Abstract
Leptospirosis is the most widespread zoonosis in the world. Current vaccines are based on whole-cell preparations that cause severe side effects and do not induce satisfactory immunity. In light of the leptospiral genome sequences recently made available, several studies aimed at identification of protective recombinant immunogens have been performed; however, few such immunogens have been identified. The aim of this study was to evaluate 27 recombinant antigens to determine their potential to induce an immune response protective against leptospirosis in the hamster model. Experiments were conducted with groups of female hamsters immunized with individual antigen preparations. Hamsters were then challenged with a lethal dose of Leptospira interrogans. Thirteen antigens induced protective immune responses; however, only recombinant proteins LIC10325 and LIC13059 induced significant protection against mortality. These results have important implications for the development of an efficacious recombinant subunit vaccine against leptospirosis.
INTRODUCTION
Leptospirosis is a disease caused by pathogenic spirochetes of the Leptospira genus (1). Transmission occurs through direct or indirect exposure to urine of mammalian reservoirs, especially during floods, occupational exposure, and the practice of water sports (3). The infection usually manifests as asymptomatic or as a self-resolving febrile illness. However, up to 15% of all human infections progress to severe leptospirosis, with complications such as kidney failure and pulmonary hemorrhage and fatality rates of up to 50% (11, 21). Mortality remains high because of delays in diagnosis due to lack of infrastructure and adequate clinical suspicion and to other poorly understood reasons, especially in underdeveloped and developing countries (3).
Although vaccination is the recommended method of prevention in at-risk settings (17), the immune response generated by most of the currently available vaccines (bacterins) is attributable to the outer membrane lipopolysaccharide (LPS) component (29). As over 250 leptospiral serovars have been identified thus far (7), with the main antigenic differences attributed to the LPS, the efficacy of such vaccines has been found to be limited to short-term, serovar-specific immunity. Bacterin-type vaccines have been approved for use in humans in Cuba, China, Japan, and France. However, bacterins induce adverse reactions and side effects and, in general, their use has been restricted to animals (21), especially dogs, cattle, and pigs (1). Therefore, considerable effort is being made to identify novel leptospiral vaccine candidates with fewer side effects that can induce a cross-protective immune response to the pathogenic serovars.
In recent years, many potential vaccine candidates have been tested in animal models, and several different approaches, including those employing subunit, DNA, adenovirus, and Mycobacterium bovis BCG constructs, have been used (2, 5, 6, 12, 13, 15, 19, 24, 26, 27). Most of these studies identified their protein targets by screening for antigenicity through the use of sera from patients with leptospirosis (14) and/or proteins with predicted surface exposure (10). However, a recent review highlighted the difficulties of evaluating the reports of efficacy for these vaccine candidates due to the different animal models and statistical methods used. The authors of the review reported that when the same method of statistical analysis of protection against mortality was used, very few candidates were found to offer significant immunoprotection (1). In the majority of reports, furthermore, protection did not induce sterilizing immunity.
Recently, our group used an approach based on reverse vaccinology to identify eight putative lipoproteins in the L. interrogans genome and to subsequently characterize those lipoproteins in terms of immunogenicity and antigenicity (16). The eight putative lipoproteins and an additional 19 proteins, predicted to be surface exposed and recognized by sera from convalescent patients with leptospirosis (14), were evaluated using a hamster model of lethal leptospirosis. The aim of the study was to identify potential vaccine candidates that could protect hamsters against lethal challenge; the endpoints used in the present study included survival and protection against mortality.
MATERIALS AND METHODS
Leptospira strains.
L. interrogans strain Fiocruz L1-130 (serogroup Icterohaemorrhagiae, serovar Copenhageni) was used in this study. Leptospires were cultivated in Ellinghausen-McCullough-Johnson-Harris (EMJH) liquid medium (Difco Laboratories) at 28°C. Growth was monitored by counting leptospires in a Petroff-Hausser chamber (Fisher) and by dark-field microscopy as described previously (11). Escherichia coli strains TOP10 (Invitrogen) and BL21(DE3) STAR (Novagen) were used in this study. They were grown in Luria-Bertani (LB) or Terrific broth (TB) media at 37°C.
Plasmid construction and expression and purification of recombinant proteins.
All proteins used in this study were identified in previous studies (14, 16, 23). The genomic DNA of L. interrogans was used as a template for amplification of the target sequences. PCR products were cloned into pAE (25), pQE30 (Qiagen), or pET100-D/TOPO (Invitrogen) plasmid vector. Those vectors contain a 6×His tag that is expressed fused to the recombinant protein. In general, primers were designed to include most of the target genes but not their highly hydrophobic signal sequences. They also included a restriction enzyme site to allow direct cloning of the PCR product. Full primer information is presented in Table 1. Recombinant plasmids were used to transform E. coli strains by electroporation, and E. coli organisms containing the constructs were then cultured at 37°C. Expression was induced by the use of IPTG (isopropyl-β-d-thiogalactopyranoside) at a 1 mM final concentration. Cells were harvested by centrifugation, resuspended in column buffer containing 8 M urea (no urea was used for soluble protein; see Table 1), and disrupted by sonication. His-tagged proteins were purified by affinity chromatography in a nickel (Ni+2)-charged Sepharose column. Columns containing bound protein were washed with 10 volumes of wash buffer containing 10 mM imidazole. His-tagged proteins were eluted from the column with elution buffer containing 250 mM imidazole. A dialysis procedure was used to remove urea and imidazole and to promote refolding of the recombinant proteins. Proteins in the final preparation were quantified by the Bradford (4) and bicinchoninic acid (BCA) (Pierce) methods (Table 1 contains further information regarding solubility, vectors, and protein size).
Table 1.
Primer and protein information
| Antigen | Primer | Molecular mass (kDa) | Vector |
|---|---|---|---|
| rLIC10191 | F CGGGATCCTCAACGCAAGAGCA | 22.9 | pAE |
| R GGGGTACCTTGTTGTGGTGCGGA | |||
| rLIC12099 | F TTGGTACCGCTCAAACGGCAAG | 52.7 | pAE |
| R GGGAAGCTTTTTATATTTGACGATGA | |||
| rLIC11947 | F CCGGATCCCCTGTGGAAAGAAA | 17.9 | pAE |
| R GGGAAGCTTTTTTTCTGGAGGAA | |||
| rLIC10011 | F TAGGTACCACGGATGGACTTTTGAA | 19.8 | pAE |
| R CGGAATTCTTATTGTTTGGAAACCTC | |||
| rLIC12730 | F GCGGATCCATTTTAGTCTTTACCTC | 57.8 | pAE |
| R CCCAAGCTTGATCAATTCCGTTC | |||
| rLIC10561 | F GCGGATCCTTAATATTTCTGGTCTTTC | 30.22 | pAE |
| R CCCAAGCTTGATCAATTCCGTTC | |||
| rLIC10508 | F CGGGATCCAATTCAATAACTATG | 22.99 | pAE |
| R GGGAAGCTTACAACCAGGACCTT | |||
| rLIC12538 | F GGGATCCGCAGACGAAAAGGAAA | 24.9 | pAE |
| R CCAAGCTTTCAGCTAGTCAGAGTAAAA | |||
| rLIC10501 | F CACCGATAACAAAGAGAAGGGAGG | 48.8 | pET100-D/TOPO |
| R CTACTCCACACATTCGGGACTATTG | |||
| rLIC13006 | F GACTCGAGAACTCTGCTTTAAGTGGCTTAA | 47 | pAE |
| R GGCCATGGTTATTGTTCTACACAAACTAAA | |||
| rLIC13306a | F CACCTCCAAAGAGAAATGTTTATTC | 17.7 | pET100-D/TOPO |
| R TCATTTCCGAACCGGATGACCGT | |||
| rLIC12253 | F TTCTCGAGGAGAAACCGGACGATACTACTT | 23.4 | pAE |
| R CCCCATGGTTAGGGAAGACTTCTAACAAC | |||
| rLIC11184 | F CACCTGTGAAGATGAAAAAAAGGA | 18.8 | pET100-D/TOPO |
| R TTAGTAACCACACTCACTCGCAGC | |||
| rLIC10645 | F CACCAAAAAAGATAAGGACGATACCTT | 42.3 | pAE |
| R TTAACGAACTAGTACAGTCGGTAAATG | |||
| rLIC10021 | F GACTCGAGAATTGTTCTGTCAAGCCC | 63.8 | pAE |
| R CCAAGCTTTCATAAATCCACGGAAGT | |||
| rLIC11859a | F CACCGAATTTATGAAGGTCACG | 30 | pET100-D/TOPO |
| R TTAAAAAGCACTTAAGGCAGCC | |||
| rLIC10325a | F CACCATTCAAGACGAAGATTCCAAAC | 40.6 | pET100-D/TOPO |
| R TCAATCCAATTTTTCGGTTTCTAG | |||
| rLIC12555 | F CATCTCGAGAGCCCAGTACAAATGAAAGT | 43 | pAE |
| R TCCATGGTTAAAGATTTGTAACGCAGATTCC | |||
| rLIC11087 | F CACCGTTGGAGATTCCAGAAAGGAA | 29.9 | pET100-D/TOPO |
| R TTAAAATAAATTACAACCAGTCTGATATAA | |||
| rLIC12632 | F GTCTCGAGTGTAAACCTGGCAAACAAAATT | 63.8 | pAE |
| R GGCCATGGCTAATGATGATAGATTAAATCT | |||
| rLIC10054 | F TTTTTTGGATCCGAGTCTAAACGAAG | 32.1 | pQE30 |
| R TTTTTTAAGCTTCACCGTATTCTTGTC | |||
| rLIC20172 | F CGGGATCCGATACGGACAAGGACGGG | 28.1 | pQE30 |
| R CCCAAGCTTTTCGGAATCCTCGTCCGG | |||
| rLIC13059 | F CGGGATCCGAATCCATGGTATATTAT | 14.4 | pQE30 |
| R CCCAAGCTTACTTTGACGAATCAATGC | |||
| rLIC11567 | F CGGGATCCAACAGATTGATTCGTAAA | 13.9 | pQE30 |
| R CCCAAGCTTCTTTTTGTATTCCACAAG | |||
| rLIC10091 | F CGGGATCCAAACTATTTTTAGCTCCTTTG | 14.6 | pQE30 |
| R CCCAAGCTTGATTTCAAAAGAAGTATG | |||
| rLIC10009 | F TTTTTTTGATCACAAGAAGCGCAGATCT | 24.8 | pQE30 |
| R CCCAAGCTTGAACTCATCCTGTTTA | |||
| rLIC13305 | F TTTTTTTGATCAAACTATGATCGTGAC | 20.3 | pQE30 |
| R TTTAAGCTTGATATCACCACCCAAA |
Expressed as soluble protein and did not require the use of urea for purification.
Protein gel electrophoresis.
All proteins were submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in order to verify molecular weight and sample purity. These gels were stained with Coomassie blue solution to reveal the proteins.
Hamster immune protection studies.
Female golden Syrian hamsters (4 to 5 weeks of age) were obtained from the animal house facility at the Federal University of Pelotas. The animals were immunized twice in the quadriceps muscle with the recombinant protein in an aluminum hydroxide solution (15%) on day 0 (zero) and day 14. All vaccine doses contained 60 μg of purified recombinant protein, with administration of a standard volume of 200 μl at a single injection site. Negative-control groups in all experiments were inoculated with a 200 μl of phosphate-buffered saline (PBS)–aluminum hydroxide (15%). Positive-control groups were immunized with 200 μl of a bacterin inoculum containing approximately 108 leptospires per dose. All hamsters were challenged on day 28 (at age 8 to 9 weeks) with an intraperitoneal inoculum of 100 leptospires (∼2 × the 50% lethal dose [LD50]) of strain Fiocruz L1-130 (28) 14 days after the last immunization. The inoculum was produced from log-phase cultures and consisted of a 1-ml dose. Hamsters were monitored daily for clinical signs of leptospirosis and euthanized when clinical signs of terminal disease appeared.
Twenty-seven recombinant proteins were tested in 11 individual experiments; each group consisted of six hamsters except where otherwise noted (Table 2). All experiments included negative-control (PBS) and positive-control (bacterin) groups. All animal studies were approved by the Ethics Committee for the Use of Experimental Animals of the Universidade Federal de Pelotas.
Table 2.
Survival timeline of vaccinated animals
| Antigen | Name or feature | No. of days to death for each animal | No. (%) of surviving animals/total no. of animals |
|---|---|---|---|
| rLIC10191 | OmpA-like | 10, 10, 10, 10, 11, 13 | 0/6 (0) |
| rLIC12099 | Hypothetical protein | 10, 13, 13, 13, 13, 14 | 0/6 (0) |
| rLIC11947 | Putative lipoprotein | 12, 12, 14, 14, 16, 17 | 0/6 (0)b |
| rLIC10011 | LipL21 | 11, 12, 13, 13, 14 | 1/6 (16.7)b |
| rLIC12730 | Hypothetical protein | 11, 11, 12, 13, 14, 14 | 0/6 (0) |
| rLIC10561 | Hypothetical protein | 11, 11, 11, 11, 13 | 1/6 (16.7) |
| rLIC10508 | Putative lipoprotein | 11, 13, 13, 13, 18 | 1/6 (16.7) |
| rLIC12538 | SecD | 11, 11, 13, 13, 14 | 1/6 (16.7)b |
| rLIC10501 | Putative lipoprotein | 10, 11, 11, 11, 13 | 1/6 (16.7)b |
| rLIC13306 | Hypothetical protein | 12, 12, 13, 14, 17 | 1/6 (16.7) |
| rLIC13006 | Putative lipoprotein | 10, 11, 11, 12, 12, 13 | 0/6 (0) |
| rLIC12253 | Putative lipoprotein | 12, 12, 12, 12, 12 | 1/6 (16.7) |
| rLIC11184 | Putative lipoprotein | 12, 12, 12, 12, 13 | 1/6 (16.7)b |
| rLIC10645 | Putative lipoprotein | 10, 11, 12, 12, 14 | 1/6 (16.7)b |
| rLIC10021 | Putative lipoprotein | 11, 11, 11, 13, 14, 20 | 0/6 (0)b |
| rLIC11859 | Hypothetical protein | 10, 11, 11, 12, 14 | 1/6 (16.7) |
| rLIC10325 | Hemolysin | 11, 12, 13, 13 | 2/6 (33.3)c |
| rLIC12555 | Hypothetical protein | 11, 12, 12, 12, 12, 15 | 0/6 (0) |
| rLIC11087 | Putative lipoprotein | 11, 11, 12, 13, 13, 14 | 0/6 (0) |
| rLIC12632 | Hemolysin | 11, 11, 12, 13, 14, 14 | 0/6 (0)b |
| rLIC10054 | Putative lipoprotein | 9, 10, 10, 10, 13 | 1/6 (16.7) |
| rLIC20172 | Lipoprotein | 10, 13, 13, 13, 13, 15, 16 | 1/8 (12.5)a |
| rLIC13059 | Putative lipoprotein | 8, 9, 10, 10 | 2/6 (33.3)c |
| rLIC11567 | Putative lipoprotein | 9, 10, 10, 10, 13, 13 | 2/8 (25)a |
| rLIC10091 | Putative lipoprotein | 10, 10, 10, 11, 11, 11, 11 | 1/8 (12.5)a |
| rLIC10009 | Putative lipoprotein | 9, 10, 10, 10, 10, 13, 14, 15 | 0/8 (0)a |
| rLIC13305 | Putative lipoprotein | 10, 10, 10, 11, 11, 11, 11 | 1/8 (12.5)a |
The experiment was conducted with eight hamsters per group.
One animal from the PBS control group survived in the corresponding experiment.
The rate of survival of vaccinated animals was significantly different from the rate seen with the PBS control group (log rank sum test).
Statistical analysis.
The log-rank test was used to determine significant differences in survival among the vaccinated and the negative-control groups. All P values were calculated using two-sided tests, and a P value of <0.05 was considered to indicate statistical significance. Prism 4 software (GraphPad Software) was used to perform the statistical analysis.
RESULTS
Production of recombinant proteins and vaccine preparation.
All PCR products were successfully cloned and expressed in E. coli as 6×His tag N-terminus fusion proteins, which allowed purification of the proteins by affinity chromatography. The recombinant proteins required urea-promoted denaturing conditions for purification (except where noted otherwise) followed by prolonged dialysis to obtain soluble protein preparations. The integrity and purity of the recombinant proteins used in this study were verified by SDS-PAGE analysis (Fig. 1). When necessary, proteins were concentrated prior to dialysis, permitting the standardization of vaccine doses at 200 μl. All vaccine doses were prepared at least 1 day prior to vaccination, and proteins were allowed to adsorb onto the aluminum hydroxide overnight.
Fig. 1.
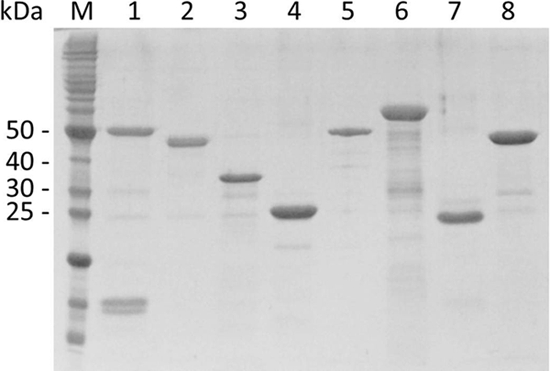
SDS-PAGE (12%) of purified proteins. Lanes: M, molecular mass marker; 1, LIC10501; 2, LIC12555; 3, LIC11087; 4, LIC12253; 5, LIC10645; 6, LIC10021; 7, LIC11184; 8, LIC13006.
Protection of hamsters immunized with recombinant proteins against lethal challenge with L. interrogans.
Immunization with the majority of the proteins did not prevent death among the challenged hamsters. Although immunization with the recombinant LIC11859 (rLIC11859), rLIC12253, rLIC10561, rLIC10508, rLIC10091, rLIC13059, rLIC10054, rLIC11567, rLIC20172, rLIC10561, and rLIC10508 proteins did result in more survivors than were seen with the negative-control groups, the differences were not significant. rLIC10325 and rLIC13059 significantly (P < 0.05) increased survival among the vaccinated hamsters (log-rank test). Table 2 shows the timeline of days to death for all the experiments. One animal survived in the negative-control group in each experiment, whereas animals in the positive-control group were fully protected in all experiments.
DISCUSSION
Several studies have employed the recombinant subunit vaccine approach to try to develop a vaccine against leptospirosis; however, the results have been variable and difficult to interpret (1). Although LigA seems to be the most promising antigen (24, 27), immune protection resulting from the use of that antigen in an adjuvant approved for human use has not been shown. Some authors reported protection induced by LipL32, LigB, and other outer membrane proteins (2, 6, 15, 19, 26); however, those proteins have yet to demonstrate practical applicability. In an effort to identify novel vaccine candidates, we evaluated 27 recombinant leptospiral proteins. In our assays, a total of 15 recombinant proteins were incapable of inducing a protective immune response to challenge with L. interrogans. A total of 12 recombinant proteins did improve survival compared to the negative-control group results. However, only two of those recombinant proteins (rLIC10325 and rLIC13059) induced survival at a rate that reached significance (P < 0.05). Those two proteins may constitute potential vaccine candidates.
The majority of the proteins evaluated in this study were identified as putative lipoproteins or hypothetical proteins (see Table 2). Of the protective antigens, LIC10325 was annotated as a hemolysin whereas LIC13059 was identified as a putative lipoprotein (23). A BLAST analysis revealed that the corresponding genes are present in all pathogenic leptospiral genomes described to date and are highly conserved among the pathogenic Leptospira spp. These are important features for antigens intended for use as vaccines aimed at affording cross-protection against different Leptospira spp. and serovars.
Previous reports have shown that vaccine preparations that include two or more proteins can be more effective than the individual components (8, 15). Several of the leptospiral virulence factors and outer membrane proteins have exhibited redundancy; this was demonstrated by the fact that knockout of some of these genes did not reduce virulence (9, 20, 22). Therefore, immunity responses directed toward one of these proteins may not be effective. Thus, further investigation of the protective proteins (fused or coadministered with each other and/or with proteins described elsewhere) identified in this study may increase the effectiveness of our preparations.
Although characterization of the immunogenicity and antigenicity of immunogens is an important step in identifying surface-exposed proteins, there is no correlation between the amplitude of the immune response and protection against leptospirosis. Highly immunogenic and antigenic surface proteins such as LipL32 do not induce protective immunity (18). For this reason, we used a challenge assay to screen for protective antigens. This approach produces relatively fast and practical results. Antigens that fail to induce a protective immune response need not be further assessed.
In this study, we identified two potentially protective antigens. These, in combination with other leptospiral antigens already described, may result in the formulation of an effective and cross-protective vaccine against human and animal leptospirosis. Studies are being conducted to test not only different immunization protocols and antigen combinations but also different adjuvants and forms of antigen presentation.
ACKNOWLEDGMENTS
We thank FAPERJ, CNPq, and CAPES for funding the research and sponsoring the researchers.
We thank the staff of the animal house of UFPel for providing and looking after the animals.
Footnotes
Published ahead of print on 26 October 2011.
REFERENCES
- 1. Adler B., de la Peña-Moctezuma A. 2010. Leptospira and leptospirosis. Vet. Microbiol. 140:287–296 [DOI] [PubMed] [Google Scholar]
- 2. Atzingen M. V., et al. 2010. Characterization of leptospiral proteins that afford partial protection in hamsters against lethal challenge with Leptospira interrogans. J. Med. Microbiol. 59:1005–1015 [DOI] [PubMed] [Google Scholar]
- 3. Bharti A. R., et al. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757–771 [DOI] [PubMed] [Google Scholar]
- 4. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 5. Branger C., et al. 2005. Protection against Leptospira interrogans sensu lato challenge by DNA immunization with the gene encoding hemolysin-associated protein 1. Infect. Immun. 73:4062–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branger C., et al. 2001. Identification of the hemolysis-associated protein 1 as a cross-protective immunogen of Leptospira interrogans by adenovirus-mediated vaccination. Infect. Immun. 69:6831–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerqueira G. M., Picardeau M. 2009. A century of Leptospira strain typing. Infect. Genet. Evol. 9:760–768 [DOI] [PubMed] [Google Scholar]
- 8. Chang Y. F., et al. 2007. Immunogenicity of the recombinant leptospiral putative outer membrane proteins as vaccine candidates. Vaccine 25:8190–8197 [DOI] [PubMed] [Google Scholar]
- 9. Croda J., et al. 2008. Targeted mutagenesis in pathogenic Leptospira species: disruption of the LigB gene does not affect virulence in animal models of leptospirosis. Infect. Immun. 76:5826–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cullen P. A., Haake D. A., Adler B. 2004. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol. Rev. 28:291–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faine S. B., Adler B., Bolin C., Perolat P. 1999. Leptospira and leptospirosis, 2nd ed. MedSci, Melbourne, Australia. [Google Scholar]
- 12. Faisal S. M., et al. 2008. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine 26:277–287 [DOI] [PubMed] [Google Scholar]
- 13. Felix S. R., et al. 2009. Leptospirosis vaccine: search for subunit candidates. Procedia Vaccinol. 1:110–114 [Google Scholar]
- 14. Gamberini M., et al. 2005. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol. Lett. 244:305–313 [DOI] [PubMed] [Google Scholar]
- 15. Haake D. A., et al. 1999. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 67:6572–6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartwig D. D., Seixas F. K., Cerqueira G. M., McBride A. J., Dellagostin O. A. 2011. Characterization of the immunogenic and antigenic potential of putative lipoproteins from Leptospira interrogans. Curr. Microbiol. 62:1337–1341 [DOI] [PubMed] [Google Scholar]
- 17. Hotez P. J., Ferris M. T. 2006. The antipoverty vaccines. Vaccine 24:5787–5799 [DOI] [PubMed] [Google Scholar]
- 18. Ko A. I., Goarant C., Picardeau M. 2009. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 7:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koizumi N., Watanabe H. 2004. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22:1545–1552 [DOI] [PubMed] [Google Scholar]
- 20. Lin Y. P., Chang Y. F. 2007. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem. Biophys. Res. Commun. 362:443–448 [DOI] [PubMed] [Google Scholar]
- 21. McBride A. J., Athanazio D. A., Reis M. G., Ko A. I. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376–386 [DOI] [PubMed] [Google Scholar]
- 22. Murray G. L., et al. 2009. Genome-wide transposon mutagenesis in pathogenic Leptospira species. Infect. Immun. 77:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nascimento A. L., et al. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palaniappan R. U., et al. 2006. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect. Immun. 74:1745–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramos C. R., Abreu P. A., Nascimento A. L., Ho P. L. 2004. A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz. J. Med. Biol. Res. 37:1103–1109 [DOI] [PubMed] [Google Scholar]
- 26. Seixas F. K., et al. 2007. Recombinant Mycobacterium bovis BCG expressing the LipL32 antigen of Leptospira interrogans protects hamsters from challenge. Vaccine 26:88–95 [DOI] [PubMed] [Google Scholar]
- 27. Silva E. F., et al. 2007. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine 25:6277–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva E. F., et al. 2008. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine 26:3892–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z., Jin L., Wegrzyn A. 2007. Leptospirosis vaccines. Microb. Cell Fact. 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]


