Abstract
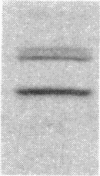
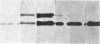
We immunized rabbits with purified guanine nucleotide-binding proteins (G proteins) from bovine brain and obtained an antiserum, RV3, that reacts specifically with the alpha subunit (39 kDa) of a G protein of unknown function, termed Go, as well as with the beta subunit (35 kDa) common to all G proteins. RV3 showed no crossreactivity with the alpha subunits of the stimulatory (Gs) or inhibitory (Gi) G proteins associated with adenylate cyclase, nor with that of the rod outer segment G protein, transducin. Immunoblots with crude and affinity-purified antiserum showed that RV3 specifically recognizes the Go alpha subunit and the beta subunit in crude brain membranes. Using RV3, we found approximately equal amounts of Go in brain membranes from frog, chicken, rat, cow, and man. Quantitative immunoblotting gave Go alpha subunit/ beta subunit ratios approximately equal to 1 in cerebral cortex, raising the possibility that free Go alpha subunit (unassociated with beta subunit) may exist in brain. The concentration of Go alpha subunit in cortex is about 5 times that of Gi alpha subunit. The results show that Go is an immunochemically distinct, highly conserved protein distributed throughout the brain, with particularly high concentrations in forebrain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Codina J., Hildebrandt J. D., Sekura R. D., Birnbaumer M., Bryan J., Manclark C. R., Iyengar R., Birnbaumer L. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylyl cyclases. Purification of the human erythrocyte proteins without the use of activating regulatory ligands. J Biol Chem. 1984 May 10;259(9):5871–5886. [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Seamon K. B. Activation of cyclic AMP-generating systems in brain membranes and slices by the diterpene forskolin: augmentation of receptor-mediated responses. J Neurochem. 1982 Feb;38(2):532–544. doi: 10.1111/j.1471-4159.1982.tb08660.x. [DOI] [PubMed] [Google Scholar]
- Florio V. A., Sternweis P. C. Reconstitution of resolved muscarinic cholinergic receptors with purified GTP-binding proteins. J Biol Chem. 1985 Mar 25;260(6):3477–3483. [PubMed] [Google Scholar]
- Flynn D. D., Potter L. T. Different effects of N-ethylmaleimide on M1 and M2 muscarine receptors in rat brain. Proc Natl Acad Sci U S A. 1985 Jan;82(2):580–583. doi: 10.1073/pnas.82.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierschik P., Codina J., Simons C., Birnbaumer L., Spiegel A. Antisera against a guanine nucleotide binding protein from retina cross-react with the beta subunit of the adenylyl cyclase-associated guanine nucleotide binding proteins, Ns and Ni. Proc Natl Acad Sci U S A. 1985 Feb;82(3):727–731. doi: 10.1073/pnas.82.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Huff R. M., Axton J. M., Neer E. J. Physical and immunological characterization of a guanine nucleotide-binding protein purified from bovine cerebral cortex. J Biol Chem. 1985 Sep 5;260(19):10864–10871. [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Garciá-Sáinz J. A. Pertussis toxin catalyzes the ADP-ribosylation of two distinct peptides, 40 and 41 kDa, in rat fat cell membranes. FEBS Lett. 1984 Oct 29;176(2):301–306. doi: 10.1016/0014-5793(84)81184-9. [DOI] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Pines M., Gierschik P., Milligan G., Klee W., Spiegel A. Antibodies against the carboxyl-terminal 5-kDa peptide of the alpha subunit of transducin crossreact with the 40-kDa but not the 39-kDa guanine nucleotide binding protein from brain. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4095–4099. doi: 10.1073/pnas.82.12.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M., Gierschik P., Spiegel A. The tryptic and chymotryptic fragments of the beta-subunit of guanine nucleotide binding proteins in brain are identical to those of retinal transducin. FEBS Lett. 1985 Mar 25;182(2):355–359. doi: 10.1016/0014-5793(85)80332-x. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Gierschik P., Levine M. A., Downs R. W., Jr Clinical implications of guanine nucleotide-binding proteins as receptor-effector couplers. N Engl J Med. 1985 Jan 3;312(1):26–33. doi: 10.1056/NEJM198501033120106. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]