Abstract
Old tuberculin (OT) and purified protein derivative (PPD) are widely used for tuberculin skin testing (TST) in diagnosis of tuberculosis (TB) but often yield poor specificity and anergy in reaction. Therefore, it is necessary to develop new serological methods as a possible auxiliary diagnostic method for TB. In this study, we characterized the dynamic antibody responses of 10 purified recombinant antigens, PPD, and OT in rhesus monkeys experimentally infected with Mycobacterium tuberculosis and analyzed the time to antibody detection, antibody levels, and their association with the infectious doses. The antibodies were detected as early as 4 weeks after infection in response to 5 antigens (CFP10, CFP10-ESAT-6, U1, MPT64, and Ag85b). Antibodies against most of the other antigens were detected between 4 and 12 weeks after infection. The levels of antibodies were dose dependant. We further evaluated the serodiagnostic potential of these antigens by using indirect enzyme-linked immunosorbent assay in 71 TST-positive and 90 TST-negative serum samples from monkeys. For all 12 antigens, the median optical density values of TST-positive monkeys were statistically significantly higher than those of TST-negative monkeys (P < 0.001). Among those antigens, Ag85b and CFP10 showed higher diagnostic potential than others. A combination of results from Ag85b, the 38-kDa antigen (Ag38kDa), and Ag14kDa reaches a sensitivity of 95.77%, indicating that these antigens may be ideal cocktails in TB diagnosis.
INTRODUCTION
Tuberculosis (TB) is a bacterial disease which causes serious health problems to both humans and nonhuman primates (NHPs). The zoonotic potential of TB and its potential transmission to laboratory NHPs are major concerns for researchers. Outbreaks of TB in laboratory monkey colonies are economically costly due to animal losses as well as increased expenses by disrupted research, lost time, and even delayed release of new products into the market. Though many strict control guidelines have been implemented, the absence of accurate diagnostic methods prevents effective TB control. Current TB diagnosis of NHPs largely depends on old tuberculin (OT) tuberculin skin testing (TST) and purified protein derivative (PPD) TST (OT-TST and PPD-TST, respectively), which have several serious limitations (7, 18, 23), including poor specificity, anergy in reaction, and intermittent positive results on repeated testing. A new method, which is based on detection of gamma interferon in whole blood (6, 22) has been developed for diagnosing TB in living NHPs. However, its sensitivity and application are still under evaluation.
With the development of the cloning and expressing of M. tuberculosis-specific antigens, a serological test becomes an attractive diagnostic method for its convenience, robustness, and easy implementation, as well as for the absence of a requirement for living peripheral blood mononuclear cells (PBMCs). Several seroantigens, such as ESAT-6, CFP10, and the 38-kDa antigen (Ag38kDa), have been identified and included in many commercial immunochromatographic test kits for human and primate TB diagnosis (15, 24). Other antigens, including MTB48 (13, 26) and Mtb81 (10), are immunogenic and become potential targets for serology-based tests. However, sensitivity and specificity of serological tests have not been determined yet, largely because the great heterogeneity of the antibody response occurs in TB cases. In the present study, we characterize the antibody responses to 10 M. tuberculosis purified proteins, PPD, and OT in rhesus monkeys (Macaca mulatta) naturally and experimentally infected with Mycobacterium and identify these 12 antigens as serological targets. The characterizations of antibodies against multiple M. tuberculosis antigens are valuable for the rapid, early, and accurate diagnosis of primate TB.
MATERIALS AND METHODS
Antigens.
Antigens used in this study are listed in Table 1. PPD was purchased from the Harbin Pharmaceutical Group Bio-vaccine Co., Ltd., and OT was from Synbiotics Corp. Ten recombinant M. tuberculosis proteins were purified to near homogeneity from Escherichia coli as described previously (1, 5, 11, 16, 21, 26, 27).
Table 1.
Specific antigens of M. tuberculosis used in this study
| Antigen | Rv no. of gene | Molecular mass (kDa) | Expression vector | Coating concn | Serum dilution | Reference for sequence |
|---|---|---|---|---|---|---|
| PPD | 400 IU/ml | 1:50 | ||||
| OT | 400 IU/ml | 1:50 | ||||
| Ag85b | Rv1886c | 30 | pET-24b | 0.5 μg/ml | 1:20 | 11 |
| MPT64L | Rv1980c | 23 | pET-24b | 0.5 μg/ml | 1:20 | 27 |
| U1 | Rv1932 | 16.8 | pET-28a | 0.5 μg/ml | 1:20 | 16 |
| Ag16kDa | Rv1926c | 16 | pET-15b | 1 μg/ml | 1:20 | 27 |
| TB16.3 | Rv2185c | 16.3 | pET-24b | 0.5 μg/ml | 1:20 | 1 |
| Ag38kDa | Rv0934 | 35.9 | pET-24b | 1 μg/ml | 1:20 | 5 |
| Ag14kDa | Rv2031c | 14 | pET-22b | 1 μg/ml | 1:20 | 21 |
| CFP10 | Rv3874 | 11 | pET-24b | 0.5 μg/ml | 1:20 | 27 |
| ESAT-6 | Rv3875 | 6 | pET-24b | 1 μg/ml | 1:20 | 27 |
| CFP10-ESAT-6 | Rv3874/Rv3875 | 66 | pET-28a | 0.5 μg/ml | 1:20 | 26 |
Experimental infection and sample collection.
Four rhesus monkeys aged 3 to 4 years were obtained from Gaoyao Kangyuan Laboratory Animal Science & Technology Co., Ltd. [license no. SCXK (Yue)2009-0009] and routinely tested negative for monkey B virus, simian immunodeficiency virus (SIV), and simian T-cell leukemia virus 1 (STLV-1) by enzyme-linked immunosorbent assay (ELISA) and simian retrovirus (SRV) by immunofluorescence. The monkeys were quarantined for 1 month after arrival at the facility and were evaluated extensively for the absence of tuberculosis by biweekly repeated TST during the quarantine period. Two monkeys (06-1519R and 06-1523R) were infected intratracheally with 500 CFU of M. tuberculosis H37Rv, and the other two monkeys (06-1411R and 06-1445R) were infected intratracheally with 50 CFU of M. tuberculosis H37Rv too. Daily clinical assessment, TST, gross and microscopic examination at necropsy, and bacteriologic culture were performed to ensure the infection status.
At the interval of 2 to 4 weeks, all monkeys were anesthetized intramuscularly with ketamine in combination with Sumianxin II (846 composition group) for blood collection. Ten milliliters of blood was collected from the femoral vein. Sera were separated by centrifugation and stored at −80°C.
Animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Guangdong Laboratory Animal Monitor Institute in accordance with the Guide for the Care and Use of Laboratory Animals (17). Animal work was conducted using biosafety level 3 operating procedures and policies in an ABSL-3 facility with approval of and oversight by the Institutional Environmental Health and Safety Office.
Naturally TST-positive and -negative sample collection.
In the past 5 years, 71 rhesus monkeys were detected with at least one instance of TST-positive reaction by routine quarantines, and 62 of these monkeys were further confirmed TB positive by necropsies, while the other 9 monkeys were not euthanatized to confirm TB infection by necropsies. The whole blood was collected from 62 monkeys via the carotid artery after anesthesia, and 5 ml of blood was collected from the other 9 monkeys via the femoral vein. Ninety blood samples were collected from rhesus monkeys negative for TST in routine quarantines in recent 3 years. All sera were separated by centrifugation and stored at −80°C.
Antibody detection by ELISA.
The antigen coating concentrations and serum dilutions are listed in Table 1. The ELISA procedure was performed as follows: 96-well polystyrene microtitration plates were coated overnight at 4°C with 100 μl of antigen solution in 0.1 M carbonate-bicarbonate buffer, pH 9.6. After washing, the plates were blocked with 150 μl 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) at 37°C for 1 h. After washing again, the wells were filled with 100-μl individual serum samples diluted in PBS and incubated at 37°C for 1 h. After the plates were washed, the wells were incubated for 1 h with 100 μl of goat anti-monkey IgG antibody conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) diluted 1:15,000. Enzyme activity was assayed by TMB (3,3′,5,5′-tetramethylbenzidine) peroxidase kit (Dream Biotechnology Co., Ltd., Guangdong, China). To terminate the reaction, 50 μl of 2 M sulfuric acid was added, and then the optical density (OD) was measured at 450 nm in a microplate reader (Bio-Rad Co., Japan). The positive and negative controls were mixtures of 10 naturally TST-positive sera and 10 naturally TST-negative sera, respectively.
Data analysis.
For experimentally infected monkeys, the ELISA results were used to analyze the time course of antibody responses, the time to antibody detection, and the relations between infection doses and antibody responses.
Data are presented as the mean and standard deviation (SD). Student's t test was used to compare the differences in the OD values between naturally TST-positive and -negative monkeys. The receiver operating characteristic (ROC) curves of the OD values were plotted using SAS8.01 software, and the area under the curve (AUC) and 95% confidence intervals (95% CIs) were calculated. The Z test was employed to analyze the AUCs between two antigens. In addition, the optimal cutoff values were chosen when the positive likelihood ratio [sensitivity/(1 − specificity)] was maximum. According to cutoff values, all monkeys were divided into true positive and true negative groups. Differences of sensitivities and specificity between two antigens were analyzed by the χ2 test. P values of less than 0.05 were considered statistically significant.
RESULTS
Experimental infection status.
No obvious clinical symptoms were observed except occasional cough for all monkeys, and all animals gained weight during the infection period. At week 4 postinfection, results of TST were positive for 3 monkeys and suspected for monkey 06-1411R (50 CFU). All monkeys tested positive at week 6 postinfection. At necropsy, all animals showed visibly bilaterally enlarged hilar lymph nodes. Three animals (all except 06-1519R [500 CFU]) were characterized by the presence of multiple large and small granulomas in both lungs, and granulomas showed conglomeration to larger caseous areas in the left-middle lung lobe of animal 06-1523R (500 CFU). Granulomas were also present in the spleen of the animal 06-1523R (500 CFU). M. tuberculosis was readily cultured from lung and hilar lymph nodes but was cultured in lesser numbers or not at all from the spleen or other extrapulmonary organs. Histopathologically, a mixture of subacute and chronic granulomas with central necrosis was present in the lungs of 3 animals (all except 06-1519R [500 CFU]), the hilar lymph nodes of all the animals, and in the spleen of animal 06-1523R (500 CFU), while only granulomas without central necrosis were present in lung of animal 06-1519R (500 CFU). In the spleen of animal 06-1445R (50 CFU), the periarteriolar lymphocyte sheaths (PALS) were clear with diffuse lymphatic tissue, but no germinal centers were exhibited within spleen. Evaluations of pathological changes showed no significant correlation with infectious dose.
Time course of antibody response in experimentally infected monkeys.
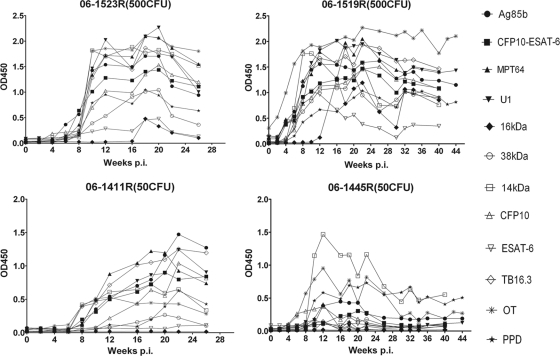
The time course of antibody production in response to the antigens during experimental infection was characterized by indirect ELISA (Fig. 1). Among the four monkeys, antibodies were produced earliest in monkey 06-1519R (500 CFU), who developed brachychronic and severe disease, and latest in monkey 06-1445R, who showed a low immunity, which was demonstrated by the presence of only a few germinal centers within spleen and outside lymph nodes.
Fig. 1.
Dynamic antibody responses of selected antigens in experimentally infected monkeys. The levels of antibodies were dose dependent, and the high-dose infections showed earlier serum reactivity than the low-dose infections. The antibody response in monkey 06-1445R (50 CFU) was different from that of the others, with only a brief peak.
In animals infected with 500 CFU of M. tuberculosis (06-1519R and 06-1523R), antibody production in response to all antigens except the 16-kDa protein was characterized by a sharp rise during the first 4 to 10 weeks of infection and a steady plateau for at least 10 to 14 weeks followed by a slow decline. In contrast, the antibody production in response to the 16-kDa protein started at 12 to 18 weeks after infection and declined rapidly after the maximum level.
In monkeys infected with 50 CFU of M. tuberculosis, antibody response was different from those infected with 500 CFU of M. tuberculosis. For monkey 06-1411R, the rise in antibody levels was gentle, and the highest levels were significantly lower than those of animals infected with 500 CFU of M. tuberculosis. And there were no antibody responses to 2 antigens (ESAT-6 and Ag16kDa). While the antibody responses in monkey 06-1445R had distinctive characteristics, antibodies to most antigens in this animal declined to preinfection levels after a brief peak of antibody responses and stayed at a very low level during the course of infection. In addition, the monkey presented negative antibody responses to 3 antigens (ESAT-6, Ag16kDa, and Ag38kDa).
Time to antibody detection.
The time to antibody detection was determined as the earliest time point at which serum showed a positive ELISA result. Antibodies to most antigens tested positive between week 4 and week 12 of infection (Table 2). Among all antigens, CFP10 showed great value in early diagnosis, followed by CFP10-ESAT-6, U1, MPT64, and Ag85b. ESAT-6 alone showed early serum reactivity in animals infected with 500 CFU of M. tuberculosis but presented seronegative in animals infected with 50 CFU of M. tuberculosis.
Table 2.
Time to antibody detectiona
| Antigen | Seropositive wk |
|||
|---|---|---|---|---|
| 06-1523R (500 CFU) | 06-1519R (500 CFU) | 06-1445R (50 CFU) | 06-1411R (50 CFU) | |
| PPD | 8 | 6 | 8 | 12 |
| OT | 8 | 0b | 8 | 8 |
| Ag85b | 6 | 4 | 12 | 10 |
| MPT64L | 8 | 4 | 10 | 8 |
| U1 | 6 | 4 | 8 | 8 |
| Ag16kDa | 18 | 14 | NEc | NEc |
| TB16.3 | 8 | 0b | 10 | 8 |
| Ag38kDa | 10 | 6 | NEc | 20 |
| Ag14kDa | 10 | 6 | 10 | 8 |
| CFP10 | 4 | 4 | 4 | 8 |
| ESAT-6 | 8 | 6 | NEc | NEc |
| CFP10-ESAT-6 | 6 | 4 | 10 | 8 |
Antibodies against most antigens tested positive between week 4 and week 12 of infection, and CFP10 showed great value in early diagnosis, followed by CFP10-ESAT-6, U1, MPT64, and Ag85b.
Seropositive to this antigen preinfection.
NE, negative.
Antibody responses in TST-positive and -negative monkeys.
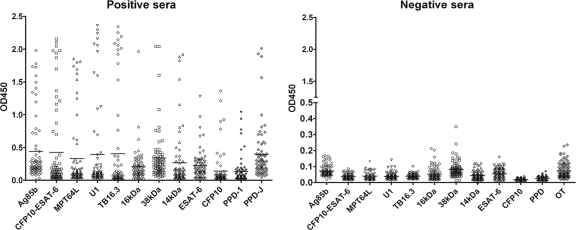
Serum antibodies against 10 recombined antigens, OT, and PPD were measured by indirect ELISA in TST-positive and -negative monkeys (Fig. 2), and the OD values are listed in Table 3. The levels of antibodies against these antigens in TST-positive monkeys were significantly higher than those in TST-negative ones (P < 0.001, Student's t test).
Fig. 2.
Distribution of OD values of 71 TST-positive and 90 TST-negative serum samples. Sera from 71 TST-positive and 90 TST-negative individuals were evaluated for antibody reactivity with 12 antigens using indirect ELISA. The levels of antibodies against these antigens in TST-positive monkeys were significantly higher than those in TST-negative ones (P < 0.001, Student's t test).
Table 3.
Serum antibody responses to specific antigens of M. tuberculosis
| Group | Serum antibody response (mean OD450 ± SD) to indicated specific antigen |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPD | OT | Ag85b | MPT64L | U1 | Ag16kDa | TB16.3 | Ag38kDa | Ag14kDa | CFP10 | ESAT-6 | CFP10-ESAT-6 | |
| TST positive | 0.129 ± 0.193a | 0.396 ± 0.415a | 0.440 ± 0.496a | 0.331 ± 0.527a | 0.394 ± 0.671a | 0.209 ± 0.253a | 0.408 ± 0.722a | 0.340 ± 0.382a | 0.267 ± 0.433a | 0.149 ± 0.268a | 0.225 ± 0.222a | 0.395 ± 0.671a |
| TST negative | 0.025 ± 0.010 | 0.073 ± 0.048 | 0.073 ± 0.032 | 0.039 ± 0.020 | 0.038 ± 0.012 | 0.053 ± 0.038 | 0.039 ± 0.012 | 0.081 ± 0.041 | 0.044 ± 0.027 | 0.023 ± 0.006 | 0.055 ± 0.031 | 0.037 ± 0.015 |
Significantly high levels compared to TST-negative individuals are indicated (P < 0.001; Student's t test).
Furthermore, all OD values of antibodies were used to generate ROC curves, and AUCs of ROC curves were all above 0.700 (Table 4). All antigens were ranked from maximum AUC to minimum as follows: CFP10, Ag85b, OT, MPT64L, CFP10-ESAT-6, Ag38kDa, PPD-1, U1, Ag16kDa, ESAT-6, Ag14kDa, and TB16.3. Sensitivities and specificities of these 12 antigens are listed in Table 4. Among these 12 antigens, Ag85b and CFP10 showed the highest sensitivity (above 70%), and CFP10-ESAT-6 and ESAT-6 ranked next (66.20% and 67.61%, respectively). The 16-kDa protein showed the lowest sensitivity of 33.80%. The sensitivities of other antigens ranged from 40% to 60%.
Table 4.
Parameters obtained from ROC analysis of antibody responses to M. tuberculosis-specific antigens
| Antigen | AUCa | SEMb | 95% CIc | Positive likelihood ratio | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| PPD | 0.897 | 0.025 | 0.849–0.945 | 53.24 | 0.052 | 59.15 | 98.89 |
| OT | 0.935 | 0.018 | 0.901–0.970 | 51.97 | 0.232 | 57.75 | 97.78 |
| Ag85b | 0.947 | 0.021 | 0.905–0.986 | 67.18 | 0.166 | 74.65 | 98.89 |
| MPT64L | 0.912 | 0.020 | 0.877–0.957 | 53.24 | 0.087 | 59.15 | 98.89 |
| U1/TPX | 0.878 | 0.029 | 0.820–0.935 | 39.30 | 0.102 | 43.66 | 98.89 |
| Ag16kDa | 0.877 | 0.028 | 0.822–0.932 | 31.69 | 0.208 | 33.80 | 98.89 |
| TB16.3 | 0.719 | 0.044 | 0.632–0.805 | 40.56 | 0.070 | 45.07 | 98.89 |
| Ag38kDa | 0.907 | 0.025 | 0.860–0.957 | 44.37 | 0.242 | 49.30 | 98.89 |
| Ag14kDa | 0.836 | 0.031 | 0.774–0.899 | 39.30 | 0.122 | 43.66 | 98.89 |
| CFP10 | 0.973 | 0.010 | 0.954–0.992 | 64.65 | 0.043 | 71.83 | 98.89 |
| ESAT-6 | 0.846 | 0.035 | 0.781–0.916 | 60.85 | 0.118 | 67.61 | 98.89 |
| CFP10-ESAT-6 | 0.910 | 0.023 | 0.865–0.954 | 59.58 | 0.075 | 66.20 | 98.89 |
AUC, area under the curve.
SEM, standard error of the mean.
CI, confidence interval.
In Fig. 3, positive antibody responses to different antigens in the same animals are presented with black spaces. Only four animals were seropositive to all antigens, two animals were seronegative to all antigens, and two animals were seropositive for only one antigen. Most of the animals (46 out of 71) were seropositive for at least six antigens.
Fig. 3.
Distribution of true positives in antibodies of 71 TST-positive monkeys. Four animals (numbered 3, 10, 27, and 28) were seropositive to all antigens, two animals (numbered 36 and 69) were seronegative to all antigens, and two animals (numbered 12 and 38) were seropositive for only one antigen (Ag38kDa and Ag14kDa, respectively). Forty-six out of 71 animals were seropositive for at least six antigens.
Serodiagnostic potential of M. tuberculosis antigens.
Accuracy is commonly used to evaluate the serodiagnostic potential of the antigen in tuberculosis diagnosis. To compare the accuracies of different antigens, the AUCs were analyzed by the Z test (Table 5). CFP10 was significantly higher than all other antigens except Ag85b and OT (P < 0.05), and Ag85b was significantly higher than 4 antigens; OT and MPT64L were significantly higher than 2 antigens. TB16.3 showed the lowest accuracy. The remaining antigens showed no significant differences between each other.
Table 5.
Results of Z test for each of two antigens' AUCsa
| Antigen | PPD | OT | Ag85b | MPT64L | U1 | Ag16kDa | TB16.3 | Ag38kDa | Ag14kDa | CFP10 | ESAT-6 | CFP10-ESAT-6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPD | — | 0.2174 | 0.1257 | 0.6394 | 0.6197 | 0.5942 | 0.0004 | 0.7773 | 0.1256 | 0.0048 | 0.2357 | 0.7020 |
| OT | 1.234 | — | 0.6644 | 0.3927 | 0.0949 | 0.0814 | <0.0001 | 0.3634 | 0.0057 | 0.0650 | 0.0237 | 0.3920 |
| Ag85b | 1.531 | 0.434 | — | 0.2275 | 0.0540 | 0.0455 | <0.0001 | 0.2205 | 0.0030 | 0.2636 | 0.0133 | 0.2348 |
| MPT64L | 0.469 | 0.855 | 1.207 | — | 0.3345 | 0.3091 | 0.0001 | 0.8759 | 0.0394 | 0.0064 | 0.1016 | 0.9477 |
| U1 | 0.496 | 1.670 | 1.927 | 0.965 | — | 0.9802 | 0.0026 | 0.4488 | 0.3225 | 0.0020 | 0.4814 | 0.3873 |
| Ag16kDa | 0.533 | 1.742 | 2.000 | 1.017 | 0.0248 | — | 0.0024 | 0.4242 | 0.3264 | 0.0012 | 0.4892 | 0.3624 |
| TB16.3 | 3.517 | 4.544 | 4.676 | 3.993 | 3.017 | 3.030 | — | 0.0002 | 0.0297 | <0.0001 | 0.0239 | 0.0001 |
| Ag38kDa | 0.283 | 0.909 | 1.225 | 0.156 | 0.757 | 0.799 | 3.715 | — | 0.0746 | 0.0142 | 0.1561 | 0.9296 |
| Ag14kDa | 1.532 | 2.762 | 2.964 | 2.060 | 0.989 | 0.981 | 2.174 | 1.783 | — | <0.0001 | 0.8306 | 0.0552 |
| CFP10 | 2.823 | 1.845 | 1.118 | 2.728 | 3.097 | 3.229 | 5.629 | 2.451 | 4.206 | — | 0.0005 | 0.0120 |
| ESAT-6 | 1.186 | 2.261 | 2.474 | 1.637 | 0.704 | 0.692 | 2.259 | 1.418 | 0.214 | 3.489 | — | 0.1265 |
| CFP10-ESAT-6 | 0.383 | 0.856 | 1.188 | 0.0656 | 0.865 | 0.911 | 3.847 | 0.0883 | 1.917 | 2.512 | 1.528 | — |
The data below — are |Z| values and those above are P values. The AUCs were analyzed by the Z test. The AUC of CFP10 was significantly larger than those of all other antigens except Ag85b and OT, that of Ag85b was significantly larger than those of 4 antigens, and those of OT and MPT64L were significantly larger than those of 2 antigens, while TB16.3 showed the lowest AUC.
Differences for the sensitivities between each of two antigens were analyzed by the χ2 test in naturally TST-positive sera (Table 6). Ag85b showed higher sensitivities than 7 antigens (OT, MPT64L, U1, Ag16kDa, TB16.3, Ag38kDa, and Ag14kDa); CFP10, ESAT-6, CFP10-ESAT-6, and MPT64 showed higher sensitivities than 4 antigens (U1, Ag16kDa, TB16.3, and Ag14kDa); PPD showed higher sensitivities than 3 antigens (Ag16kDa, TB16.3, and Ag14kDa); OT and Ag38kDa showed higher sensitivities than the same single antigen (Ag16kDa); and U1 protein, Ag16kDa, TB16.3, and Ag14kDa showed the lowest sensitivities. Among ESAT-6, CFP10, and CFP10-ESAT-6, no significant difference was found, suggesting that the fusion protein did not have any higher sensitivity than the single proteins.
Table 6.
Results of χ2 test for each of two antigensa
| Antigen | PPD | OT | Ag85b | MPT64L | U1 | Ag16kDa | TB16.3 | Ag38kDa | Ag14kDa | CFP10 | ESAT-6 | CFP10-ESAT-6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPD | — | 0.8084 | 0.0555 | 1.0000 | 0.1060 | 0.0010 | 0.0499 | 0.4795 | 0.0343 | 0.0947 | 0.2393 | 0.3173 |
| OT | 0.0588 | — | 0.0233 | 0.8618 | 0.0679 | 0.0011 | 0.0947 | 0.6394 | 0.0863 | 0.0679 | 0.1936 | 0.2008 |
| Ag85b | 3.6667 | 5.1429 | — | 0.0278 | <0.0001 | <0.0001 | <0.0001 | 0.0044 | 0.0005 | 0.6374 | 0.2513 | 0.1797 |
| MPT64L | 0.0000 | 0.0303 | 4.8400 | — | 0.0076 | 0.0015 | 0.0124 | 0.4328 | 0.0411 | 0.0606 | 0.2207 | 0.2513 |
| U1 | 2.6129 | 3.3333 | 17.2857 | 7.1176 | — | 0.2498 | 0.4054 | 0.2623 | 1.0000 | 0.0009 | 0.0031 | <0.0001 |
| Ag16kDa | 10.8000 | 10.7037 | 19.5581 | 10.1250 | 1.3243 | — | 0.1573 | 0.0082 | 0.1779 | <0.0001 | 0.0136 | 0.0002 |
| TB16.3 | 3.8462 | 2.7931 | 16.3333 | 6.2500 | 0.6923 | 2.0000 | — | 0.2888 | 0.8658 | 0.0006 | 0.0025 | 0.0018 |
| Ag38kDa | 0.5000 | 0.2195 | 8.1000 | 0.6154 | 1.2564 | 7.0000 | 1.1250 | — | 0.2087 | 0.2743 | 0.5164 | 0.0578 |
| Ag14kDa | 4.4815 | 2.9412 | 12.1000 | 4.1724 | 0.0000 | 1.8148 | 0.0286 | 1.5806 | — | 0.0002 | 0.0016 | 0.0077 |
| CFP10 | 2.7931 | 3.3333 | 0.2222 | 3.5217 | 11.1111 | 22.0909 | 11.6452 | 1.1951 | 14.2857 | — | 0.4669 | 0.3938 |
| ESAT-6 | 1.3846 | 1.6897 | 1.3158 | 1.5000 | 8.7576 | 6.0952 | 9.1429 | 0.4211 | 9.9655 | 0.5294 | — | 0.8185 |
| CFP10-ESAT-6 | 1.0000 | 1.6364 | 1.8000 | 1.3158 | 16.0000 | 14.2973 | 9.7826 | 3.6000 | 7.1111 | 0.7273 | 0.0526 | — |
The data below — are χ2 values and those above are P values. The sensitivities of two antigens were compared by χ2 test in naturally TST-positive sera. Ag85b showed higher sensitivities than 7 antigens; CFP10, ESAT-6, CFP10-ESAT-6, and MPT64 showed higher sensitivities than 4 antigens; PPD showed higher sensitivities than 3 antigens; and OT and Ag38kDa showed higher sensitivities than the same single antigen.
DISCUSSION
TB diagnosis of living NHPs largely depends on clinical assessment, radiographic (X-ray) examination, and TST. Bacterial culture is not practical as a first-line test due to the long culture period and the fact that few sputum samples collected from monkeys are available. Serological tests have been widely studied and used in the detection of TB infection for its convenience, robustness, and easy implementation (13, 19, 20). Single proteins and cocktails of several proteins as well as genetically engineered fusion molecules with several antigens have been used to improve the sensitivity and specificity to detect TB infection. In this study, we evaluated 10 purified M. tuberculosis proteins as well as OT and PPD as serodiagnostic biomarkers by ELISA in primate sera. PPD and OT were selected as candidate antigens for its commercial maturation, robustness, and easy implementation. Ag38kDa, ESAT-6, CFP10, and fusion proteins of CFP10-ESAT-6 are frequently studied in human and bovine TB as well as in NHPs (2), but their use in NHPs still needs further evaluation with naturally TST-positive monkeys. Ag85b, an abundantly secreted protein in M. tuberculosis culture, is not valuable in human TB diagnosis due to its high cross-reactivity with mycobacterial species but may be valuable in NHP TB diagnosis due to its high sensitivity. There are few reports on other antigens in NHP TB diagnosis, but some, such as TB16.3, have proved to be potent antigens for special use in human TB diagnosis. TB16.3 could be recognized by more than 85% of the samples from TB patients coinfected with human immunodeficiency virus for which it is in general difficult to detect M. tuberculosis-specific antibodies (25).
Analysis with experimental infection models reveals the relationship among the time course of antibody responses, antibody levels, and infection doses. It is still unclear whether the time to antibody detection and antibody levels can indicate the TB infection in the host, largely because the role of humoral immunity in host defense against M. tuberculosis is poorly understood. The time to antibody detection is critical for assay evaluation and early diagnosis to prevent asymptomatic tuberculosis from being released from quarantine. Some researches reported that antibody was detected between day 30 and day 60 after infection (3). Time to antibody detection in naturally infected monkeys may be longer due to a lower infectious dose. In this study, we found that antibodies against some antigens (CFP10, CFP10-ESAT-6, U1, MPT64, and Ag85b) are detected as early as 4 weeks after infection in monkeys infected with 500 CFU of M. tuberculosis and are not detected or are detected at least 2 weeks later in monkeys infected with 50 CFU of M. tuberculosis. Some few antigens, such as the 16-kDa protein, induce antibodies much later than others. In addition, we also identify that the levels of antibodies depend on the infectious doses.
We further evaluate the diagnostic potential of these M. tuberculosis antigens by ELISA in sera from TST-positive and -negative monkeys. We find that the levels of antibodies against all antigens in TST-positive sera are much higher than those in TST-negative sera, consistent with previous reports on humans (14, 26), suggesting their potential values in the TB diagnosis of nonhuman primates.
We also evaluate the sensitivity and specificity of these antigens. Ag85b and CFP10 show the highest sensitivities of 74.65% and 71.83% respectively, followed by ESAT-6 and CFP10-ESAT-6, while the sensitivities of U1 protein, Ag16kDa, TB16.3, Ag38kDa, and Ag14kDa are below 50%. Though PPD is developed and used for a long period, it shows only a moderately high sensitivity of 59.15%. The sensitivity and specificity of these antigens are not the same as those in human patients (25, 28).
In addition, we reveal the differences of serodiagnostic potential between two antigens by the Z test and the χ2 test. Ag85b and CFP10 show higher serodiagnostic potential than other antigens, while U1 protein, Ag16kDa, TB16.3, and Ag14kDa show lower values for discriminating TST-negative and -positive monkeys. Though the 38-kDa protein presents a low sensitivity of below 50%, it showed poorer sensitivity than Ag85b only, suggesting its great value in combination with other antigens. A combination of results from Ag85b and Ag38kDa may reach a high sensitivity of 90.14%, and if the 14-kDa protein is added, only 3 of 71 monkeys were seronegative, indicating these proteins' great potential in serodiagnostic cocktails. Currently, more proteins combined in various cocktail kits are under testing in order to find ideal kits with high sensitivity and specificity (under evaluation). Compared with cocktail antigens, the fusion proteins show higher specificity, but it is unclear whether they can increase the sensitivity. In our study, the fusion protein of ESAT-6 and CFP10 (CFP10-ESAT-6) does not have higher sensitivity than any single protein. However, the sensitivity of the fusion protein may be affected by many factors such as antigen sources and posttranslational modification of the antigen (5, 9).
In conclusion, we have characterized the antibody responses of 10 purified proteins and two kinds of tuberculin, PPD and OT. Most of the selected antigens show great serodiagnostic potential for primate TB and can be used to increase the sensitivity and specificity for the diagnosis of primate TB. However, the sensitivity and specificity of these antigens are needed to be tested in more naturally TB-positive monkeys. Further study will be conducted to screen and evaluate more antigens and to identify the optimal combinations of antigens for the sensitive and specific diagnosis of primate TB.
ACKNOWLEDGMENTS
This work was supported by grants 2006B20801004, 2005B60302002, and 2009B060300017 of the Guangdong Provincial Science and Technology Project and by the Guangdong Provincial Key Laboratory of Laboratory Animals (grant 2007B060101002).
We are grateful to Tie Wu for helpful discussions and expert technical assistance.
Footnotes
Published ahead of print on 28 October 2011.
REFERENCES
- 1. Aagaard C., et al. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abebe F., et al. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand. J. Immunol. 66:176–191 [DOI] [PubMed] [Google Scholar]
- 3. Brusasca P. N., et al. 2003. Antigen recognition by serum antibodies in nonhuman primates experimentally infected with Mycobacterium tuberculosis. Comp. Med. 53:12–19 [PubMed] [Google Scholar]
- 4. Reference deleted.
- 5. Espitia C., et al. 1989. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin. Exp. Immunol. 77:373–377 [PMC free article] [PubMed] [Google Scholar]
- 6. Fu R. L., et al. 2009. An improved whole-blood gamma interferon assay based on the CFP21-MPT64 fusion protein. Clin. Vaccine Immunol. 16:686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia M. A., et al. 2004. Diagnosis of tuberculosis in macaques, using whole-blood in vitro interferon-gamma (PRIMAGAM) testing. Comp. Med. 54:86–92 [PubMed] [Google Scholar]
- 8. Reference deleted.
- 9. Harboe M., Wiker H. G. 1992. The 38-kDa protein of Mycobacterium tuberculosis: a review. J. Infect. Dis. 166:874–884 [DOI] [PubMed] [Google Scholar]
- 10. Hendrickson R. C., et al. 2000. Mass spectrometric identification of Mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langermans J. A. M., et al. 2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23:2740–2750 [DOI] [PubMed] [Google Scholar]
- 12. Reference deleted.
- 13. Lodes M. J., et al. 2001. Serological expression cloning and immunological evaluation of MTB48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyashchenko K., et al. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyashchenko K. P., et al. 2007. PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin. Vaccine Immunol. 14:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mukherjee S., et al. 2004. Potential serological use of a recombinant protein that is a replica of a Mycobacterium tuberculosis protein found in the urine of infected mice. Clin. Diagn. Lab. Immunol. 11:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 18. O'Reilly L. M. 1995. Tuberculin skin tests: sensitivity and specificity, p. 85–91 In Thoen C. O., Steele J. H. (ed.), Mycobacterium bovis infection in animals and humans. Iowa State University Press, Ames, IA [Google Scholar]
- 19. Pottumarthy S., Wells V. C., Morris A. J. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samanich K. M., et al. 2000. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 7:662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verbon A., et al. 1992. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J. Bacteriol. 174:1352–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vervenne R. A. W., et al. 2004. TB diagnosis in non-human primates: comparison of two interferon-g assays and the skin test for identification of Mycobacterium tuberculosis infection. Vet. Immunol. Immunopathol. 100:61–71 [DOI] [PubMed] [Google Scholar]
- 23. Walsh G. P., et al. 1996. The Philippine cynomolgus monkey (Macaca fascicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat. Med. 2:430–436 [DOI] [PubMed] [Google Scholar]
- 24. Wang H. B., Zhu Z. Y., Xie Y. 2005. Clinical application of the protein chip for detection of the antibody to multiple Mycobacterium tuberculosis antigens. China Trop. Med. 5:317–318 [Google Scholar]
- 25. Weldingh K., et al. 2005. Assessing the serodiagnostic potential of 35 Mycobacterium tuberculosis proteins and identification of four novel serological antigens. J. Clin. Microbiol. 43:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu X. Q., et al. 2010. Humoral immune responses against the Mycobacterium tuberculosis 38-kilodalton, MTB48, and CFP-10/ESAT-6 antigens in tuberculosis. Clin. Vaccine Immunol. 17:372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X. Q., et al. 2010. Comparison of antibody responses to seventeen antigens from Mycobacterium tuberculosis. Clin. Chim. Acta 411:1520–1528 [DOI] [PubMed] [Google Scholar]
- 28. Zhang G. Q., et al. 2009. Screening and assessing 11 Mycobacterium tuberculosis proteins as potential serodiagnostical markers for discriminating TB patients from BCG vaccinees. Genomics Proteomics Bioinformatics 7:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]