Abstract
Immune responses against colonization factors (CFs) and the nontoxic B component of the enterotoxigenic Escherichia coli (ETEC) heat-labile toxin (LTB) are considered to be important for immunity against diarrhea caused by ETEC. Individual live attenuated ETEC derivatives that have had their toxin genes removed and whose aroC, ompC, and ompF genes are deleted have shown promise as vaccines against ETEC. The development of such strains has culminated in the testing of a three-strain-combination live attenuated vaccine known as ACE527, comprised of strains ACAM2025 expressing colonization factor antigen I (CFA/I) and LTB; ACAM2022, expressing CS5, CS6, and LTB; and ACAM2027, expressing CS1, CS2, CS3, and LTB. The recombinant CF and LTB genes expressed in the three strains were inserted into the bacterial chromosome to ensure their stable inheritance and expression without the requirement for any selection. ACE527 has been tested in a randomized placebo-controlled, double-blind, phase I safety and immunogenicity study in healthy adult volunteers and proved to be well tolerated and immunogenic at dose levels of 1010 and 1011 total CFU. There was no indication of strain interference on the basis of fecal shedding patterns, with all three being detected in the feces of 50% and 83% of low- and high-dose vaccine recipients, respectively. Similarly, strong immune responses to LTB and to CFs expressed on all three constituent strains were induced, with at least 50% of subjects in the high-dose group responding to LTB, CFA/I, CS3, and CS6.
INTRODUCTION
In regions of the world where sanitation and clean water supplies are inadequate, diarrheal diseases are the second highest cause of mortality in children under the age of 5 years, after pneumonia. Recently published estimates of the number of deaths due to diarrhea worldwide in children under age 5 years are 1.3 million (95% confidence interval [CI], 0.8 million to 2.0 million) (4) and 1.9 million (95% CI, 1.6 million to 2.2 million) (5), with 237,000 (4) to 535,000 (5) deaths occurring in India alone. Additional health burdens on both individuals and their families due to the effect of multiple diarrheal episodes early in life, which cause impairment of physical, intellectual, and economic development, are increasingly being recognized (11, 23, 27).
Enterotoxigenic Escherichia coli (ETEC) is estimated to cause 10% to 20% of diarrheal cases in developing countries (2), every year accounting for 280 million to 400 million cases in children under age 5 years and an additional 100 million and 400 million cases in children over age 5 years and adults, respectively (3). These infections are estimated to cause between 300,000 and 500,000 deaths annually, mostly in children. ETEC also remains a major cause of diarrhea in travelers to such areas, including military personnel, resulting in an estimated 10 million cases annually (38, 46, 47).
For general information about ETEC, the reader is referred to two excellent reviews (24, 31). ETEC expresses fimbriae called colonization factor antigens (CFAs), which enable the organism to adhere to the small intestine mucosa, where it secretes toxins, causing the symptoms of diarrhea. The genes coding for these virulence factors are usually on plasmids. CFA/I, CFA/II, and CFA/IV are among the most prevalent CFAs expressed by ETEC (49). CFA/II consists of three different fimbriae, called coli surface antigens (CSs), CS1, CS2, and CS3. However, natural CFA/II ETEC strains express only CS3 alone or express CS3 with CS1 or CS2. Likewise, CFA/IV consists of CS4, CS5, and CS6, with CS6 being expressed alone or with either CS4 or CS5. Many studies suggest that immune responses against colonization factors may protect against ETEC infection (13, 16, 35, 39, 48). In some cases, the titer of CFA-specific serum antibody has been shown to predict a reduced risk or severity of ETEC illness (21, 32).
ETEC also expresses one or more toxins, including heat-stable toxin (ST), heat-labile toxin (LT), and enteroaggregative Escherichia coli heat-stable toxin 1 (EAST1) (22, 36). ST and EAST1 are short polypeptides and are poor antigen candidates. LT is similar to cholera toxin (CT), consisting of a pentamer of B subunits (LTB), which acts as a carrier for a single, toxic subunit, subunit A (33). Like cholera toxin, LTB without the A subunit is nontoxic but is immunogenic and therefore is another good candidate for inclusion in a vaccine against ETEC. A recent phase II field study in travelers to Central America demonstrated significant protection against diarrheal disease in subjects vaccinated with LT delivered via a skin patch before travel (12) This approach was further tested in a phase III trial, in which 60% protection was observed against LT-only ETEC strains; however, protection against all ETEC strains or any cause of diarrhea was not observed (15). Earlier studies with the cholera vaccine Dukoral also indicate that anti-CT immunity can provide at least some degree of protection against ETEC in the field due to the immunological cross-reactivity with LT (7, 25, 37).
Alternative vaccine strategies against ETEC have been thoroughly reviewed (45). One promising approach is the development of oral live attenuated vaccines. However, in order for a live vaccine to be effective, an immune response to a variety of ETEC strains is likely to be necessary. A vaccine containing CFA/I, CFA/II, and CFA/IV plus LTB may provide protection against up to 90% of ETEC strains in most areas (31, 49). To address this unmet need, a vaccine designated ACE527 that contains three live attenuated ETEC vaccine strains collectively expressing CFA/I, CS1, CS2, CS3, CS5, CS6, and LTB is being developed. This report describes the safety and immunogenicity results from the first-in-human dose-escalation trial of ACE527.
MATERIALS AND METHODS
Primary and secondary objectives.
There were two primary objectives of this dose-escalation study. The safety objective was to evaluate the tolerability of ascending doses of ACE527 versus placebo when administered orally at 0 and 21 days. The immunogenicity objective was to evaluate the induction of systemic and mucosal immunity by assessing anti-CFA/I-, anti-CS3-, anti-CS6-, and anti-LTB-specific responses postvaccination. The frequency of mucosal IgA responses to the CFA/I, CS3, and CS6 antigens (≥4-fold rise in ≥50% of the subjects) was used as the criterion for determining whether the ACE527 vaccine would advance to a phase IIB immunization and challenge study after the completion of the phase I study. The secondary objective of the study was to evaluate the excretion profiles of the three individual strains comprising ACE527 in stools of the subjects to assess potential strain interference as well as to confirm that excretion is self-limiting.
Production of vaccine.
The ETEC strains and associated plasmids used to produce the vaccine are described briefly in Table 1 and in more detail elsewhere (41, 42). The original ETEC clinical isolates, from which the attenuated vaccine strains were derived, were collected as part of a large epidemiological study of diarrhea in children residing in Abu Homos, Egypt (26), and were kindly provided by the United States Naval Medical Research Unit 3 (NAMRU3), Cairo, Egypt. All the strains were attenuated by the same strategy to generate strains ACAM2025, ACAM2022, and ACAM2027, which together comprise the vaccine. Briefly, all genes encoding known or putative enterotoxins (ST, LT, or EAST1) were removed; all antibiotic resistance determinants were removed; and three deletion mutations were introduced into the chromosomes to remove genes aroC, ompC, and ompF. These mutations were previously shown to yield attenuated ETEC strains which were well tolerated and immunogenic at high doses of up to 2 × 1010 CFU in healthy adult subjects (10, 19, 43).
Table 1.
Bacterial strains
| Strain name | Phenotype and genotypea | Reference |
|---|---|---|
| WS-2773E | O141:H5 CS5 CS6 LT ST EAST1 (clinical isolate) | NMRCb |
| ACAM2022 | O141:H5 CS5 CS6 ΔastA ΔeltAB ΔestA ΔompF::Ptac-LTB ΔaroC Δphage | 25, 42 |
| WS-3504D | O39:H12 CS2 CS3 LT ST EAST1 Apr (clinical isolate) | NMRC |
| ACAM2007 | O39:H12 CS2 CS3 ΔastA ΔeltAB ΔestA ΔompC ΔompF ΔaroC | 5, 25, 42 |
| ACAM2017 | O39:H12 CS2 CS3 ΔastA ΔeltAB ΔestA ΔompC::CS1 ΔompF ΔaroC | 5, 25, 42 |
| ACAM2027 | O39:H12 CS2 CS3 ΔastA ΔeltAB ΔestA ΔompC::CS1 ΔompF::Ptac-LTB ΔaroC | 42 |
| WS-1858B | O71:H− CFA/I ST EAST1 Apr Tpr (clinical isolate) | NMRC |
| ACAM2010 | O71:H− CFA/I ΔastA ΔestA ΔaroC ΔompC ΔompF | 24, 25 |
| ACAM2025 | O71:H− CFA/I ΔastA ΔestA ΔaroC ΔompC ΔompF::pLLTB | 42 |
Antibiotic resistance: Apr, ampicillin; Tpr, trimethoprim.
NMRC, provided by S. Savarino, Naval Medical Research Center, Silver Spring, MD.
Each of the three vaccine strains was grown under conditions of good manufacturing practice (GMP) in large shake flask cultures in media guaranteed to be free from all animal components. Cells were harvested, washed, and resuspended in phosphate-buffered saline (PBS) containing 20% glycerol and then frozen at −80°C for long-term storage. These vials constituted both the GMP master cell banks (MCBs) for future development of the vaccine and the direct source of cells for dosing.
ACE527 was administered orally as a liquid formulation. Individual vaccine doses were prepared by pooling and mixing appropriate volumes from a number of vials of each of the MCBs and then combining appropriate volumes from each strain to form a single 10-ml suspension. This suspension contained approximately 3 × 109 CFU and 3 × 1010 CFU of each of the three strains for cohorts 1 and 2, respectively; thus, the doses are referred to as 1010 CFU and 1011 CFU total dose, respectively. Following administration of each dose to a group of subjects, the actual viable dose of each strain given was determined by serial dilution and plating of an aliquot of the suspension for quality control purposes and confirmation that it was consistent with the intended level. Placebo preparations consisted of 10 ml of PBS alone. At no more than 2 h before the time of dosing, each dose of ACE527 or placebo was mixed with 190 ml of the commercial vaccine buffer solution CeraVacx (Cera Products, Columbia, MD). Subjects were required to fast for 90 min before and after vaccination and were observed closely for 60 min postvaccination for safety and to ensure no regurgitation of study product. The first dose was administered to each cohort on the morning of the second day of a 4-day inpatient period, and the second dose was administered on an outpatient basis 21 days later.
Study design.
ACE527 protocol 101 was a single-center, randomized, double-blind, placebo-controlled trial to evaluate increasing dosages of ACE527 in healthy adults. After a comprehensive screening evaluation, two sequential cohorts of 18 subjects were enrolled to receive either placebo or ACE527 at approximate dosages of 1010 or 1011 CFU total for cohort 1 and cohort 2, respectively. Within cohorts, subjects were randomized to receive vaccine or placebo in a 2:1 ratio, such that 12 subjects received vaccine and 6 received placebo. Subjects received two doses of ACE527 or placebo at 3-week intervals between doses, were actively monitored for up to 28 days after the second dose, and then were followed up with a telephone call to check for any serious emergent medical conditions after 3 months. The clinical protocol was performed under BB-IND 13,982, was approved by the appropriate institutional review boards of the Johns Hopkins Bloomberg School of Public Health and PATH, and was conducted in accordance with principles of good clinical practice.
Healthy adults between 18 and 50 years of age with body mass indices (BMIs) of between 19.0 and 34.0 kg/m2 were eligible. Breast-feeding women or women who were or planned to become pregnant during the study period were excluded. Other exclusion criteria included immunoglobulin A deficiency; alcohol or drug dependence within the preceding 3 years; vaccination or receipt of an investigational product in the preceding month; employment as a food handler, child care provider, or health care worker; having household contacts who were immunocompromised, <2 years old, or >80 years old; abnormal hematology/serum chemistry; positive screen for HIV or hepatitis virus B/C; having abnormal stool patterns (less than 3 per week or more than 3 per day); history of diarrhea in the 7 days prior to vaccination; regular use of laxatives or antacids; history of vaccination against or ingestion of ETEC, cholera toxin, or LT within 3 years; symptoms consistent with traveler's diarrhea concurrent with travel to countries where ETEC is endemic within 2 years; and known allergy to quinolones, trimethoprim-sulfamethoxazole, or penicillins. Each participant signed an informed-consent form and was required to score ≥70% on a study comprehension assessment prior to undergoing study-specific procedures.
Safety evaluation.
The primary safety endpoints were adverse events (AEs) in the 7 days following each dose evaluated via focused medical interviews, standardized vaccination report cards (VRCs), laboratory tests, and physical examinations. Of particular interest were AEs expected to be associated with the ingestion of attenuated ETEC strains, including loose stools, diarrhea, intestinal hyperactivity, nausea, vomiting, abdominal pain or cramping, defecation urgency, or constipation. Other systemic AEs, including fever, headache, malaise, chills, anorexia, light-headedness, and myalgia, were also collected. Diarrhea during the period postvaccination was defined as three or more loose or liquid stools within 24 h; other unformed bowel movements were recorded as loose stools. Secondary safety endpoints included the duration and maximum quantitative level of shedding of each of the three vaccine strains.
Clinical monitoring.
The first immunization for all subjects was administered in an inpatient unit. Vital signs were obtained at 60 min pre- and postvaccination and 3 times daily during the 4-day inpatient stay. On study days 0 through 3, a daily medical interview and physical exam were conducted by the principal investigator (PI). To capture anticipated and unanticipated AEs postdischarge, subjects completed VRCs on study days 4 to 7. The second immunization was given in an outpatient clinic on study day 21, and vital signs were measured at 60 min pre- and postvaccination. Participants completed VRCs on study days 21 to 28 after the second vaccination. Laboratory parameters for hematology and clinical chemistries were monitored at designated time points throughout the study. The following parameters were monitored: basophils (%), eosinophils (%), hematocrit, hemoglobin, lymphocytes (%), monocytes (%), neutrophils (%), platelet count, red blood cells (RBCs), white blood cells (WBCs), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, bilirubin, creatinine, gamma-glutamyl transpeptidase (GGT), potassium, sodium, and blood urea nitrogen (BUN). Abnormal laboratory values were evaluated for clinical significance by the PI and graded as AEs using U.S. Food and Drug Administration toxicity table guidelines. Before escalating to the higher dose level, safety data through study day 7 of the lower-dose cohort had to be reviewed and approved by an independent safety committee (ISC), comprised of 3 independent members unaffiliated with the sponsor or the clinical site. Similarly, before subjects within a cohort could receive their second dose, safety data through study day 7 were reviewed and approved by the ISC.
Stool microbiology.
Stool samples were collected at days 3, 7, 10, 14, 21, 28, 31, 35, and 49 after the first vaccination for evaluation of vaccine shedding. ACE527 colonies were identified by spreading samples on MacConkey agar plates (typical lactose-positive pink appearance) and replica picking up to 50 colonies and placing them onto M9 minimal medium agar plates with and without supplementation of aromatic compounds (19); the aroC auxotrophic mutation prevents growth on M9 alone. Pools of aromatic-dependent colonies were then made and screened in a multiplex PCR to identify the presence or absence of the three individual vaccine strains (primers used are listed in Table 2). On days 3 and 23, initial serial dilutions were made and plates were inoculated to allow quantitative estimates of the shedding level of individual strains, expressed as numbers of CFU per gram of stool, with individual colonies identified by dot blots with specific antisera which had been raised in rabbits by hyperimmunization with heat-killed whole bacterial cells.
Table 2.
Oligonucleotides used for PCR identification of vaccine strains
| Name | Nucleotide sequence (5′ → 3′) | Target locus |
|---|---|---|
| 4738 | GGAAAGAGAGTATATCTATGTAACGC | CS5 (ACAM2022) |
| 4739 | CGGTCGAGTAATAAGCTGTACTCTGC | CS5 (ACAM2022) |
| 4729 | AAAGAATAGATTAGTCGTAGCG | cfaA (ACAM2025) |
| 4730 | TTCTTCACGAACTAATTGAGTG | cfaA (ACAM2025) |
| 4712 | GTAACTGCTAGCGTTGATCC | CS2 (ACAM2027) |
| 4713 | CCTTGATAGTACCAGCTAC | CS2 (ACAM2027) |
Immunogenicity evaluation.
This first-in-human study was designed to evaluate initial safety and immunogenicity; however, it was not specifically powered to draw conclusions about the level or frequency of responses. Systemic and mucosal antibody responses to LTB, CFA/I, CS3, and CS6 were evaluated to monitor the immunogenicity overall and the immunogenicity of the three individual strains. Serum IgG and IgA responses were measured on days 0, 21, 31, and 49 and were analyzed by the level of response (geometric mean titers) as well as the frequency with which subjects seroconverted, as measured by a 2.5-fold or more increase over the preimmunization levels.
Mucosal immune responses were evaluated using the antibody from lymphocyte supernatant (ALS) assay (6), which measures the specific IgA secreted by peripheral blood mononuclear cells (PBMCs) circulating to the mucosal inductive sites, peaking at 7 to 10 days following oral immunization. PBMCs were isolated on the day of and at 7 and 10 days following each immunization. When preparing the ALS specimens, 107 PBMCs/ml were incubated in RPMI medium (Lonza, Walkersville, MD) with 10% fetal bovine serum (SAFC Biosciences, Lenexa, KS) in a 5% CO2 incubator at 37°C for 72 h with no stimulation. The supernatant was harvested, and aliquots were kept at −80°C until the enzyme-linked immunosorbent assay (ELISA) was carried out. ALS responses were considered positive if, at any time point after vaccination, they reached 4-fold or more over baseline at day 0.
ELISAs of serum or ALSs were performed according to standard protocols using peroxidase-labeled antihuman isotype-specific detecting antibodies (KPL, Baltimore, MD). CFA/I, CS3, and CS6 antigens were supplied by E. Oak at the Walter Reed Army Institute for Research (WRAIR), and LTB was purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analyses.
Clinical and safety data were captured using electronic case report forms (CRFs). The intention-to-treat (ITT) analysis set comprised all subjects who received at least one administration of ACE527 or placebo. The per protocol (PP) analysis set comprised all subjects who received both administrations of ACE527 or placebo. Immune responses were compared by dosage level. The threshold for definition of a positive serological response was derived by investigating the apparent level of responses in the placebo recipients. The fold increases in serum IgG responses to CFA/I and LTB over baseline (day 0) at each of days 21, 31, and 49 were analyzed by log transform of individual data points and calculation of their means and standard deviations (SD). A significant positive response was then defined as a response more than 3 SDs above the mean. For CFA/I and LTB, this corresponded to 2.35-fold and 1.76-fold increases, respectively. Accordingly, to give a further level of confidence, the threshold was set at 2.5-fold; by this criterion, no positive responses to any antigen were then seen in any placebo recipients at any time point.
RESULTS
Subject disposition and demographics.
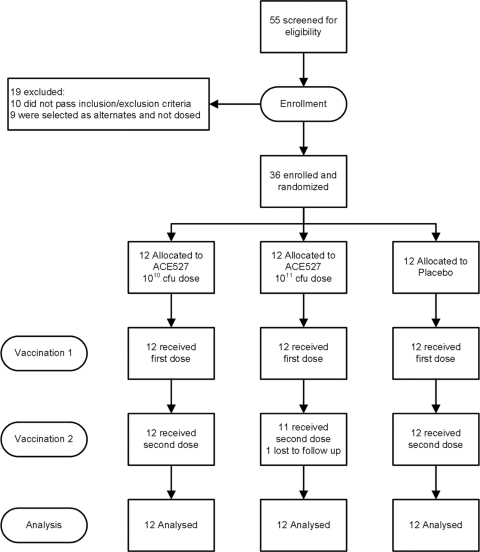
Fifty-five healthy adult volunteers were screened for eligibility, with 36 enrolled, as illustrated in Fig. 1. Ten subjects failed to meet the screening criteria. Eight failed either to meet the inclusion criterion requiring general good health or to show full comprehension of the informed-consent material. Five met one or more of the following exclusion criteria: significant medical condition, abnormal hematology or clinical chemistry values, BMI out of range, or allergy to antibiotics. In addition, one subject had an abnormal electrocardiogram, and another was screened after enrollment closed. Nine subjects who passed all screening criteria were selected as alternates and received no study treatments. The age range of the subjects enrolled was from 18 to 50 years; the mean age was 34.5 years. The majority of the subjects (26 of 36; 72.2%) were black or African American. There were 27 males who participated in the study (75%). The demographics, by study group, are shown in Table 3. A total of 36 subjects received the first dose of vaccine or placebo per protocol, 35 subjects completed the study through study day 80, and 34 subjects completed the study through the final follow-up call on study day 98. One subject (in the high-dose ACE527 group) was lost to follow-up, having received a single dose of vaccine and provided samples for analysis at days 7 and 10; an additional subject was lost to follow-up after day 80, having received two doses of placebo.
Fig. 1.
Subject disposition from screening.
Table 3.
Subject demographics, by group
| Characteristic | ACE527, 1010 CFU (n = 12) | ACE527, 1011 CFU (n = 12) | Placebo (pooled; n = 12) | Total (n = 36) |
|---|---|---|---|---|
| No. (%) of subjects | ||||
| Sex | ||||
| Male | 7 (58.3) | 10 (83.3) | 10 (83.3) | 27 (75.0) |
| Female | 5 (41.7) | 2 (16.7) | 2 (16.7) | 9 (25.0) |
| Race | ||||
| Black or African American | 8 (66.7) | 11 (91.7) | 7 (58.3) | 26 (72.2) |
| White | 4 (33.3) | 0 (0.0) | 3 (25.0) | 7 (19.4) |
| Other | 0 (0.0) | 1 (8.3) | 0 (0.0) | 1 (2.8) |
| Asian | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (2.8) |
| American Indian or Alaska Native | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (2.8) |
| Ethnicity | ||||
| Not Hispanic or Latino | 12 (100.0) | 11 (91.7) | 12 (100.0) | 35 (97.2) |
| Hispanic or Latino | 0 (0.0) | 1 (8.3) | 0 (0.0) | 1 (2.8) |
| Age (yr) | ||||
| Mean (SD) | 31.9 (10.63) | 34.6 (6.69) | 36.9 (11.34) | 34.5 (9.71) |
| Range | 19–50 | 23–46 | 18–50 | 18–50 |
Safety results.
No subject experienced an immediate postvaccination reaction, and no serious adverse events occurred. The frequency of adverse events in the high-dose group was 91.7%, compared to 66.7% each in the low-dose and placebo groups. Most adverse events were equally distributed across treatment groups. Of note, however, were the AEs attributable to gastrointestinal (GI) disorders, which were predominately (31/38, 81.6%) of mild severity (Table 4). GI AEs were more common in the high-dose ACE527 group than in either the low-dose ACE527 group or the placebo group. Of the 12 subjects who reported GI complaints, 8 were in the high-dose group, 2 were in the low-dose group, and 2 were in the placebo group. The most frequent treatment-related GI disorder was flatulence, which was observed more frequently in high-dose ACE527 recipients. There was no significant difference in the incidence of diarrhea or nausea between treatment groups, although there was a suggestion of more loose stools possibly or probably associated with vaccination in the high-dose ACE527 subjects than in the other treatment groups combined (3 of 12 [2 with loose stools and 1 with diarrhea] versus 1 of 24 [loose stools only]; P = 0.10, Fisher's exact test). There were no clinically significant or reproducible changes in clinical safety lab test results in any group or at any time point in the study. There was one subject in cohort 2 whose AST level was abnormal on day 0 and through day 49, but this was considered unlikely to be related to ACE527 by the investigator. There were no clinically significant or reproducible changes in hematology in any group or at any time point in the study; hemoglobin, hematocrit, and red blood cells had several low values, but these were equally distributed across groups and time points.
Table 4.
Subjects with treatment-related adverse events by group
| Adverse event | No. (%) of subjectsa |
||
|---|---|---|---|
| ACE527, 1010 CFU | ACE527, 1011 CFU | Placebo | |
| General symptoms | |||
| Malaise (felt unwell) | 0 (0) | 1 (8.3) | 0 (0) |
| Headache | 0 (0) | 2 (16.7) | 2 (16.7) |
| Chills | 0 (0) | 0 (0) | 1 (8.3) |
| Anorexia (loss of appetite) | 0 (0) | 0 (0) | 1 (8.3) |
| Gastrointestinal symptoms | |||
| Flatulence | 2 (16.7) | 8 (66.7) | 2 (16.7) |
| Diarrhea (≥3 loose stools in 24 h) | 0 (0) | 1 (8.3) | 0 (0) |
| Loose stools not meeting diarrhea definition | 0 (0) | 2 (16.7) | 1 (8.3) |
| Nausea | 0 (0) | 2 (16.7) | 1 (8.3) |
| Abdominal pain | 0 (0) | 1 (8.3) | 1 (8.3) |
| GI sounds abnormal (gurgling stomach) | 0 (0) | 2 (16.7) | 0 (0) |
| Urgency of defecation | 0 (0) | 0 (0) | 1 (8.3) |
| Gastrointestinal disorder | 0 (0) | 1 (8.3) | 0 (0) |
Each group had 12 subjects.
Vaccine shedding results.
Colonization by the vaccine was self-limiting. All subjects were culture negative for ACE527 by study day 49 (4 weeks after the second vaccination), and no subject required treatment with antibiotics to terminate shedding of the vaccine. Both the duration and the magnitude of shedding were higher in the high-dose ACE527 cohort, which received a 10-fold higher dose than the low-dose ACE527 cohort. Table 5 shows the number of subjects shedding each vaccine strain or ACE527 (i.e., any of the three strains) by study day and the mean log shedding level of each strain and ACE527 in only those subjects who were positive on study days 3 and 23. For the recipients of the higher dose of vaccine, the median time to negative stool cultures was estimated to be 6 to 7 days following the first dose and 4 to 5 days following the second. Encouragingly, there was no evidence for interference between the three strains in the vaccine. In recipients of the higher dose, 6/12 shed detectable levels of all three strains after the first dose and 9/11 shed detectable levels of all three strains after the second dose, and altogether, 10 subjects were observed to shed all three strains on at least one occasion. The corresponding figures for recipients of the lower dose level were 4/12 after the first dose and 4/12 after the second dose, and 6 subjects shed all three strains at least once.
Table 5.
Shedding of vaccine strains in cohort 1 (low dose) and cohort 2 (high dose)
| Cohort and strain | No. of subjects shedding on daya: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 9 | 14 | 21 | 23 | 28 | 30 | 34 | 49 | |
| Cohort 1 | ||||||||||
| ACAM2022 | 8 (4.52) | 1 | 1 | 0 | 0 | 7 (4.48) | 0 | 0 | 0 | 0 |
| ACAM2025 | 5 (3.56) | 3 | 1 | 0 | 0 | 5 (4.35) | 0 | 0 | 0 | 0 |
| ACAM2027 | 7 (4.66) | 1 | 1 | 0 | 0 | 8 (5.12) | 1 | 1 | 1 | 0 |
| ACE527 | 8 (4.99) | 3 | 1 | 0 | 0 | 9 (5.18) | 1 | 1 | 1 | 0 |
| Cohort 2 | ||||||||||
| ACAM2022 | 8 (5.42) | 2 | 1 | 0 | 0 | 9 (5.96) | 0 | 0 | 0 | 0 |
| ACAM2025 | 6 (4.71) | 2 | 0 | 0 | 1 | 9 (5.69) | 1 | 0 | 1 | 0 |
| ACAM2027 | 10 (5.38) | 3 | 1 | 0 | 1 | 10 (6.06) | 0 | 1 | 1 | 0 |
| ACE527 | 10 (5.62) | 5 | 1 | 0 | 2 | 10 (6.34) | 1 | 1 | 1 | 0 |
The number of subjects shedding each strain was determined by PCR, and the number shedding ACE527 is defined as the number shedding at least one of the constituent strains. For quantitative determinations, the individual strains were identified by dot blots with specific antisera. For cohort 1, 12 subjects were evaluated on each day. For cohort 2, 12 subjects were evaluated on days 3, 7, and 9 and 11 subjects were evaluated on days 14, 21, 23, 28, 30, 34, and 49. Data in parentheses represent the mean log10 CFU/g in positive subjects.
Immunogenicity results.
The rates of response in serum and ALS are shown in Table 6. Clearly, the strongest systemic and mucosal responses were observed against LTB; 100% of subjects at both dose levels gave a significant rise in ALS titer. The serum response, however, was dose dependent, with 7 and 10 subjects responding with increased IgA and IgG serum titers, respectively, following receipt of the high dose but only 5 subjects mounting an IgG response to the low dose and no subjects mounting IgA responses.
Table 6.
Frequency of serum antibody and mucosal responses by dosing group
| Antibody | Group (no. of CFU) | No. (%) of respondersa |
Pb |
|
|---|---|---|---|---|
| Placebo | ACE527 dose | |||
| Anti-LTB | ||||
| ALS IgA | Placebo | 1 (8) | ||
| ACE527 (1010) | 12 (100) | <0.0001 | ||
| ACE527 (1011) | 12 (100) | <0.0001 | ||
| Serum IgA | Placebo | 0 | ||
| ACE527 (1010) | 0 | |||
| ACE527 (1011) | 7 (58) | 0.005 | 0.005 | |
| Serum IgG | Placebo | 0 | ||
| ACE527 (1010) | 5 (42) | 0.037 | ||
| ACE527 (1011) | 10 (83) | <0.0001 | 0.089 | |
| Anti-CFA/I | ||||
| ALS IgA | Placebo | 2 (17) | ||
| ACE527 (1010) | 8 (67) | 0.036 | ||
| ACE527 (1011) | 9 (82)c | 0.003 | ||
| Serum IgA | Placebo | 0 | ||
| ACE527 (1010) | 0 | |||
| ACE527 (1011) | 3 (27)c | 0.093 | 0.093 | |
| Serum IgG | Placebo | 0 | ||
| ACE527 (1010) | 0 | |||
| ACE527 (1011) | 1 (9)c | |||
| Anti-CS3 | ||||
| ALS IgA | Placebo | 2 (8) | ||
| ACE527 (1010) | 6 (50) | |||
| ACE527 (1011) | 6 (55)c | 0.089 | ||
| Serum IgA | Placebo | 0 | ||
| ACE527 (1010) | 0 | |||
| ACE527 (1011) | 0c | |||
| Serum IgG | Placebo | 0 | ||
| ACE527 (1010) | 0 | |||
| ACE527 (1011) | 1 (9)c | |||
| Anti-CS6 | ||||
| ALS IgA | Placebo | 2 (17) | ||
| ACE527 (1010) | 5 (42) | |||
| ACE527 (1011) | 10 (83) | 0.003 | 0.089 | |
| Serum IgA | Placebo | 0 | ||
| ACE527 (1010) | 0 | |||
| ACE527 (1011) | 4 (33) | 0.093 | 0.093 | |
| Serum IgG | Placebo | 0 | ||
| ACE527 (1010) | 2 (17) | |||
| ACE527 (1011) | 1 (9)c | |||
Thresholds, 2.5× for serum responses and 4× for ALS responses.
P value using Fisher exact test for difference between each dose level of ACE527 and placebo individually or between the 2 dose levels of ACE527, respectively. Numbers of responders are compared at the 2.5× threshold for serum antibody responses and at the 4× threshold for ALS responses. Only values of <0.1 are shown.
One subject dropped out after receiving the first dose. If the subject did respond to the first dose then n was equal to 12; if the subject did not, then for this analysis, n was equal to 11, as the subject may have responded to the second dose.
The magnitude of the responses to LTB in the sera of vaccinees was impressive and comparable to that seen in subjects challenged with virulent ETEC strain H10407 (14). Responses are plotted in Fig. 2. In the high-dose recipients, 7/12 gave a positive response after the first dose and 3/12 additional subjects seroconverted after the second dose. There was a trend toward higher titers following the second dose, with maximal titers reached by day 31. Of the responses to the CFAs expressed by the vaccine strains, the strongest were to CFA/I and CS6, with mucosal responses detected in over 80% of subjects at the higher vaccine dose; 55% of subjects showed a positive response to CS3. The magnitudes of the ALS responses to LTB, CFA/I, CS3, and CS6, by group, are shown in Fig. 3. Among the responders to either dose level, the peak geometric mean increases in specific IgA ALS titers compared with the baseline titers to each of the antigens were as follows: LTB, 18-fold (95% CI, 7- to 42-fold); CFA/I, 22-fold (95% CI, 13- to 38-fold); CS3, 7-fold (95% CI, 5- to 9-fold); and CS6, 13-fold (95% CI, 7- to 24-fold).
Fig. 2.
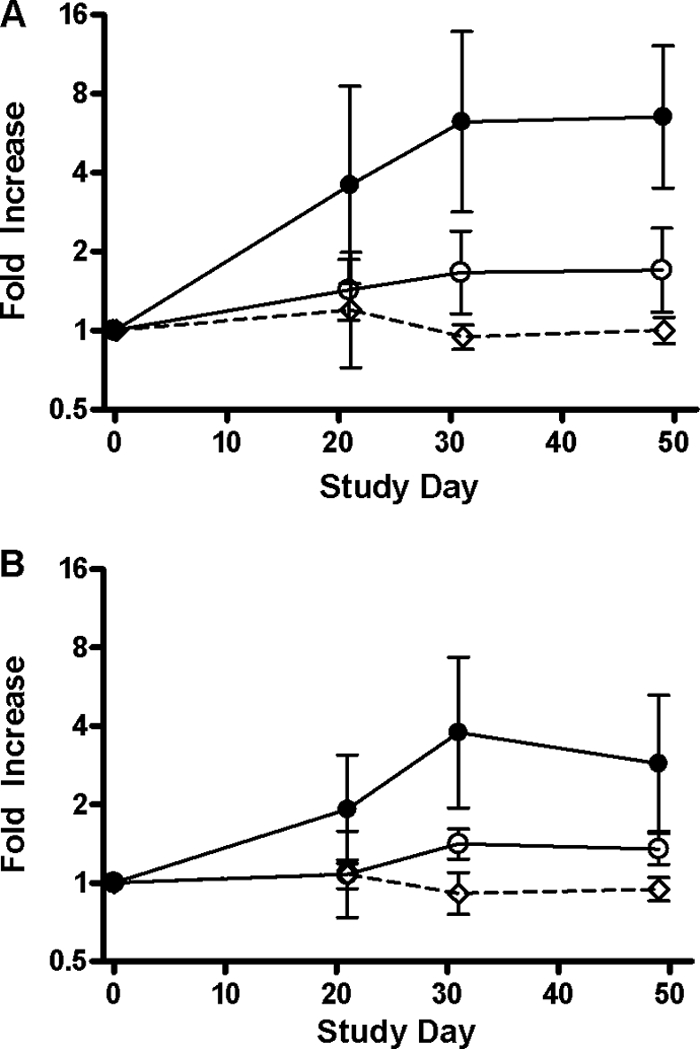
Serum antibody responses to LTB. Graphs show geometric means on a log2 scale of the fold increase over preimmunization titers, with error bars representing 95% confidence intervals. (A) Serum IgG titers; (B) serum IgA titers. Filled circles, 1011 CFU ACE527; open circles, 1010 CFU ACE527; open diamonds with dashed line, placebo.
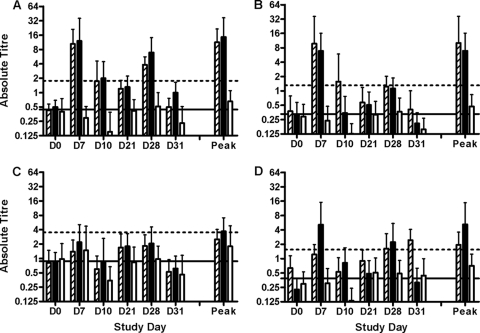
Fig. 3.
Magnitude of ALS responses to LTB and colonization factors. Graphs show the mean specific IgA titer in ALSs on a log2 scale for all subjects in a group, with error bars indicating standard deviations. Responses are plotted for each individual day (days 0, 7, 10, 21, 28, and 31), as is the peak response for each subject on any day. (A) LTB responses; (B) CFA/I responses; (C) CS3 responses; (D) CS6 responses. Empty bars, hatched bars, and solid bars, data for placebo, low-dose, and high-dose groups, respectively; solid horizontal lines, mean preimmunization titers for all subjects for each specific antigen; dotted horizontal lines, four times the mean preimmunization titers for all subjects for each specific antigen.
There were no apparent differences between the dose groups with respect to the frequency and magnitude of ALS responses to the four individual antigens, although a trend was observed in the case of CS6, suggesting better responses at the higher dose. Overall, vaccination with ACE527 induced significant LTB-, CFA/I-, CS3-, and CS6-specific IgA ALS responses in 24 of 24 (100%), 17 of 23 (74%), 12 of 23 (52%), and 15 of 24 subjects (63%) subjects, respectively (Table 6). The lower apparent rate of response to CS3 may be due in part to the higher baseline titer of antibody to this antigen, as shown in Fig. 3.
The breadth of the cumulative mucosal response (i.e., the number of subjects responding in the ALS assay to 0, 1, 2, or 3 of the CFAs after the first and either the first or second vaccination) is shown in Table 7. The frequency of the observed responses was dose dependent, and the number of positive responses increased following the second dose at both dose levels.
Table 7.
Breadth of mucosal anti-CFA responses by dose and group
| Group | No. (%) of subjects |
|||
|---|---|---|---|---|
| Cohort 1 (low dose) |
Cohort 2 (high dose) |
|||
| First dose (n = 12) | By the second dose (n = 12) | First dose (n = 12) | By the second dose (n = 11)a | |
| Subjects who did not respond to any of the CFA antigens | 5 (42) | 3 (25) | 1 (8) | 1 (9) |
| Subjects who responded to only one CFA antigen | 3 (25) | 1 (8) | 4 (33) | 1 (9) |
| Subjects who responded to two CFA antigens | 3 (25) | 6 (50) | 4 (33) | 4 (36) |
| Subjects who responded to all three CFA antigens | 1 (8) | 2 (17) | 3 (25) | 5 (46) |
| Subjects who responded to at least two CFA antigens | 4 (33) | 8 (67) | 7 (58) | 9 (82) |
One subject who responded to 1/3 CS antigens after the first dose was lost to follow-up before the second dose.
DISCUSSION
This report describes the first evaluation in humans of a trivalent, live attenuated ETEC vaccine. ACE527 was developed with the express purpose of overcoming the dual hurdles of inducing immune responses with sufficient magnitude and breadth while maintaining an acceptable reactogenicity profile (45). In this initial phase I study, ACE527 was safe and well tolerated. Ingestion of ACE527 was not associated with any immediate postvaccination reactions, and serious adverse events were not encountered. There were no unexpected adverse events, on the basis of prior experience with similarly attenuated ETEC strains (10, 19, 43). Nonserious adverse events occurred with similar frequencies across study groups, except for gastrointestinal symptoms. There was an observed increase in the frequency of gastrointestinal adverse events in the ACE527 high-dose group, but nearly all of these events were mild in severity and all were self-limited. Flatulence was the most common gastrointestinal symptom in this group, reported by nearly two-thirds of high-dose ACE527 recipients. This study therefore extends previous observations with precursor and similarly attenuated ETEC strains and confirms their safety and tolerability up to doses of 1011 CFU (10, 43). The most significant AEs which were seen (loose stools and nausea) were of a similar frequency and intensity seen in subjects administered equivalent doses of whole-cell killed ETEC vaccines (8, 35).
ACE527 was immunogenic following two immunizations given 3 weeks apart, inducing strong immune responses to both toxin and colonization factor antigens at doses of 1010 CFU and 1011 CFU and with dose-dependent increases in the magnitude of anti-LTB and the frequency of anti-CF responses being detected. The predominant systemic immune response was against LTB. A clear dose-response was seen in serum anti-LTB responses, with 10/12 recipients of the higher dose developing at least 2.5-fold rises in IgG. Of these responders, 7 seroconverted after the first dose of vaccine and an additional 3 subjects did so after the second dose. There was no significant further rise in geometric mean titer in the subjects who seroconverted after the first dose after they received the second dose. Mucosal anti-LTB IgA ALS responses were robust, reflecting the strategic design of the three vaccine strains. LTB expression in the three constructs is a mixture of cytoplasmic and periplasmic; there are no specific signal sequences provided to attempt to secrete the product or attach it to the external surface of the cell. Instead, the high level of constitutive expression seen in strains ACAM2022 and ACAM2027 (in which expression is driven by the Ptac promoter) and the inducible expression in ACAM2025 (where the native LT promoter is used) are relied upon to deliver a dose of antigen sufficient to be immunogenic (41, 42). This is clearly a successful approach, as 100% of subjects mounted a positive mucosal response to LTB at both dose levels of ACE527, in contrast to the low antibody secreting cells (ASC) response to CT seen following vaccination with Peru15, the live cholera vaccine expressing CTB (29). This observation suggests that these ETEC vaccine strains may have the potential to also serve as vectors for the expression of additional antigens from other enteric pathogens. The second dose of vaccine increased both the magnitude and breadth of the immune responses generated, supporting the value of a two-dose regimen in travelers; the potential for further increasing the immunogenicity with a third dose should be explored in future studies in children in countries where ETEC is endemic and where an ETEC vaccine is likely to be given according to the routine Expanded Program on Immunization (EPI) schedule.
The dose-response observed here is consistent with the higher responses to a 1010-CFU dose than to a 109-CFU dose reported previously with similar strains (10). There was no obvious relationship between the frequency of immune responses and the number of days on which the vaccine was shed or the magnitude of that shedding in subjects who received the same dose level of ACE527. A similar lack of correlation was seen in phase I studies with a live attenuated cholera vaccine (34).
The ability of this vaccine to induce strong anti-LTB and anti-colonization factor mucosal immune responses (Table 6) is particularly encouraging since animal model, human challenge, and field trial data all indicated that both responses can play an important role in protection against ETEC (1, 13, 30). The induction of significant anti-LTB, anti-CFA/I, anti-CS3, and anti-CS6 IgA ALS mucosal immune responses in most vaccinees suggests that the vaccine may be capable of inducing local intestinal antibody responses against most of the ETEC strains that may be encountered by travelers or infants and young children living in areas where ETEC is endemic. Although serum antibody responses, particularly to colonization factor antigens, tended to be less frequent than mucosal responses to the same vaccine components (Table 6), it was also encouraging that ACE527 induced anti-LTB, anti-CFA/I, and anti-CS6 serum responses that are in the same range as those observed among subjects experimentally infected with wild-type ETEC strains in human challenge studies. Seroconversion of IgA and IgG CFA/I-specific antibodies occurred in 43% and 23% of subjects, respectively, fed H10407 in a study performed by some of the present authors in a similar population and using the identical assay methods (14); the rates were 27% and 9%, respectively, at the high dose in the present study. In an independent study, the rates of IgA and IgG seroconversion to CS6 in subjects fed the virulent strain B7A were 31% and 25%, respectively (9), compared to 33% and 9%, respectively, at the high dose in the present study. This study is the first time that an oral cellular ETEC vaccine has been shown to induce both mucosal and serum anti-CS6 responses. Seroconversion to CS3 was not detected in subjects, even though 55% and 50% of vaccinees in the high- and low-vaccine-dose groups, respectively, mounted ALS responses to this antigen. However, baseline serum antibody titers to this antigen tended to be higher than those to CFA/I and CS6, making it more difficult to demonstrate a significant raise following vaccination (data not shown), as previously reported in a similar study population (21).
All three vaccine strains were shed in comparable amounts and with comparable durations. The quantitative level of excretion was evaluated 3 days after the first dose and 2 days after the second, with higher counts observed in samples from the latter evaluation, suggesting that excretion is of a short duration, with a peak occurring in the first 2 days after vaccination. The shedding levels are approximately 10-fold higher in the second cohort, in proportion to the higher dose that they received. The peak level observed, 2.2 × 106 CFU of ACE527 per gram of stool, corresponds to 1/50,000 of the administered dose of vaccine per gram of fecal material, reducing the risk of transmission of an effective dose of vaccine to an unintended recipient to essentially zero. This level of shedding of the attenuated vaccine is about 1% of that observed for virulent ETEC strains, such as H10407 (13, 14) and E24377A (20, 21). At the higher dose level, the median time to negative stool cultures was less than 7 days after either dose, and the longest time after a vaccine dose where a positive culture was obtained was 21 days. These results are broadly in line with those seen with other live bacterial vaccines, e.g., the cholera vaccine Peru15, where 53% and 13% of vaccinees were excreting vaccine at 7 and 10 days after dosing, respectively (34), and a newer cholera vaccine candidate, vaccine 638, shown to be shed at a peak of 1.8 × 104 CFU/g, or 1/110,000 of an effective dose per gram (44). All three strains in ACE527 demonstrate a sufficient duration of colonization for effective antigen expression and induction of immune responses but adequate attenuation to preclude persistent shedding. This correlates with the observation that immune responses to the key antigens expressed on all three strains (CFA/I, CS3, CS6) were seen in the majority of subjects.
Live attenuated vaccines against a number of enteric bacterial diseases have been successfully developed (Vivotif for typhoid fever and Orochol for cholera), with other candidates being in various stages of clinical development (17, 18, 28, 44). Previously, attenuated ETEC strains expressing different colonization factors have been produced and tested in phase I trials at maximum doses of 2 × 1010 CFU (10, 40). However, each of these previous studies used single strains of attenuated ETEC, none of which expressed an antigen capable of inducing antitoxin immunity. Since LT is expressed by the majority of ETEC strains and has been shown to be both immunogenic and protective as a stand-alone vaccine antigen, an effective vaccine will be required to induce immune responses to LT (or the nontoxic antigenic B subunit). However, the frequency of strains which express ST either alone or alongside LT makes it unlikely that this will be sufficient to confer broad protection. To protect against these strains, it will also be necessary to induce effective responses to the most prevalent colonization factor antigens, particularly CFA/I, CFA/II, and CFA/IV.
ACE527 shows promise as a well-tolerated live attenuated vaccine that could provide protection against a broad spectrum of ETEC strains. Thus, this vaccine has potential as a public health intervention for both travelers and children at risk for ETEC diarrhea and warrants further development and clinical evaluation. The strong systemic and mucosal antitoxin responses coupled with mucosal responses against principal colonization factor antigens and the positive safety findings in this study support further clinical testing of ACE527 at the 1011-CFU dose in a phase II proof-of-concept vaccination and challenge trial.
ACKNOWLEDGMENTS
The contributions of members of the clinical and research laboratory teams of the Johns Hopkins University Bloomberg School of Public Health and Hye Kim for research pharmacy assistance are gratefully acknowledged.
Andrea Feller was supported by U.S. Department of Health and Human Services, National Institutes of Health, National Eye Institute, training grant number EY07127, Clinical Trials Training Program in Vision Research.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Ahren C. M., Svennerholm A. M. 1982. Synergistic protective effect of antibodies against Escherichia coli enterotoxin and colonization factor antigens. Infect. Immun. 38:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous. 1999, posting date The evolution of diarrheal and acute respiratory disease control at WHO. WHO, Geneva, Switzerland [Google Scholar]
- 3. Anonymous. 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly. Epidemiol. Rec. 81:97–104 [PubMed] [Google Scholar]
- 4. Black R. E., et al. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987 [DOI] [PubMed] [Google Scholar]
- 5. Boschi-Pinto C., Velebit L., Shibuya K. 2008. Estimating child mortality due to diarrhea in developing countries. Bull. World Health Organ. 86:710–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carpenter C. M., et al. 2006. Comparison of the antibody in lymphocyte supernatant (ALS) and ELISPOT assays for detection of mucosal immune responses to antigens of enterotoxigenic Escherichia coli in challenged and vaccinated volunteers. Vaccine 24:3709–3718 [DOI] [PubMed] [Google Scholar]
- 7. Clemens J. D., et al. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372–377 [DOI] [PubMed] [Google Scholar]
- 8. Cohen D., et al. 2000. Safety and immunogenicity of two different lots of the oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Israeli young adults. Infect. Immun. 68:4492–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coster T. S., et al. 2007. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect. Immun. 75:252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daley A., et al. 2007. Genetically modified enterotoxigenic Escherichia coli vaccines induce mucosal immune responses without inflammation. Gut 56:1550–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dillingham R., Guerrant R. L. 2004. Childhood stunting: measuring and stemming the staggering costs of inadequate water and sanitation. Lancet 363:94–95 [DOI] [PubMed] [Google Scholar]
- 12. Frech S. A., et al. 2008. Use of a patch containing heat-labile toxin from Escherichia coli against travellers' diarrhea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet 371:2019–2025 [DOI] [PubMed] [Google Scholar]
- 13. Freedman D. J., et al. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 177:662–667 [DOI] [PubMed] [Google Scholar]
- 14. Harro C., et al. 2011. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin. Vaccine Immunol. 18:1719–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Intercell 2010. Intercell provides update on clinical trials for the patch-based travelers' diarrhea vaccine. Intercell, Vienna, Austria: http://www.intercell.com/main/forbeginners/news/news-full/article/intercell-provides-update-on-clinical-trials-for-the-patch-based-travelers-diarrhea-vaccine/ [Google Scholar]
- 16. Levine M. 1990. Vaccines against enterotoxigenic Escherichia coli infections, p. 649–660 In Levine M. M., Woodrow C. G. (ed.), New generation vaccines. Marcel Dekker, Inc., New York, NY [Google Scholar]
- 17. Lyon C. E., et al. 2010. In a randomized, double-blinded, placebo-controlled trial, the single oral dose typhoid vaccine, M01ZH09, is safe and immunogenic at doses up to 1.7 × 10(10) colony-forming units. Vaccine 28:3602–3608 [DOI] [PubMed] [Google Scholar]
- 18. Mahalanabis D., et al. 2009. Randomized placebo controlled human volunteer trial of a live oral cholera vaccine VA1.3 for safety and immune response. Vaccine 27:4850–4856 [DOI] [PubMed] [Google Scholar]
- 19. McKenzie R., et al. 2006. Comparative safety and immunogenicity of two attenuated enterotoxigenic Escherichia coli vaccine strains in healthy adults. Infect. Immun. 74:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKenzie R., et al. 2007. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 25:3684–3691 [DOI] [PubMed] [Google Scholar]
- 21. McKenzie R., et al. 2008. A double-blind, placebo-controlled trial to evaluate the efficacy of PTL-003, an attenuated enterotoxigenic E. coli (ETEC) vaccine strain, in protecting against challenge with virulent ETEC. Vaccine 26:4731–4739 [DOI] [PubMed] [Google Scholar]
- 22. McVeigh A., et al. 2000. IS1414, an Escherichia coli insertion sequence with a heat-stable enterotoxin gene embedded in a transposase-like gene. Infect. Immun. 68:5710–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore S. R., et al. 2010. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology 139:1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nataro J. P., Kaper J. B. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peltola H., et al. 1991. Prevention of travellers' diarrhea by oral B-subunit/whole-cell cholera vaccine. Lancet 338:1285–1289 [DOI] [PubMed] [Google Scholar]
- 26. Peruski L. F., Jr., et al. 1999. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J. Clin. Microbiol. 37:2974–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petri W. A., Jr., et al. 2008. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Invest. 118:1277–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qadri F., et al. 2007. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 25:231–238 [DOI] [PubMed] [Google Scholar]
- 29. Qadri F., et al. 2005. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J. Infect. Dis. 192:573–579 [DOI] [PubMed] [Google Scholar]
- 30. Qadri F., et al. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun. 75:3961–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qadri F., Svennerholm A. M., Faruque A. S., Sack R. B. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao M. R., et al. 2005. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J. Infect. Dis. 191:562–570 [DOI] [PubMed] [Google Scholar]
- 33. Rappuoli R., Pizza M., Douce G., Dougan G. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493–500 [DOI] [PubMed] [Google Scholar]
- 34. Sack D. A., et al. 1997. Evaluation of Peru-15, a new live oral vaccine for cholera, in volunteers. J. Infect. Dis. 176:201–205 [DOI] [PubMed] [Google Scholar]
- 35. Sack D. A., et al. 2007. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhea of travellers to Guatemala and Mexico. Vaccine 25:4392–4400 [DOI] [PubMed] [Google Scholar]
- 36. Savarino S. J., et al. 1996. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative E. coli. J. Infect. Dis. 173:1019–1022 [DOI] [PubMed] [Google Scholar]
- 37. Scerpella E. G., et al. 1995. Safety, immunogenicity, and protective efficacy of the whole-cell/recombinant B subunit (WC/rBS) oral cholera vaccine against travelers' diarrhea. J. Travel Med. 2:22–27 [DOI] [PubMed] [Google Scholar]
- 38. Steffen R., Castelli F., Dieter Nothdurft H., Rombo L., Jane Zuckerman N. 2005. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. J. Travel Med. 12:102–107 [DOI] [PubMed] [Google Scholar]
- 39. Svennerholm A. M., Tobias J. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev. Vaccines 7:795–804 [DOI] [PubMed] [Google Scholar]
- 40. Turner A. K., et al. 2006. Construction and phase I clinical evaluation of the safety and immunogenicity of a candidate enterotoxigenic Escherichia coli vaccine strain expressing colonization factor antigen CFA/I. Infect. Immun. 74:1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner A. K., Greenwood J., Stephens J. C., Beavis J. C., Darsley M. J. September 2010. Attenuated bacteria useful in vaccines. Patent EP 1,425,038
- 42. Turner A. K., et al. 2011. Generation and characterization of a live attenuated enterotoxigenic Escherichia coli combination vaccine expressing six colonization factors and heat-labile toxin subunit B. Clin. Vaccine Immunol. 18:2128–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turner A. K., Terry T. D., Sack D. A., Londono-Arcila P., Darsley M. J. 2001. Construction and characterization of genetically defined aro omp mutants of enterotoxigenic Escherichia coli and preliminary studies of safety and immunogenicity in humans. Infect. Immun. 69:4969–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valera R., et al. 2009. Randomized, double-blind, placebo-controlled trial to evaluate the safety and immunogenicity of live oral cholera vaccine 638 in Cuban adults. Vaccine 27:6564–6569 [DOI] [PubMed] [Google Scholar]
- 45. Walker R. I., Steele D., Aguado T. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545–2566 [DOI] [PubMed] [Google Scholar]
- 46. Wang M., Szucs T. D., Steffen R. 2008. Economic aspects of travelers' diarrhea. J. Travel Med. 15:110–118 [DOI] [PubMed] [Google Scholar]
- 47. WHO 2009, posting date Diarrheal diseases; enterotoxigenic Escherichia coli (ETEC). WHO, Geneva, Switzerland [Google Scholar]
- 48. Wiedermann G., Kollaritsch H., Kundi M., Svennerholm A. M., Bjare U. 2000. Double-blind, randomized, placebo controlled pilot study evaluating efficacy and reactogenicity of an oral ETEC B-subunit-inactivated whole cell vaccine against travelers' diarrhea (preliminary report). J. Travel Med. 7:27–29 [DOI] [PubMed] [Google Scholar]
- 49. Wolf M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569–584 [DOI] [PMC free article] [PubMed] [Google Scholar]