Abstract
Effective immunoglobulin responses play a vital role in protection against most pathogens. However, the molecular mediators and mechanisms responsible for signaling and selective expression of immunoglobulin types remain to be elucidated. Previous studies in our laboratory have demonstrated that protein kinase R (PKR) plays a crucial role in IgE responses to double-stranded RNA (dsRNA) in vitro. In this study, we show that PKR plays a critical role in IgG expression both in vivo and in vitro. PKR−/− mice show significantly altered serum IgG levels during respiratory syncytial virus (RSV) infection. IgG2a expression is particularly sensitive to a lack of PKR and is below the detection level in mock- or RSV-infected PKR−/− mice. Interestingly, we show that upon activation by anti-CD40 and gamma interferon (IFN-γ), B cells from PKR−/− mice show diminished major histocompatibility complex class II (MHC II), CD80, and CD86 levels on the cell surface compared to wild-type (WT) mice. Our data also show that PKR is necessary for optimal expression of adhesion molecules, such as CD11a and ICAM-1, that are necessary for homotypic aggregation of B cells. Furthermore, in this report we demonstrate for the first time that upon CD40 ligation, PKR is rapidly phosphorylated and activated, indicating that PKR is an early and novel downstream mediator of CD40 signaling pathways.
INTRODUCTION
B cells play a critical role in humoral immune responses by making antibodies to identify and neutralize pathogens such as viruses and bacteria. Mature B cells express IgM and IgD on their surfaces; upon activation by a specific antigen, they undergo class switch recombination (CSR). During CSR, the constant heavy chain of an antibody is changed from the μ isotype (which generates IgM) to other isotypes, such as γ, α, and ε (to generate IgG1, IgG2a, IgG2b, IgG3, IgA, or IgE) (16, 31). CSR is extremely important in humoral immune responses because it generates a repertoire of antibodies with the same antigen specificities but with different effector functions. This process is crucial for establishing immunity against many lethal pathogens. It is well known that patients who are selectively deficient in CSR suffer from recurrent and severe infections (7).
During viral infections, the type of antibody response generated against specific viral proteins is important in determining how effectively the virus can be neutralized. Mainly IgG and IgA antibody responses have been found to be effective in neutralizing and reducing viral loads (8). Furthermore, IgG subclasses (IgG2a, IgG2b, IgG1, and IgG3) show marked differences in their specificities for neutralizing viruses and lysing infected cells. IgG2a has been shown to be responsible predominantly for effective host defense against viral infections (14). Cytokines such as interleukin-4 (IL-4), gamma interferon (IFN-γ), and transforming growth factor beta (TGF-β) play critical roles in directing the isotype (IgE, IgG, and IgA) and immunoglobulin (Ig) subclass specificity of CSR. For example, IL-4 drives the switch to IgG1 and IgE (21), IFN-γ has been shown to promote IgG2a and IgG3 immune responses (3, 29), and TGF-β directs the switch to IgG2b and IgA (12, 18).
Additional important signals in inducing B cells to switch to antibody-producing plasma cells are triggered through binding of several costimulatory molecules on the surfaces of B cells with their corresponding molecules on T cells. Among these cellular interactions, engagement of CD40-expressing B cells and CD154-expressing T cells is critical for initiation of the T cell-dependent CSR (1, 17). CD40-CD154 has emerged as a key system in inducing the functional outcomes that are essential for generating and maintaining the humoral immune response, such as B cell proliferation, Ig class switching, and memory B cell responses (17). CD40 also plays a role in the induction of homotypic adhesion of B cells that is essential for robust activation of B cells (2).
CD40 is a 47- to 50-kDa type I transmembrane glycoprotein (4) belonging to the superfamily of tumor necrosis factor (TNF) receptors (9). Engagement of the CD40 receptor on B cells with the CD40 ligand (CD40L) leads to multimerization of the receptor and initiation of CD40-mediated signaling pathways (15). The CD40 cytoplasmic C terminus lacks kinase activity, and adaptor proteins and tyrosine kinases (Src, phosphatidylinositol 3-kinase [PI3K], c-Jun N-terminal kinase [JNK], and p38) (6, 13, 28) mediate the activation of the CD40 signaling cascade, leading to nuclear translocation of transcription factors such as NF-κB (10). However, all of the molecular mediators of CD40 signaling pathways have not been identified.
Another important enzyme that has been shown to play a role in immunoglobulin class switching is double-stranded RNA (dsRNA)-activated protein kinase (PKR) (26). PKR has two dsRNA-binding motifs, located in the NH2-terminal domain, that bind dsRNA and recruit other dsRNA-binding proteins. The carboxyl terminus of PKR contains the kinase catalytic domain. Once activated, PKR dimerizes and undergoes autophosphorylation at multiple sites (34). The phosphorylated PKR can then activate its cellular substrates to downregulate protein synthesis and induce inflammatory immune responses. Upon activation, PKR functions as one of the mediators of antiviral and antiproliferative activities of type I interferons (11). In addition to its well-established role in inhibition of viral replication, PKR has been found to be a critical mediator of many inflammatory pathways following bacterial infections and of cytokine-mediated signaling pathways, as well as its role in immunoglobulin class switching (24, 26). Also, PKR has been shown to play a role in activation of mitogen-activated protein kinases (MAPKs) such as JNK, p38, and extracellular signal-regulated kinase (ERK) following treatment with bacterial cell wall components such as lipopolysaccharide (LPS) and dsRNA (24, 32). Furthermore, in vitro studies have demonstrated that PKR can inactivate the inhibitor of NF-κB, IκB, by phosphorylation. It is well known that NF-κB plays an important role in B cell effector functions such as proliferation, differentiation, and immunoglobulin class switching (30). In particular, both canonical and noncanonical NF-κB pathways have been shown to be necessary for CD40-mediated B cell activation (35).
A previous report from our group demonstrated that PKR is important for induction of IgE class switching in human B cells (26). This finding led us to further investigate the role of PKR in immunoglobulin expression. In the present study, we show that a lack of PKR in vivo results in altered immunoglobulin G secretion in response to respiratory syncytial virus (RSV) infection, while in vitro, PKR−/− splenocytes show altered IgG2a, IgG2b, IgG3, and IgG1 class switching expression upon CD40 ligation in the presence of cytokines. More importantly, in this report we show for the first time that PKR is rapidly phosphorylated and activated following CD40 ligation, suggesting an early and likely critical role of PKR in CD40 signaling pathways.
MATERIALS AND METHODS
Cell line, culture conditions, and reagents.
The human Burkitt's lymphoma cell line Ramos.2G6.4C10 was purchased from the ATCC (Manassas, VA), and the cells were maintained in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS) and 100 units/ml of penicillin-streptomycin at 37°C in a 5% CO2 humidified chamber.
Mice, virus, and ELISA.
Male and female B6/SV129/F1 and PKR−/− mice (Jackson Laboratory, Bar Harbor, ME) at 8 to 12 weeks of age were used in all experiments. The mice were maintained under specific-pathogen-free conditions in the animal facility at the National Institute of Environmental Health Sciences (NIEHS). All experiments were performed in accordance with the Animal Welfare Act and the U.S. Public Health Service policy on humane care and use of laboratory animals after review of the protocol by the animal care and use committee of the NIEHS. Mice were anesthetized using isoflurane and then were treated with either phosphate-buffered saline (PBS) or 107 PFU of RSV-A2/mouse by intratracheal aspiration. Blood samples were collected from mice at days 4, 7, and 14. Serum was obtained from blood by centrifugation and was stored at −80°C until use.
Sera were tested for total IgM, IgG2a, IgG2b, IgG3, and IgG1 concentrations by using respective enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocol (Bethyl Laboratories, Montgomery, TX). Briefly, the serum was diluted 1:4,000. The wells were coated with the corresponding capture immunoglobulin and sequentially incubated with diluted serum, biotinylated antibody for the relevant Ig, and streptavidin-conjugated horseradish peroxidase (HRP) for color development. Standard curves were obtained by use of recombinant IgM, IgG2a, IgG2b, IgG3, and IgG1. The lower detection limits of the Ig ELISAs were 1.37 ng/ml (IgM), 0.69 ng/ml (IgG2a), 3.12 ng/ml (IgG2b), and 1.03 ng/ml (IgG1).
For RSV titration, mice were euthanized at 4 days postchallenge and lungs were removed and quick-frozen. PFU of RSV were measured by plaque assay on Hep-2 cell monolayers.
Spleen lymphocyte isolation.
Mice were sacrificed by pentobarbital administration. The spleens from one or two mice were pooled, homogenized, and passed through a nylon filter. The cell suspension was then carefully overlaid on Histopaque 1077 (Sigma, St. Louis, MO) and centrifuged at 1,600 rpm for 20 min at room temperature, and the splenic mononuclear cells were carefully collected from the medium-Ficoll interphase, resuspended in RPMI 1640, and counted for culture.
Western blots.
One-microliter samples of sera from infected and noninfected wild-type (WT) and PKR−/− mice were analyzed by Western blotting. For RSV-specific immunoglobulins, cell lysates from RSV-infected Hep-2 cells were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was placed in a Surf-Blot antibody screening system (Idea Scientific Company, Minneapolis, MN). Immunoglobulins from serum samples from mock- and RSV-infected mice were introduced into the channels and allowed to bind to RSV proteins. The nitrocellulose membrane was then probed with anti-IgG-HRP. As a positive control, one channel was probed with anti-RSV polyclonal antibody (Fitzgerald Industries Limited, North Acton, MA). The immunoblotted proteins were then visualized by use of enhanced chemiluminescence reagents (GE Healthcare Biosciences, Piscataway, NJ).
For in vitro PKR activation, Ramos cells or WT splenocytes were exposed to anti-CD40 monoclonal antibody (MAb) for the indicated periods. Cell lysates were prepared using 1× Laemmli sample buffer containing 1% SDS and 2-mercaptoethanol and were analyzed by Western blotting as described above. The nitrocellulose membrane was probed with anti-phospho-PKR MAb (Epitomics Inc., Burlingame, CA).
Splenocyte isolation and stimulation.
Mice were sacrificed by pentobarbital administration. The spleens from one or two animals were pooled, homogenized, and passed through a nylon filter. The cell suspension was then carefully overlaid on Histopaque 1077 (Sigma) and centrifuged at 1,600 rpm for 20 min at room temperature, and the splenic mononuclear cells were carefully collected from the medium-Ficoll interphase and resuspended in RPMI 1640. Splenocytes (1 × 106) were then stimulated with IFN-γ (20 or 50 ng/ml), IL-4 (50 ng/ml), or TGF-β (2 ng/ml) (Sigma), with or without 1 μg/ml anti-CD40 MAb (eBiosciences, San Diego, CA). At days 4 and 7, the supernatants were collected and frozen at −80°C until further analysis of IgG2a, IgG2b, IgG1, and IgG3 concentrations by ELISA according to the manufacturer's protocol (Bethyl Laboratories).
Flow cytometry.
Splenocytes were cultured at 2 × 106 cells/ml in RPMI 1640 (GibcoBRL, Grand Island, NY) with 10% fetal bovine serum (FBS; GibcoBRL) for 4 and 7 days in the presence of 1 μg/ml anti-CD40 MAb and IFN-γ, IL-4, or TGF-β. Cells were then counted and stained with a rat anti-mouse MAb. Specific MAbs to B220 (conjugated to allophycocyanin [APC]), CD86 (conjugated to fluorescein isothiocyanate [FITC]), major histocompatibility complex class II (MHC II) (conjugated to efluor 450), CD11a (conjugated to phycoerythrin [PE]), and CD44 (conjugated to APC-Cy7) (eBiosciences) were added and incubated for 30 min on ice. Cell acquisition and analysis were performed on a BD LSR-II flow cytometer using FACS Diva software (version 4.1.2; Becton Dickinson). Compensation of the spectral overlap for each fluorochrome was calculated using cells stained with each fluorophore.
Statistical analysis.
For each parameter, the values for individual mice were averaged and the standard error was calculated. The significance of differences between exposure groups was determined by two-way analysis of variance (ANOVA) in conjunction with Bonferroni's post hoc analysis, where appropriate. All ANOVA models were performed with Prism software, version 5 (GraphPad Prism, San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
PKR plays a role in IgG expression in RSV-infected mice.
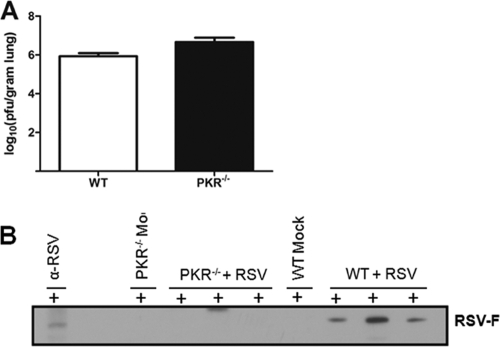
RSV infection is known to induce a robust protective immunoglobulin response characterized by the induction of predominantly IgG antibodies (25). To determine whether PKR has an integral function in IgG responses to infectious viruses, we infected WT and PKR−/− mice with RSV. To examine the viral load, viral titers in the lung tissue were determined at day 4 postchallenge (peak viral load) (Fig. 1A). Next, to assess the immunoglobulin responses, serum levels of IgM and IgG were determined in WT and PKR−/− mice by Western blot analysis. IgM serum levels were found to be comparable between the two types of mice (data not shown). However, total serum IgG levels were diminished in both untreated and RSV-infected mice (data not shown). To further analyze the role of PKR in immunity against specific RSV proteins, we analyzed the serum samples from each mouse for RSV-specific IgG. The results show that sera from infected PKR−/− mice demonstrated diminished IgG, especially against RSV F protein (Fig. 1B). Taken together, the results suggest that PKR deficiency affects the basal physiological levels of IgG. Also, protective IgG responses following RSV infection are impaired in PKR−/− mice compared to those in WT mice.
Fig. 1.
PKR−/− mice show no difference in RSV titer but diminished serum IgG levels specific to RSV F protein. (A) WT and PKR−/− mice (4 to 6 of each) were euthanized on day 4 post-RSV challenge. Viral titers in the lung tissue were measured by plaque assay on subconfluent Hep-2 cell monolayers. Data represent the means and standard deviations (SD) of the log10 PFU/g of lung tissue. (B) RSV-specific IgG detection in sera of individual noninfected and RSV-infected mice. RSV-infected Hep-2 cell lysates were probed with sera from individual mice for binding of RSV-specific IgG antibodies in separate channels. Each lane represents RSV-specific IgG in the serum from an individual mouse at day 7 postinfection. PKR−/− mice showed an impaired IgG response to RSV F protein compared to WT mice. Representative blots from two separate experiments are shown.
Absence of IgG2a response in RSV-infected PKR−/− mice.
To further confirm the diminished basal levels of IgG and to look at the differences in specific IgG subclasses, serum samples from mock- and RSV-infected mice were collected on days 4, 7, and 14 and analyzed for IgG2a, IgG2b, and IgG1 levels by ELISA. Among the IgG subclasses, the immunoglobulin response to a live virus is characterized by induction of predominantly IgG2a antibodies (14). Interestingly, the IgG2a level was below the detection level even in sera of mock-infected PKR−/− mice, in contrast to the case for WT mice (Fig. 2A). However, basal levels of IgG2b and IgG1 were similar in WT and PKR−/− mice (Fig. 2B). Furthermore, regardless of exposure to RSV, the PKR−/− mice showed a persistent absence of an IgG2a response. However, post-RSV infection, both IgG2b and IgG1 responses were increased significantly in RSV-infected PKR−/− mice compared with WT mice (Fig. 2B and C). The results demonstrate that the absence of PKR affects predominantly the IgG2a response in vivo. Also, the undetectable levels of basal IgG2a strongly supported the possibility that there are intrinsic defects in B cell functionality in PKR-deficient mice.
Fig. 2.
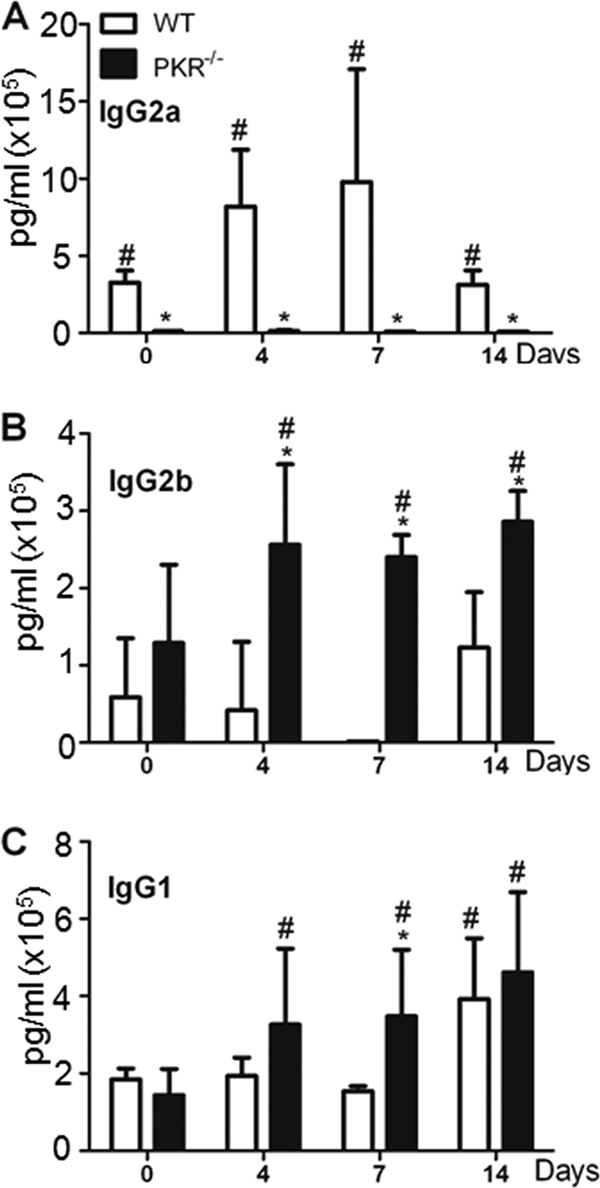
PKR−/− mice show an altered serum IgG subclass profile following RSV infection. PKR−/− or WT mice were infected with RSV at 1 × 107 PFU/ml. After 4, 7, and 14 days, mice were euthanized and blood was collected. The serum levels of IgG subclasses were measured by ELISA. (A) IgG2a; (B) IgG2b; (C) IgG1. Data represent means and SD for duplicate samples from 5 different mice. *, P < 0.05 versus corresponding WT control; #, P < 0.05 versus untreated or “0” control.
Absence of PKR affects IgG expression in vitro.
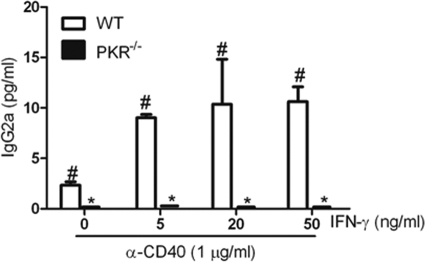
To explore the influence of PKR deficiency on IgG secretion in vitro, we cultured splenocytes from WT and PKR−/− mice with anti-CD40 MAb and IFN-γ, TGF-β, or IL-4. Consistent with the in vivo studies, PKR−/− splenocyte cultures showed a complete absence of IgG2a on day 7, in contrast to WT splenocytes (Fig. 3). Increasing concentrations of IFN-γ had no effect on IgG2a secretion, confirming the lack of IgG2a response in PKR−/− splenocytes (Fig. 3). This lack of response was not due to IFN-γ responses, since detectable IgG3 expression was observed in the same cultures (Fig. 4C). When splenocytes from WT and PKR−/− mice were cultured with anti-CD40 and TGF-β to stimulate IgG2b production, the results showed that there was decreased IgG2b secretion in PKR−/− splenocytes (Fig. 4A). Also, PKR−/− splenocytes stimulated with anti-CD40 MAb and IL-4 or IFN-γ showed diminished production of IgG1 and IgG3 compared to WT splenocytes (Fig. 4B and C). This observation indicates that an in vitro absence of PKR leads to diminished levels of IgG subclasses irrespective of the stimulant cytokines. PKR deficiency appeared to specifically abrogate IgG2a expression, while IgG2b, IgG3, and IgG1 expression was diminished significantly in PKR−/− splenocytes following CD40 stimulation in vitro. These findings suggest that PKR may play a role in CD40 signaling independent of the signaling initiated by cytokines, emphasizing the potential intrinsic defects in B cell activation in PKR−/− mice.
Fig. 3.
PKR−/− splenocytes show levels of IgG2a below the detection limit in vitro. Splenocytes from WT and PKR−/− mice were treated with anti-CD40 MAb and different concentrations of IFN-γ. The culture supernatants were harvested on day 7, and IgG2a levels were determined using ELISA. Data are means and SD for 3 different experiments performed with duplicate samples. *, P < 0.05 versus corresponding WT control; #, P < 0.05 versus untreated or “0” control.
Fig. 4.
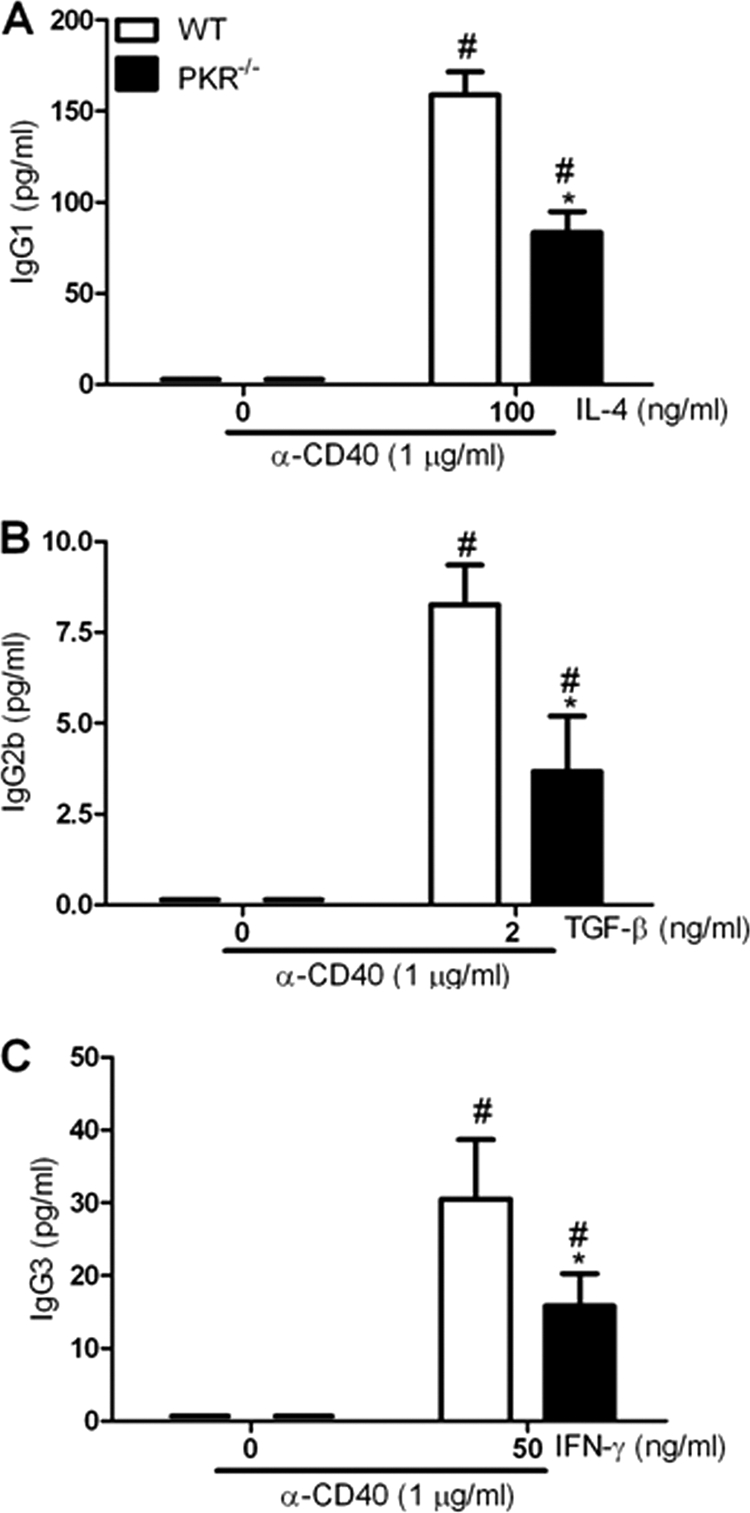
CD40 ligation in PKR−/− splenocytes leads to diminished IgG2b, IgG3, and IgG1 expression in vitro. Splenocytes from WT and PKR−/− mice were treated with anti-CD40 MAb and TGF-β, IFN-γ, or IL-4. On day 7, levels of IgG2b (B), IgG3 (C), and IgG1 (A) in the cell culture supernatants were determined using ELISA. Data are means and SD for 3 different experiments performed with duplicate samples. *, P < 0.05 versus corresponding WT control; #, P < 0.05 versus untreated or “0” control.
Increased MHC II and costimulatory molecule expression on B cells.
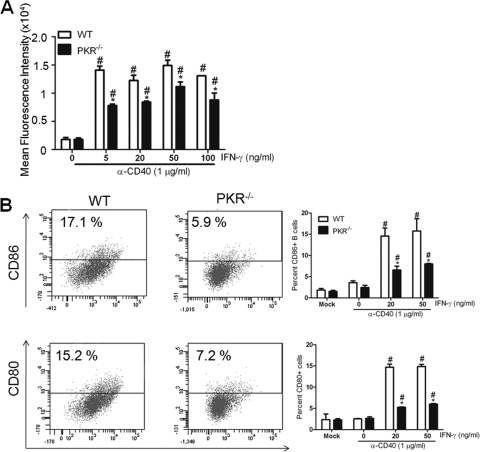
To further investigate the role of PKR in B cell activation, we looked at the cell surface expression of activation markers on WT and PKR−/− B cells. An increase in MHC II expression is a classic marker of B cell activation. Upon CD40 ligation in the presence of IFN-γ, WT B cells showed a robust increase in MHC II expression, while PKR−/− B cells showed a relatively diminished expression (Fig. 5A). It is well known that interaction between costimulatory molecules such as CD80 and CD86 on B cells and their counterparts on T cells is important for an efficient immune response. Furthermore, the interaction is critical for production of neutralizing antibodies (22). The expression of these costimulatory molecules is greatly enhanced upon activation of B cells. To determine the consequence of the absence of PKR on costimulatory molecule expression and B cell activation, we treated the splenocytes from WT and PKR−/− mice with anti-CD40 MAb and IFN-γ in vitro. On day 4, B cells from WT mice showed enhanced CD80 and CD86 cell surface expression compared with those from PKR−/− mice (Fig. 5B). In order to confirm that the observed differences were not due to altered proliferation of cells or diminished expression of CD40 on B cells, we quantified the cell numbers and CD40 expression following treatments with CD40 MAb. It has been shown previously that treatment with CD40 MAb leads to proliferation of B cells, which is a marker of B cell activation (1). Upon CD40 ligation with anti-CD40 MAb, B cells from PKR−/− mice demonstrated no difference in proliferation compared to those from WT mice (data not shown). Also, the expression of CD40 was comparable between WT and PKR−/− B cells (data not shown), suggesting that the alterations in IgG2a, IgG2b, and IgG1 did not result from diminished expression of the CD40 receptor.
Fig. 5.
Decreased MHC II and costimulatory marker expression on PKR−/− B cells. Splenocytes from WT and PKR−/− mice were cultured with anti-CD40 MAb and IFN-γ for 3 days. Splenic B cells from WT and PKR−/− mice were analyzed for surface activation markers such as MHC II (A) and for CD80 and CD86 expression (B). Data are means and SD for 3 different experiments with single samples. *, P < 0.05 versus corresponding WT control; #, P < 0.05 versus untreated or “0” control.
PKR plays a role in homotypic aggregation of B cells.
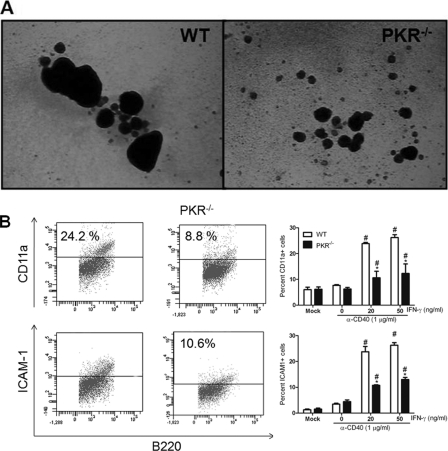
CD40-CD40L interaction is known to be a key step in lymphocyte activation and immunoglobulin class switching (1). Once B cells are stimulated with anti-CD40 and cytokines, they form homotypic aggregates. These homotypic aggregates are essential for optimal B cell activation (2). To test the role of PKR in CD40-mediated B cell homotypic aggregation, resting mature lymphocytes were purified from the spleen and cultured with anti-CD40 MAb and IFN-γ or IL-4 for 4 days. In the presence of IFN-γ or IL-4, the anti-CD40 MAb-treated splenocytes from WT mice formed large B cell aggregates from day 3 to day 5. However, PKR−/− B cell aggregates were markedly smaller than those of WT B cells, indicating less-than-optimal B cell activation (Fig. 6A and B). Similar results were observed when the splenocytes were stimulated with anti-CD40 MAb and IL-4 (data not shown). It has been shown previously that homotypic aggregates of B cells are formed through interactions of CD11a/CD18 (LFA-1) and CD54 (ICAM-1) adhesion molecules expressed on the cell surface (2, 23). The CD11a/CD18 adhesion system has been shown to play an important role in many lymphocyte interactions, including T cell-dependent B cell antibody responses (20). Both B and T cells constitutively express CD11a and ICAM-1. Upon flow cytometry analysis, expression of both CD11a and ICAM-1 was reduced on the PKR−/− B cell surface following activation with anti-CD40 MAb and IFN-γ (Fig. 6B). These findings suggest that PKR plays a pivotal role in lymphocyte interactions.
Fig. 6.
CD40-mediated homotypic aggregation and optimal adhesion molecule expression on B cells requires PKR. Splenocytes from WT and PKR−/− mice were stimulated in a 6-well format for 3 days with anti-CD40 MAb and IFN-γ. (A) Homotypic aggregation is shown. Pictures are of representative fields at a magnification of ×4. (B) Cell surface expression of adhesion molecules such as CD11a and ICAM1 was analyzed on stimulated splenic B cells. Data are means and SD for 3 different experiments with single samples. *, P < 0.05 compared with the PKR−/− splenocytes.
PKR is activated by CD40 ligation.
CD40 ligation on B cells is known to phosphorylate and activate multiple signaling molecules, including NF-κB, MAPKs, and PI3Ks (24). Based on the previous data, we hypothesized that PKR is a mediator of CD40 signaling. Ramos cells were treated with 1 μg/ml of anti-CD40 antibody for various times and then were lysed and used for Western blot analysis. PKR was activated at very early time points after CD40 engagement (Fig. 7). The CD40 ligation-induced PKR activation was further confirmed with primary murine splenocytes from WT mice. The WT splenocytes were cultured with 1 μg/ml of anti-CD40 MAb. There was a similar rapid activation and phosphorylation of PKR in the primary murine splenocytes (Fig. 7). In both cell types, PKR was activated as early as 1 min following CD40 ligation. These data imply for the first time that PKR is an important early component of the CD40 signaling pathway and plays a vital role in Ig production by B cells.
Fig. 7.
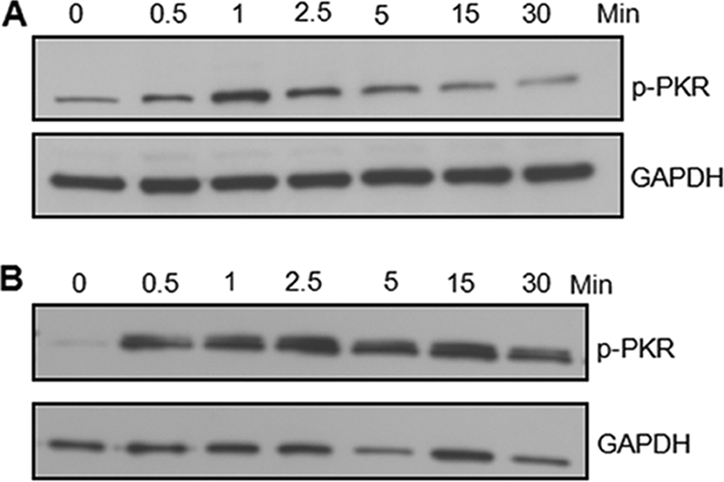
PKR is phosphorylated and activated at early time points upon CD40 ligation. Human Ramos cells (A) and primary murine splenocytes (B) were stimulated with anti-CD40 MAb for the indicated times. Denatured protein extracts were analyzed using Western blotting. Representative images of 3 different experiments are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
PKR has been shown to play an important role in several pathways triggered following virus infection, exposure to cytokines, and other cellular stresses (33). This enzyme has been demonstrated to affect a variety of cellular processes, such as antiviral protection, growth regulation, signal transduction, and differentiation (24). A previous report from our group demonstrated that PKR plays a role in IgE class switching in vitro in response to dsRNA in human B cells (26). In this study, we extend this finding and show that PKR plays a critical role in IgG expression both following RSV challenge in vivo and following CD40 ligation in vitro. This study contributes to our understanding of the molecular mediators that facilitate immunoglobulin responses elicited during natural infection or after vaccination. CD40-CD154 interactions are an integral part of the immunoglobulin class switching process (17). In this report, we show for the first time that PKR is phosphorylated and activated at early times following CD40 ligation.
IgG is the most abundant class of circulating immunoglobulins and plays a vital role in antiviral immune responses (5, 14). We found that serum IgG levels were constitutively lower in PKR−/− mice than in WT mice, thereby suggesting a fundamental defect in IgG responses. RSV infection failed to increase the levels of total circulating IgG in PKR−/− mice (data not shown). In particular, PKR−/− mice showed diminished serum levels of IgG antibodies against RSV F protein, which have previously been shown to neutralize virus both in vitro and in vivo (27). This diminished IgG response in PKR−/− mice was not due to decreased viral replication, as the viral titers in the lungs of WT and PKR−/− mice were comparable (Fig. 1A). A prominent effect of PKR was on the serum level of IgG2a. Interestingly, the level of IgG2a in mock-infected mice was below the detection limit. This is particularly intriguing and suggests that PKR is required for switching to IgG2a in vivo. However, there appears to be a PKR-independent compensatory immune mechanism to enhance IgG2b and IgG1 responses against RSV challenge. Clearly, robust IgG and IgA responses are required for mounting an effective protective immune response against both viruses and bacteria.
To further investigate the role of PKR from a broader perspective, we speculated that PKR may be an important player in CD40-mediated IgG subclass expression. We investigated the role of PKR following in vitro CD40 ligation in the presence of cytokines and in the absence of pathogen challenge. The results from the in vitro IgG subclass expression studies demonstrated that all IgG subclass levels were diminished in activated PKR−/− cell supernatants. This effect appeared to be cytokine independent, since levels of IgG3, IgG2b, and IgG2a were also decreased in the PKR-deficient cultures. These observations further strengthened the possible role of PKR in CD40 signaling. It is important that in contrast to the in vivo results, the supernatants from PKR−/− splenocyte cultures showed diminished levels of IgG2b in comparison to those from WT cells. This could be attributed to a more diverse environment in in vivo studies, with many immune cells and signaling pathways that could influence the immunoglobulin responses. Dendritic cells have previously been shown to play a role in CD40-independent immunoglobulin class switching (19). These CD40-independent signaling pathways may be involved in inducing a compensatory increase in IgG2b and IgG1 serum levels in vivo following RSV infection.
It is well established that signaling via CD40 ligation together with cytokines activates and stimulates immunoglobulin expression in B cells (1, 17). The absence of PKR diminished the CD40-induced activation and homotypic aggregation of B cells in the presence of both IFN-γ and IL-4 (data not shown). The expression of adhesion molecules that mediate homotypic aggregation, such as CD11a and ICAM-1, was also diminished significantly on PKR−/− B cells. The basal CD40 expression on PKR−/− B cells was comparable to the expression of CD40 on WT cells. Therefore, we hypothesize that PKR plays a crucial role in CD40-mediated B cell interaction and activation.
CD40 ligation transduces signals by phosphorylation and activation of adaptor proteins and enzymes to affect downstream transcription factors, including the NF-κB and MAPK pathways. This study demonstrated that PKR was rapidly and potently activated following CD40 ligation both in primary murine splenocytes and in a human B cell line. Since phosphorylation of PKR occurs at an early time point following CD40 ligation, it is possible that PKR may interact directly with CD40 or through the adaptor protein TRAF2. Further experiments are necessary to investigate the exact role of PKR in CD40 signaling.
Our data indicate that PKR plays a critical role in immunoglobulin expression as a novel downstream effector molecule for CD40 signaling. This highlights the possibility of PKR as a target of adjuvants in antiviral vaccine development. This likely will be accomplished by using double-stranded RNA, which is the most potent activator of PKR. Further work is necessary to elucidate the use of PKR as a target in vaccine development and the role of PKR in CD40 signaling.
ACKNOWLEDGMENTS
This research was supported entirely by the intramural research program of the NIH, National Institute of Environmental Health Sciences.
We thank Barney Graham (Vaccine Research Center, NIAID, NIH) for the generous gift of an RSV stock.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Banchereau J., et al. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12:881–922 [DOI] [PubMed] [Google Scholar]
- 2. Barrett T. B., Shu G., Clark E. A. 1991. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J. Immunol. 146:1722–1729 [PubMed] [Google Scholar]
- 3. Bossie A., Vitetta E. S. 1991. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell. Immunol. 135:95–104 [DOI] [PubMed] [Google Scholar]
- 4. Braesch-Andersen S., et al. 1989. Biochemical characteristics and partial amino acid sequence of the receptor-like human B cell and carcinoma antigen CDw40. J. Immunol. 142:562–567 [PubMed] [Google Scholar]
- 5. Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Craxton A., et al. 1998. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J. Immunol. 161:3225–3236 [PubMed] [Google Scholar]
- 7. Durandy A., Honjo T. 2001. Human genetic defects in class-switch recombination (hyper-IgM syndromes). Curr. Opin. Immunol. 13:543–548 [DOI] [PubMed] [Google Scholar]
- 8. Fisher R. G., Crowe J. E., Jr., Johnson T. R., Tang Y. W., Graham B. S. 1999. Passive IgA monoclonal antibody is no more effective than IgG at protecting mice from mucosal challenge with respiratory syncytial virus. J. Infect. Dis. 180:1324–1327 [DOI] [PubMed] [Google Scholar]
- 9. Foy T. M., Aruffo A., Bajorath J., Buhlmann J. E., Noelle R. J. 1996. Immune regulation by CD40 and its ligand GP39. Annu. Rev. Immunol. 14:591–617 [DOI] [PubMed] [Google Scholar]
- 10. Garceau N., et al. 2000. Lineage-restricted function of nuclear factor kappaB-inducing kinase (NIK) in transducing signals via CD40. J. Exp. Med. 191:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia M. A., et al. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 70:1032–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reference deleted.
- 13. Hodson D. J., Turner M. 2009. The role of PI3K signalling in the B cell response to antigen. Adv. Exp. Med. Biol. 633:43–53 [DOI] [PubMed] [Google Scholar]
- 14. Jegerlehner A., et al. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a. J. Immunol. 178:2415–2420 [DOI] [PubMed] [Google Scholar]
- 15. Kehry M. R. 1996. CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J. Immunol. 156:2345–2348 [PubMed] [Google Scholar]
- 16. Kinoshita K., Honjo T. 2000. Unique and unprecedented recombination mechanisms in class switching. Curr. Opin. Immunol. 12:195–198 [DOI] [PubMed] [Google Scholar]
- 17. Kitchell J. A., Clark D. L., Gombos A. M. 1986. Biologic selectivity of extinction: a link between background and mass extinction. Ann. Paleontol. 1:3–23 [Google Scholar]
- 18. Lebman D. A., Edmiston J. S. 1999. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 1:1297–1304 [DOI] [PubMed] [Google Scholar]
- 19. Litinskiy M. B., et al. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Makgoba M. W., et al. 1988. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature 331:86–88 [DOI] [PubMed] [Google Scholar]
- 21. Maliszewski C. R., et al. 1993. Recombinant CD40 ligand stimulation of murine B cell growth and differentiation: cooperative effects of cytokines. Eur. J. Immunol. 23:1044–1049 [DOI] [PubMed] [Google Scholar]
- 22. McAdam A. J., Farkash E. A., Gewurz B. E., Sharpe A. H. 2000. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mentzer S. J., Faller D. V., Burakoff S. J. 1986. Interferon-gamma induction of LFA-1-mediated homotypic adhesion of human monocytes. J. Immunol. 137:108–113 [PubMed] [Google Scholar]
- 24. Ogunsola O. I., Williams P. C. 1998. Particle size effects on compositional analyses of Nigerian tarsands. J. Afr. Earth Sci. Middle East 8:40–42 [Google Scholar]
- 25. Olson M. R., Varga S. M. 2008. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines 7:1239–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rager K. J., et al. 1998. Activation of antiviral protein kinase leads to immunoglobulin E class switching in human B cells. J. Virol. 72:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Routledge E. G., et al. 1988. The purification of four respiratory syncytial virus proteins and their evaluation as protective agents against experimental infection in BALB/c mice. J. Gen. Virol. 69:293–303 [DOI] [PubMed] [Google Scholar]
- 28. Sakata N., et al. 1995. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J. Biol. Chem. 270:30823–30828 [DOI] [PubMed] [Google Scholar]
- 29. Snapper C. M., et al. 1992. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 175:1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snapper C. M., et al. 1996. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 156:183–191 [PubMed] [Google Scholar]
- 31. Stavnezer J. 1996. Immunoglobulin class switching. Curr. Opin. Immunol. 8:199–205 [DOI] [PubMed] [Google Scholar]
- 32. Stewart M. J., Kulkarni S. B., Meusel T. R., Imani F. 2006. c-Jun N-terminal kinase negatively regulates dsRNA and RSV induction of tumor necrosis factor-alpha transcription in human epithelial cells. J. Interferon Cytokine Res. 26:521–533 [DOI] [PubMed] [Google Scholar]
- 33. Tan S. L., Katze M. G. 1999. The emerging role of the interferon-induced PKR protein kinase as an apoptotic effector: a new face of death?. J Interferon Cytokine Res. 19:543–554 [DOI] [PubMed] [Google Scholar]
- 34. Taylor S. S., Haste N. M., Ghosh G. 2005. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell 122:823–825 [DOI] [PubMed] [Google Scholar]
- 35. Zarnegar B., et al. 2004. Unique CD40-mediated biological program in B cell activation requires both type 1 and type 2 NF-kappaB activation pathways. Proc. Natl. Acad. Sci. U. S. A. 101:8108–8113 [DOI] [PMC free article] [PubMed] [Google Scholar]