference antigen and SRID-calibrated antisera (NIBSC) to the homologous CA/07, was found to be 0.6 mg/ml. VLP and commercial monovalent split vaccine doses with same amount of HA were used to immunize groups of ferrets by the i.m. route.
Antibody responses to VLP vaccines.
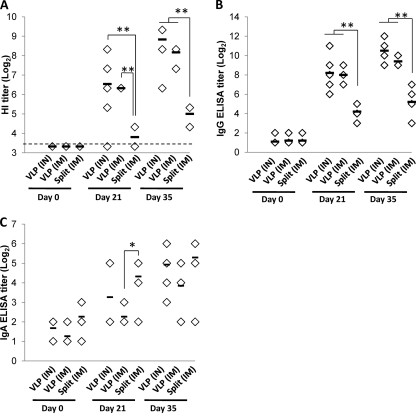
Ferrets were negative for influenza virus-specific responses prior to immunization. After immunization, ferrets responded to a single dose of H1N1pdm VLPs, delivered by either i.m. (3 μg) or i.n. (15 μg) inoculation, with moderate serum antibody titers of HI to the homologous TX/5 virus (Fig. 1A). High HI titers developed in serum after a second dose of VLPs. In comparison, the split H1N1pdm commercial vaccine was only moderately immunogenic after two doses of 3 μg HA given i.m., reaching lower HI titers than either route of VLP (P < 0.01, Mann-Whitney U test) (Fig. 1A). Serum antibody responses to homologous virus measured by ELISA plates coated with whole virus antigen paralleled HI titers, with VLPs (either i.n. or i.m.) inducing moderate titers of antiviral antibodies to the homologous TX/5 virus after a single immunization, and high titers after booster vaccination. The split vaccine induced moderate levels of antibodies to the homologous antigen after the booster dose (Fig. 1B), but these titers were significantly lower than either route of VLP immunization (P < 0.01, Mann-Whitney U test). Specific IgA antibodies were detectable after immunization with all three vaccines, although titers were lower and more variable than with i.n. or i.m. delivery (Fig. 1C). These results suggest that VLP vaccines are more immunogenic than split vaccines.
Fig. 1.
Antibody responses to homologous virus after vaccination. Ferrets were immunized on days 0 and 21 with A/California/04/2009 (H1N1pdm) HA VLPs (15 μg given i.n.[IN] or 3 μg given i.m. [IM]) or commercial split vaccine A/California/07/2009 (H1N1pdm) (3 μg given i.m.). Serum was measured preimmunization (day 0), prebooster (day 21), or 14 days after booster (day 35) using HI (A) or IgG (B) or IgA (C) ELISAs using β-propiolactone-inactivated homologous virus (A/Texas/5/2009 (H1N1pdm)) as the antigen, as described in the text. Each marker represents an individual ferret given the indicated treatment; horizontal bars indicate the average titer for each group. The dashed line in panel A represents the limit of detection. Significant differences (Mann-Whitney U test) of 0.01 < P < 0.05 and P < 0.01 are indicated by * and **, respectively. With the exception of the split vaccine at day 21, titers after immunization (days 21 and 35) were all significantly greater than preimmunization titers (P < 0.05, Mann-Whitney U test), and these differences are not indicated on the charts.
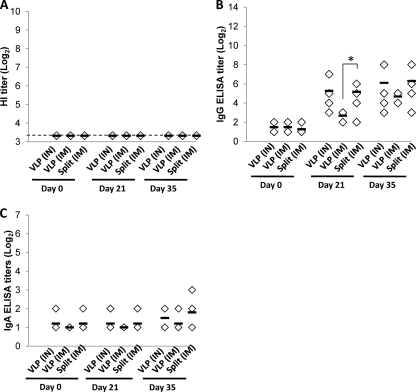
No cross-reactivity of sera from H1N1pdm-immunized ferrets with heterologous seasonal H1N1 influenza antigen (BR/59) was observed by HI assays (Fig. 2A): HI titers to BR/59 were below the limit of detection (<10), regardless of the route or form of immunization. In contrast to HI titers, ferrets immunized with H1pdm VLPs or split vaccine did develop moderate levels of antibodies that cross-reacted with BR/59 in ELISA with whole-virus antigen (Fig. 2B). In this assay, the split vaccine induced titers equivalent to i.n. VLPs and higher than i.m. VLPs, presumably because the split vaccine includes the neuraminidase (NA) antigen as well as HA and is therefore able to target more antigens in the whole-virus ELISA. However, no cross-reactive IgA response was detectable (Fig. 2C).
Fig. 2.
Antibody responses to heterologous virus after vaccination. Ferrets were immunized on days 0 and 21 with A/California/04/2009 (H1N1pdm) HA VLPs (15 μg given i.n. [IN]or 3 μg given i.m. [IM]) or commercial split vaccine A/California/07/2009 (H1N1pdm) (3 μg given i.m.). Serum responses preimmunization (day 0), prebooster (day 21), or 14 days after booster (day 35) were measured in HI (A) or IgG (B) or IgA (C) ELISAs using β-propiolactone-inactivated heterologous virus [A/Brisbane/59/2007 (H1N1)] as the antigen, as described in the text. Each marker represents an individual ferret given the indicated treatment; horizontal bars indicate the average titer for each group. The dashed line in panel A represents the limit of detection. Significant differences (Mann-Whitney U test) of 0.01 < P < 0.05 are indicated by *. In panel B, titers after immunization (days 21 and 35) were all significantly greater than preimmunization titers (P < 0.05, Mann-Whitney U test), and these differences are not indicated on the charts.
Protection against morbidity after homologous viral challenge.
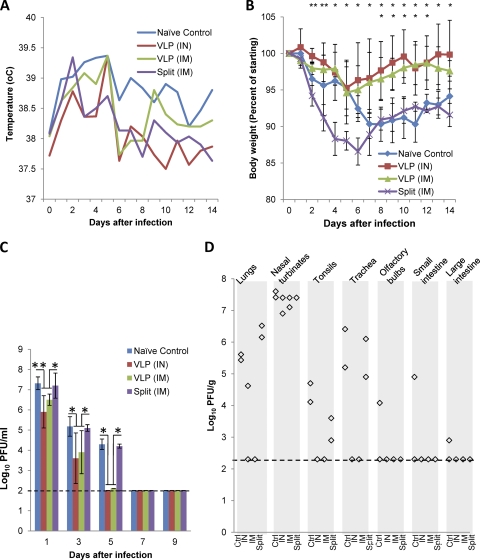
To assess the induction of protective immunity, vaccinated and naïve control ferrets were challenged with 106 PFU of homologous NY/18 or heterologous BR/59 virus intranasally at 19 days after the booster vaccination (40 days after the initial vaccination). After challenge, all ferrets showed a trend toward a moderate increase of temperature for 2 to 4 days (Fig. 3A). Nonimmunized ferrets lost approximately 10% of their body weight during the first 2 weeks after challenge, whereas ferrets vaccinated with VLPs (either i.n. or i.m.) showed only a transient and moderate weight loss (approximately 5% of body weight), recovering the lost weight rapidly (Fig. 3B). Unexpectedly, the split vaccine conferred little protection against disease symptoms, as ferrets receiving this immunization lost weight to a similar extent as naïve controls (Fig. 3B). Results from homologous virus challenge suggest that VLP vaccines might provide better protection than the split vaccine, as evidenced by less body weight loss.
Fig. 3.
Effect of challenge with homologous virus on VLP-vaccinated ferrets. Groups of 5 ferrets were vaccinated twice with A/California/04/2009 (H1N1pdm) HA VLPs (15 μg given i.n. [IN] or 3 μg given i.m. [IM]) or commercial split vaccine A/California/07/2009 (H1N1pdm) (3 μg given i.m.), or were not immunized (naïve control). Nineteen days after the second vaccination, ferrets were challenged intranasally with homologous virus [A/New York/18/2009 (H1N1pdm)]. (A) Ferret temperatures were monitored daily. Average temperatures for each group of ferrets are shown. (B) Ferret body weight was monitored daily. Shown are average body weights for each group. (C) On days 1, 3, 5, 7, and 9 after challenge, ferrets were sedated and nasal washes were collected. Virus titers (shown as log10 PFU/ml) were determined. The average virus titer for each group ± 1 standard deviation (SD) is shown. The limit of detection (100 PFU/ml) is indicated with a dashed line. Significant differences (Mann-Whitney U test) of 0.01 < P < 0.05 and P < 0.01 are indicated by * and **, respectively (upper row, split vaccine i.m. versus VLPs both i.m. and i.n.; lower row, naïve control versus VLPs both i.m. and i.n.). (D) Three days after infection two ferrets per group were euthanized. Organ samples, as indicated, were collected, and virus titers were determined. The limit of detection (200 PFU/g tissue) is indicated with a dashed line. Other tissues (brain, blood, liver, kidney, and spleen) were also tested and were negative (data not shown). Ctrl, control.
Protection against homologous viral replication and shedding.
VLP vaccination also provided significant protection against viral shedding. Even on the first day after challenge, when all animals shed high titers of virus in nasal washes, ferrets vaccinated with VLP by either route (i.n. and i.m.) showed significantly reduced virus shedding (P < 0.05, Mann-Whitney U test). By the fifth day postchallenge, virus was undetectable in the ferrets immunized with VLPs, while easily detectable levels of virus were still being shed by naïve ferrets as well as those immunized with split vaccine (Fig. 3C).
To further assess the impact of vaccination on virus replication, at 3 days after infection, two ferrets were sacrificed, and viral titers were measured in the olfactory bulb, brain, nasal turbinate, tonsils, trachea, lung, and small and large intestines. Naïve ferrets had easily detectable levels of virus in all organs tested except for the brain (Fig. 3D). All three vaccination regimens (VLPs and split vaccine) reduced to undetectable levels viral titers in large and small intestines and olfactory bulbs. Ferrets receiving either i.n. or i.m. VLPs also had undetectable levels of virus in tonsils and trachea, while those immunized with split vaccine had detectable virus in tonsils, but at lower levels than naïve ferrets. Ferrets receiving VLPs, but not those receiving split vaccine, had reduced viral titers in lungs and nasal turbinates compared to naïve controls (Fig. 3D). One ferret receiving i.n. VLPs had detectable virus in the lungs, while both ferrets receiving i.m. VLP had no detectable virus in the lungs (Fig. 3D). These results indicate that immunization with either VLPs or split vaccine prevented the spread of the virus to the peripheral tissues such as olfactory bulb and intestines and that VLPs were more effective in restricting the viral spread to the nasal turbinates, tonsils, and trachea.
Lack of protection against challenge with heterologous virus.
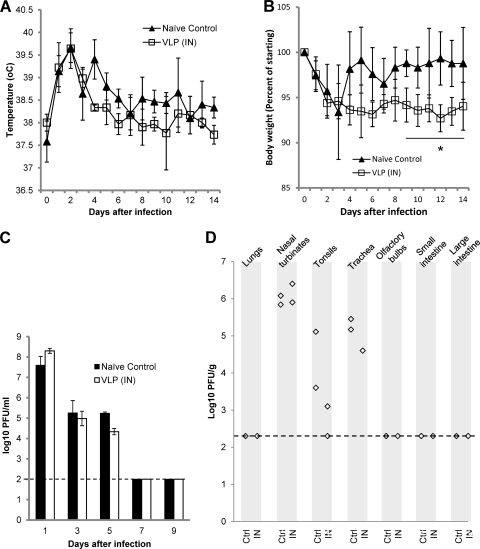
In contrast to the challenge with homologous virus, and consistent with the undetectable HI antibody response to heterologous BR/59 virus, VLPs conferred little or no protection against challenge with heterologous virus. Compared to naïve controls, vaccinated ferrets challenged with BR/59 had similar temperature (Fig. 4A) and lost weight to at least a similar extent (Fig. 4B). Vaccinated ferrets showed marginally greater weight loss than the naïve control (P = 0.04, Mann-Whitney U). Replication of the virus was also not affected by vaccination: Naïve ferrets and ferrets vaccinated by i.n. VLPs, which were subsequently challenged with BR/59 virus, shed similar amounts of virus in nasal washes for similar periods (Fig. 4C). Three days after challenge, two ferrets were sacrificed, and virus titers in internal organs were measured. Virus recovered from nasal turbinate, tonsils, and trachea reached similar titers in vaccinated and naïve animals (Fig. 4D).
Fig. 4.
Effect of challenge with heterologous virus on VLP-vaccinated ferrets. Groups of 5 ferrets were vaccinated twice with 15 μg A/California/04/2009 (H1N1pdm) HA VLPs intranasally (i.n. [IN]) or were not immunized (naïve control). Nineteen days after the second vaccination, ferrets were challenged intranasally with heterologous virus [A/Brisbane/59/2007 (H1N1)]. (A) Ferret temperatures were monitored daily (average temperatures for each group of ferrets ± 1 SD). (B) Ferret body weight was monitored daily (average body weights for each group ± 1 SD, as a percentage of the body weight on day 0). Significant differences (Mann-Whitney U test) of P = 0.04 are indicated by *. (C) On days 1, 3, 5, 7, and 9 after challenge, ferrets were sedated and nasal washes were collected. Virus titers (shown as log10 PFU/ml) were determined. The average virus titer for each group ± 1 SD is shown. The limit of detection (100 PFU/ml) is indicated by a dashed line. (D) Three days after infection, two ferrets per group were euthanized. Organ samples, as indicated, were collected, and virus titers were determined. The limit of detection (200 PFU/g tissue) is indicated by a dashed line. Other tissues (brain, blood, liver, kidney, and spleen) were also tested and were negative (data not shown). Ctrl, control.
DISCUSSION
VLPs are a promising method of vaccination that combine high immunogenicity with safety and rapid, efficient production. VLPs have been investigated for many viruses, including hepatitis C virus, several retroviruses, severe acute respiratory syndrome (SARS) coronavirus, and influenza virus A (13, 15, 20, 21, 28, 52; reviewed in reference 40). Previous experiments have demonstrated that VLPs containing influenza virus HA and NA are effective in preventing homologous and, in some cases, heterologous viral infections. The potential ease of use, safety, and immunogenicity of VLPs make them an attractive option for clinical use and could offer major advantages for mass vaccination in case of a pandemic. Intranasal (i.n.) vaccination has been demonstrated to induce a broader range of protection against lethal challenge (31). In addition, i.n. vaccination is an attractive option clinically, because it avoids injection. Here, we tested the immunogenicity and protective efficacy of a VLP vaccine containing HA and M1 from the 2009 pandemic H1N1 influenza virus, using ferrets as the most similar small animal model to the human infection and comparing i.m. and i.n. routes of immunization.
Vaccination of ferrets with VLPs containing only the M1 and HA proteins from H1N1pdm induced high antibody titers and conferred significant protection against homologous virus challenge. VLP-vaccinated animals lost less weight, shed less virus in nasal washes, and had markedly lower virus titers in all organs tested than naïve controls. A single dose of VLPs, either i.m. or i.n., induced higher levels of antibody than did two doses of commercial split vaccine, although an antibody response to the split vaccine was detectable by HI and ELISA. This is especially remarkable in that ELISA titers (which can detect antibody responses against a wide range of viral antigens) as well as HI titers were higher with the VLPs, even though the split vaccine (which includes NA as well as HA) provided an additional antigen to be targeted by the antibody response. In fact, the broader response induced by the split vaccine was observed in ELISAs against heterologous virus, in which the split vaccine induced equal titers of antibodies as the i.n. VLPs and higher than the i.m. VLPs. In these assays, antibodies might have directed at more conserved regions of HA that do not neutralize the virus or prevent hemagglutination are detected, explaining the significant ELISA titers against heterologous virus that did not correspond to detectable HI titers.
The ferrets vaccinated with split vaccine were incompletely protected against challenge. Although these animals had lower virus titers in olfactory bulbs, tonsils, and intestines, they lost weight and shed virus in nasal washes to a similar extent as naïve controls. The immunogenicity and protective efficacy of H1N1pdm split vaccine have been previously demonstrated in mice (6, 53) and humans (6, 19, 22, 24, 30, 41). In naïve ferrets, however, the split vaccine was previously shown to be only weakly immunogenic and partially protective (2, 8), consistent with our observations here. Humans vaccinated with H1N1pdm split vaccine are probably primed by previous exposure to seasonal viruses and therefore react more vigorously than naïve ferrets. The challenge dose in the present experiments, at 106 PFU, may have been too high to be controlled by the modest level of antibody induced by the split vaccine. In any case, VLP vaccination by either the i.m. or i.n. route was capable of dramatically reducing viral replication and disease even at this challenge dose, reinforcing the superior immunogenicity of VLPs.
In some previous experiments, VLP vaccines were shown to induce a relatively broad cross-protection against heterologous viruses of the same subtype in mice (4, 5, 12, 34), especially when delivered i.n. (31). We tested the ability of i.n. H1pdm VLPs to confer protection against challenge with the heterologous seasonal H1N1 influenza virus BR/59. Despite high titers of antibodies against homologous viruses by both HI and ELISAs and moderate ELISA titers against the heterologous virus, no antibody was detected in HI assays, indicating that the antibody response against heterologous virus was directed at conserved regions of HA and is not capable of blocking binding of the HA to its receptors. Consistent with this, the VLPs conferred little or no cross-protection to the challenge virus, as revealed by the lack of reduction in nasal wash virus titers, similar virus titers in organs, and lack of protection against disease, as indicated by similar levels of weight loss.
In a previous study with CA/04 VLPs, mice did develop partial cross-protection against a different heterologous virus, PR8 (37). One possibility is that mice are more readily protected against influenza virus infection, even with minimal levels of cross-reactive immune responses. For example, partial cross-protection was observed in mice where antibody titers reactive to heterologous virus were lower by more than 10-fold compared to those reactive to the homologous virus (37). It is likely that protective immunity is more difficult to achieve in ferrets, which (like humans) are intrinsically more susceptible to influenza virus than are mice. As well, the HA of the heterologous challenge virus used in this study, BR/59, is not as closely related to that of the CA/07 virus as is the PR8 challenge virus used previously (37), making cross-protection more difficult to achieve. Indeed, lack of cross-protective immunity (even after infection with wild-type viruses) between H1N1 viruses such as BR/59 and the H1N1pdm viruses may explain the rapid global spread of the H1N1pdm virus. Even though the VLPs delivered i.n. were more immunogenic than the split vaccine and did induce cross-reactive antibodies, apparently most of those antibodies lacked critical protective functions. Further study is necessary to fully understand the extent of cross-protective immunity by VLPs and other vaccines and to identify vaccination methods of consistently inducing broadly protective immune responses.
In summary, these experiments demonstrate the strong immunogenicity and protective efficacy of VLPs compared to the split vaccine and show that i.n. delivery of VLPs has the potential for highly efficacious vaccination against influenza.
ACKNOWLEDGMENTS
This work was supported in part by NIH/NIAID grants AI0680003 (R.W.C.) and AI074579 (R.W.C.). This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
We thank the staff of the Animal Resources Branch of the CDC for outstanding animal care. We thank the Center for Biologics Evaluation and Research (FDA) for providing SRID reagents.
The findings and conclusions in this report are those of the author and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
As a potential conflict of interest, S.-M.K., R.W.C., and Emory University have equity interests in Zetra Biologicals, which is developing virus-like particle technology under license from Emory University. This does not alter our adherence to all NIH policies on sharing data and materials.
Footnotes
Published ahead of print on 26 October 2011.
REFERENCES
- 1. Anonymous. 2010. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 59:1057–1062 [PubMed] [Google Scholar]
- 2. Baras B., et al. 2011. Pandemic H1N1 vaccine requires the use of an adjuvant to protect against challenge in naive ferrets. Vaccine 29:2120–2126 [DOI] [PubMed] [Google Scholar]
- 3. Bodewes R., Rimmelzwaan G. F., Osterhaus A. D. 2010. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev. Vaccines 9:59–72 [DOI] [PubMed] [Google Scholar]
- 4. Bright R. A., et al. 2008. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One 3:e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bright R. A., et al. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25:3871–3878 [DOI] [PubMed] [Google Scholar]
- 6. Caillet C., et al. 2010. AF03-adjuvanted and non-adjuvanted pandemic influenza A (H1N1) 2009 vaccines induce strong antibody responses in seasonal influenza vaccine-primed and unprimed mice. Vaccine 28:3076–3079 [DOI] [PubMed] [Google Scholar]
- 7. Crevar C. J., Ross T. M. 2008. Elicitation of protective immune responses using a bivalent H5N1 VLP vaccine. Virol. J. 5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellebedy A. H., et al. 2010. Contemporary seasonal influenza A (H1N1) virus infection primes for a more robust response to split inactivated pandemic influenza A (H1N1) virus vaccination in ferrets. Clin. Vaccine Immunol. 17:1998–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galarza J. M., Latham T., Cupo A. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18:244–251 [DOI] [PubMed] [Google Scholar]
- 10. Reference deleted.
- 11. Haynes J. R. 2009. Influenza virus-like particle vaccines. Expert Rev. Vaccines 8:435–445 [DOI] [PubMed] [Google Scholar]
- 12. Haynes J. R., et al. 2009. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27:530–541 [DOI] [PubMed] [Google Scholar]
- 13. Ho Y., Lin P. H., Liu C. Y., Lee S. P., Chao Y. C. 2004. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochem. Biophys. Res. Commun. 318:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann E., Neumann G., Kawaoka Y., Hobom G., Webster R. G. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. U. S. A. 97:6108–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeong S. H., et al. 2004. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J. Virol. 78:6995–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang S., Pushko P., Bright R., Smith G., Compans R. 2009. Influenza virus-like particles as pandemic vaccines. Curr. Top. Microbiol. Immunol. 333:269–289 [DOI] [PubMed] [Google Scholar]
- 17. Kang S. M., Song J. M., Quan F. S., Compans R. W. 2009. Influenza vaccines based on virus-like particles. Virus Res. 143:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang S. M., et al. 2009. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One 4:e4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kao T. M., et al. 2010. Immune response of single dose vaccination against 2009 pandemic influenza A (H1N1) in the Taiwanese elderly. Vaccine 28:6159–6163 [DOI] [PubMed] [Google Scholar]
- 20. Latham T., Galarza J. M. 2001. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 75:6154–6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lechmann M., et al. 2001. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology 34:417–423 [DOI] [PubMed] [Google Scholar]
- 22. Liang X. F., et al. 2010. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375:56–66 [DOI] [PubMed] [Google Scholar]
- 23. Lipatov A. S., Kwon Y. K., Pantin-Jackwood M. J., Swayne D. E. 2009. Pathogenesis of H5N1 influenza virus infections in mice and ferret models differs according to respiratory tract or digestive system exposure. J. Infect. Dis. 199:717–725 [DOI] [PubMed] [Google Scholar]
- 24. Lu C. Y., et al. 2010. Immunogenicity and safety of a monovalent vaccine for the 2009 pandemic influenza virus A (H1N1) in children and adolescents. Vaccine 28:5864–5870 [DOI] [PubMed] [Google Scholar]
- 25. Mahmood K., et al. 2008. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine 26:5393–5399 [DOI] [PubMed] [Google Scholar]
- 26. Matassov D., Cupo A., Galarza J. M. 2007. A novel intranasal virus-like particle (VLP) vaccine designed to protect against the pandemic 1918 influenza A virus (H1N1). Viral Immunol. 20:441–452 [DOI] [PubMed] [Google Scholar]
- 27. Matsuoka Y., Lamirande E. W., Subbarao K. 2009. The ferret model for influenza. Curr. Protoc. Microbiol. 13:15G.2.1–15G.2.29 [DOI] [PubMed] [Google Scholar]
- 28. Mortola E., Roy P. 2004. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 576:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ninomiya A., Imai M., Tashiro M., Odagiri T. 2007. Inactivated influenza H5N1 whole-virus vaccine with aluminum adjuvant induces homologous and heterologous protective immunities against lethal challenge with highly pathogenic H5N1 avian influenza viruses in a mouse model. Vaccine 25:3554–3560 [DOI] [PubMed] [Google Scholar]
- 30. Nolan T., et al. 2010. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 303:37–46 [DOI] [PubMed] [Google Scholar]
- 31. Perrone L. A., et al. 2009. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J. Virol. 83:5726–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pushko P., et al. 2007. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine 25:4283–4290 [DOI] [PubMed] [Google Scholar]
- 33. Quan F. S., Compans R. W., Nguyen H. H., Kang S. M. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 82:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quan F. S., Huang C., Compans R. W., Kang S. M. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 81:3514–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quan F. S., et al. 2010. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 84:7760–7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quan F. S., et al. 2008. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 26:3352–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quan F. S., Vunnava A., Compans R. W., Kang S. M. 2010. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One 5:e9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quan F. S., et al. 2009. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 83:4489–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robertson J. S., et al. 2011. The development of vaccine viruses against pandemic A(H1N1) influenza. Vaccine 29:1836–1843 [DOI] [PubMed] [Google Scholar]
- 40. Roldao A., Mellado M. C., Castilho L. R., Carrondo M. J., Alves P. M. 2010. Virus-like particles in vaccine development. Expert Rev. Vaccines 9:1149–1176 [DOI] [PubMed] [Google Scholar]
- 41. Roman F., et al. 2010. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 28:1740–1745 [DOI] [PubMed] [Google Scholar]
- 42. Ross T. M., et al. 2009. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One 4:e6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sambhara S., et al. 2001. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell. Immunol. 211:143–153 [DOI] [PubMed] [Google Scholar]
- 44. Song J. M., et al. 2010. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405:165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song J. M., et al. 2010. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 88:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szecsi J., et al. 2009. DNA vaccination with a single-plasmid construct coding for viruslike particles protects mice against infection with a highly pathogenic avian influenza A virus. J. Infect. Dis. 200:181–190 [DOI] [PubMed] [Google Scholar]
- 47. Szretter K. J., Balish A. L., Katz J. M. 2006. Unit Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. 3:15G.1.1–15G.1.22 [DOI] [PubMed] [Google Scholar]
- 48. Tao P., et al. 2009. Virus-like particle vaccine comprised of the HA, NA, and M1 proteins of an avian isolated H5N1 influenza virus induces protective immunity against homologous and heterologous strains in mice. Viral Immunol. 22:273–281 [DOI] [PubMed] [Google Scholar]
- 49. Tumpey T. M., Renshaw M., Clements J. D., Katz J. M. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. WHO 2009. World Health Organization fact sheet no. 211. Influenza (seasonal). World Health Organization, Geneva, Switzerland [Google Scholar]
- 51. Wood J. M., Mumford J., Schild G. C., Webster R. G., Nicholson K. G. 1986. Single-radial-immunodiffusion potency tests of inactivated influenza vaccines for use in man and animals. Dev. Biol. Stand. 64:169–177 [PubMed] [Google Scholar]
- 52. Yamshchikov G. V., Ritter G. D., Vey M., Compans R. W. 1995. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology 214:50–58 [DOI] [PubMed] [Google Scholar]
- 53. Yang P., et al. 2010. Response of BALB/c mice to a monovalent influenza A (H1N1) 2009 split vaccine. Cell. Mol. Immunol. 7:116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yen H. L., et al. 2007. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. J. Virol. 81:6890–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]