Abstract
Double-stranded RNA binding motif (DSRM)-containing proteins play many roles in the regulation of gene transcription and translation, including some with tandem DSRMs that act in small RNA biogenesis. We report the characterization of the genes for double-stranded RNA binding proteins 1 and 2 (DRB1 and DRB2), two genes encoding nuclear proteins with tandem DSRMs in the ciliate Tetrahymena thermophila. Both proteins are expressed throughout growth and development but exhibit distinct peaks of expression, suggesting different biological roles. In support of this, we show that expression of DRB2 is essential for vegetative growth while DRB1 expression is not. During conjugation, Drb1p and Drb2p localize to distinct nuclear foci. Cells lacking all DRB1 copies are able to produce viable progeny, although at a reduced rate relative to wild-type cells. In contrast, cells lacking germ line DRB2 copies, which thus cannot express Drb2p zygotically, fail to produce progeny, arresting late into conjugation. This arrest phenotype is accompanied by a failure to organize the essential DNA rearrangement protein Pdd1p into DNA elimination bodies and execute DNA elimination and chromosome breakage. These results implicate zygotically expressed Drb2p in the maturation of these nuclear structures, which are necessary for reorganization of the somatic genome.
INTRODUCTION
Proteins containing a double-stranded RNA (dsRNA) binding motif (DSRM) participate in diverse biological pathways in a wide range of organisms. This motif was first identified in the developmentally essential gene Staufen of Drosophila melanogaster and has since been recognized to be encoded in the genomes in all three domains of living organisms, as well as in viruses (63; reviewed in references 20 and 67). DSRM proteins commonly act in developmental pathways (e.g., RNA localization by the Staufen family and developmental transcriptional regulation by the DIP1 family) (5, 18, 62, 68) but also have ubiquitous roles in transcriptional and translational regulation (e.g., PKR family and PKR-associated proteins) (26, 45, 55, 58). Proteins vital for RNA interference (RNAi) also contain DSRMs. These include members of the RNase III family (e.g., Dicer and Drosha family proteins) and their tandem DSRM-containing partner proteins (e.g., RDE-4 of Caenorhabditis elegans, Pasha, R2D2, and Loqs in D. melanogaster, and their homologues in Homo sapiens) (4, 12, 17, 23, 27, 36, 37, 57, 65).
In the ciliate Tetrahymena thermophila, the DSRM-containing protein Dicer-like 1 (DCL1) has been shown to play a pivotal role in a process linking RNAi to heterochromatin formation and developmentally regulated DNA elimination (42, 49). Like all ciliates, T. thermophila is unicellular yet contains two distinct types of nuclei, the somatic macronucleus and the germ line micronucleus (reviewed in references 46 and 56). The polyploid micronucleus (∼50C) acts as a transcriptionally active somatic nucleus during vegetative growth, while the diploid, germ line micronucleus is transcriptionally silent (19, 70; reviewed in references 46 and 56). Under optimal growth conditions T. thermophila undergoes asexual, binary fission; however, when starved T. thermophila reproduces through the sexual process of conjugation, generating new micronuclei and macronuclei from the parental germ line micronucleus (reviewed in references 46 and 56). During the maturation of the zygotic macronuclei, the macronuclear chromosomes are fragmented at ∼180 sites, lose ∼15% of their overall genomic content, and are amplified to ∼50C (1, 7, 14, 19, 29, 69, 70). The loss of genome complexity is the result of programmed DNA rearrangements that remove specific DNA sequences, called internal eliminated sequences (IESs), from thousands of chromosomal sites (46, 56).
DNA elimination has been shown to be guided by an RNAi-related mechanism (11, 42, 47, 49). Bidirectional transcription of the germ line genome in meiotic micronuclei provides an abundant source of IES-specific dsRNA (11, 44). The resulting noncoding RNAs (ncRNAs) are processed into 27- to 30-nucleotide (nt) sRNA species, called scan RNAs (scnRNAs), by Dcl1p in the meiotic micronucleus (42, 49). These scnRNAs are exported into the cytoplasm, where they are bound by a PIWI homologue, Twi1p (47). Twi1p/scnRNA complexes are transported into the parental macronucleus, where these complexes scan macronuclear ncRNAs, and possibly mRNAs. The Twi1p/scnRNA complexes homologous to the parental macronucleus are removed from the pool of active complexes, and the remaining complexes are transported to the zygotic macronuclei upon their emergence, where they guide H3K9 and H3K27 methylation of IES-associated histones by the E(z) homologue Ezl1p (38, 47, 48). Methylated histones in zygotic macronuclei are bound by the chromo domain-containing proteins Pdd1p and Pdd3p, which along with other associated proteins form large nuclear structures called DNA elimination bodies late in conjugation (38, 40, 51, 66). DNA elimination in these bodies is catalyzed by the domesticated PiggyBac transposase Tpb2p, resulting in removal of IESs from zygotic macronuclei (13).
A second endogenous RNAi pathway that acts to silence genes and/or pseudogenes is evidenced by a class of 23- to 24-nt sRNAs that accumulate during vegetative growth (33). These sRNAs are homologous to loci clustered at ∼12 genomic positions and exhibit biased polarity, mapping to only one strand. They are produced by the essential Dicer protein Dcr2p in a coupled reaction with an RNA-dependent RNA polymerase, Rdr1p (34). This coupling likely accounts for the strand specificity observed.
As dsRNA has clear roles in regulating genome structure and activity, we characterized the two putative tandem DSRM-containing proteins, double-stranded RNA binding proteins 1 and 2 (Drb1p and Drb2p), encoded in the T. thermophila genome (21, 64). We show that both are nuclear proteins that exhibit distinct subnuclear organization. By knocking out the gene for each, we found that Drb2p is essential both during vegetative growth and also late in conjugation, where it facilitates DNA elimination body formation and subsequent RNAi-dependent DNA elimination. Drb1p, in contrast, is dispensable but is nonetheless important for efficient prezygotic development. Our data do not support that either protein acts as an essential Dicer partner protein as do tandem DSRM proteins in other eukaryotes, but instead our data suggest that these proteins have diverse roles during the T. thermophila life cycle and expose a role for dsRNA late in macronuclear development (4, 12, 17, 23, 27, 37, 57, 65).
MATERIALS AND METHODS
Tetrahymena strains and growth conditions.
Standard wild-type, laboratory T. thermophila strains CU427 (Chx/Chx [VI, cy-s]), CU428 (Mpr/Mpr [VII, mp-s]), B2086 (II), and micronucleus-defective strains B*VI (VI) and B*VII (VII) were originally obtained from Peter Bruns (Cornell University, Ithaca, NY). BVIICU427/CU427 (Chx/Chx [VII, cy-s] was generated through genomic exclusion mating between CU427 and B*VII. These strains or their transformed progeny were used for expression studies, biolistic transformations, and subsequent analyses. ΔDCL1 homozygous knockout strains were described earlier (42). Cells were grown and maintained as previously described (25, 52). Strains were starved 6 h to overnight in 10 mM Tris (pH 7.5) prior to mixing to initiate conjugation. Optical densities of cell populations were used to estimate cell numbers prior to mixing equal numbers of mating-compatible strains.
Identification of DRB1 and DRB2 sequences.
DRB1 (TTHERM_00078870; NCBI Gene ID 7837033) and DRB2 (TTHERM_00825510; NCBI Gene ID 7836999) sequences were identified by BLAST search of the T. thermophila macronuclear genome (http://www.ciliate.org) and the D. melanogaster R2D2 (accession number CG7138) and Loqs (accession number CG6866) DNA sequences. DSRMs of DRB1 and DRB2 were initially identified on the T. thermophila macronuclear genome by using InterProScan (72). Further sequence analysis included Pfam analysis of the DRB1 and DRB2 coding sequences (http://pfam.janelia.org) and alignment of DSRM sequences of DRB1 and DRB2 with those of tandem DSRM-containing proteins R2D2 (accession number NP_609152.1) and Loqs (accession number NP_609646.1) from D. melanogaster, RDE-4 (accession number NP_499265.1) from C. elegans, and TRBP2 (accession number NP_599150.1) from H. sapiens by using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and Boxshade (http://www.fr33.net/boxshadeprotein.php) for better visualization. Identification of additional homologous regions of the DRB1 and DRB2 protein sequences was carried out by protein alignment using Emboss Needle (http://www.ebi.ac.uk).
RT-PCR expression analysis.
RNA was isolated from growing, starved, and conjugating T. thermophila (CU428 × B2086) at 2-h intervals from 2 h to 14 h by RNAsol extraction (22). RNA isolation from DRB1 knockouts and DRB2 micronucleus (mic) knockouts at 4 h and 12 h after mixing was also done by RNAsol extraction. Reverse transcription-PCR (RT-PCR) of wild-type and knockout matings was done to determine expression and show loss or decrease of expression, respectively, as previously described (Table 1 provides a list of the primers used) (42).
Table 1.
Oligonucleotides used in the course of this study
| Primer purpose and name | Sequence (5′–3′) |
|---|---|
| For RT-PCR to determine expression | |
| DRB1 | |
| 1688-Loq1-836 | CGAAAAGGGGTTAGGGTTTTCTAGC |
| 1689-Loq1-1325r | CCCTTATCCCATCGTTTTCAG |
| DRB2 | |
| 1692-Loq3-1875 | GCAATAGCCAAACACAAAGAGTGTAGC |
| 1879-Loq3-2230r | GCATCAATAAGGCTACAACATCC |
| ATU1 | |
| 3364-ATU1-2391r | GTGGCAATAGAAGCGTTGACA |
| 3365-ATU1-1997 | TGCTCGATAACGAAGCCATCT |
| For gene amplification of coding sequence | |
| DRB1 | |
| 1701-Loq1X | CACCCTCGAGAAAATGAATTCTTAGCAAG |
| 1732-Loq1rH-Short | AAGCTTTAGACTTATACTTTTCATGAAAG |
| DRB2 | |
| 1887-Loq3X-Modified | CACCCTCGAGAAAATGGCGCAATCTTTTAGATTTATAG |
| 1911-Loq3rP-Full length | CTGCAGCCCATTACAAATAATTATTAAGTTATCATAAGC |
| For knockout cassette generation | |
| DRB1 upstream | |
| 1761-Loq1-2321AattB4 | GGGGACAACTTTGTATAGAAAAGTTGGTACCGGGATTACATAAAGATTTGATTCC |
| 1762-Loq1-3366rattB1 Downstream | GGGGACTGCTTTTTTGTACAAACTTGCACAATTCAATCAAAAGTGCG |
| 1763-Loq1-5746attB2 | GGGGACAGCTTTCTTGTACAAAGTGGCACTCTCATTAATGCCCCC |
| 1764-Loq1-7093AattB3 | GGGGACAACTTTGTATAATAAAGTTGGTACCAGTAAAGAGCCTAAATCAAGG |
| DRB2 upstream | |
| 2370-DRB2-1226AattB4 | GGGGACAACTTTGTATAGAAAAGTTGGTACCGAAAGCCTATGGGAGAGCAAG |
| 2372-DRB2-2595rattB1 | GGGGACTGCTTTTTTGTACAAACTTGCACTTTTAGGAAATAATGAATGTGTCAC |
| DRB2 downstream | |
| 2322-DRB2-6530attB2 | GGGGACAGCTTTCTTGTACAAAGTGGGTTGTGTTTAAAAAGAAGGTGTGTGTTATG |
| 2323-DRB2-7708ArattB3Ext | GGGGACAACTTTGTATAATAAAGTTGGTACCTTCACTTAAACCGCACCCAG |
| For knockout PCR screening | |
| DRB1 | |
| 5′ 1679-MTT1-11484r | ATTTGGAATTAAGTACTTATTTCCAAAC |
| 1946-DRB1-1086 | CGCGCACTTTTGATTGAATTGTG |
| 3′ 1866-Neo KO 2 | CGTGATATTGCTGAAGAGCTTG |
| 1867-Loq1-5353 | CAGGGGAAGATATATTTTATGAAGC |
| 1868-Loq1-5764r | GGGGGCATTAATGAGAGTG |
| DRB2 | |
| 5′ 2477-DRB2-2195 | CAATTTATCTATTAAAATACCTTTACTTAC |
| 2478-DRB2-2805r | AAAATCTGTAATTGAGAAGAAACAAAAAC |
| 3001-LIA4MTTLR | AACATTCAAACATTGTGCACTAAATA |
| 3′ 2367-p4T2-3351 | TCGCCTTCTTGACGAGTTCT |
| 2391-DRB2-6323 | GCTTAGATGATATTACACATGATAATC |
| 2392-DRB2-6721r | AAAGAGAGTGAGTTTTTCTTTTTGG |
| For assay rearrangement of IESs | |
| M | |
| 1439-M808 | ATATTGTGTGGTACAATAGGTTGTCGTAG |
| 3111-M002 | AGCTTAAACAAATGCCATATTGAG |
| 3114-M1194 | GTGGGGAGGGAGAAGGATTCAAC |
| B | |
| 3246-IES7_MDSL-112 | GGATTGATTGGCATAAATGGA |
| 3247-IES7_MDSR-158 | AAGCCCAGAATACCGCAGTTC |
Cloning of T. thermophila genes for protein localization.
Oligonucleotide primers (Table 1) were used to amplify the entire DRB1 or DRB2 coding sequences from genomic DNA by PCR. The resulting products were cloned into the Gateway recombination-compatible pENTR-D (Invitrogen) to create pENTR-D-DRB1 and pENTR-D-DRB2, respectively. Plasmids containing the DRB1 and DRB2 coding sequences were sequenced to verify coding sequence integrity. LR recombination of pENTR-D-DRB1 with pICY-GTW and of pENTR-D-DRB2 with pICC-GTW using LR clonase II (Invitrogen) fused the coding regions to yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) in pICY-DRB1 and pICC-DRB2, respectively (71). Plasmids pICY-DRB1 and pICC-DRB2 were introduced into mating wild-type cells (CU427 × CU428) by conjugative electroporation (24).
Similarly, the entire PDD1 coding sequence was amplified from genomic DNA (primers are listed in Table 1) and cloned into pENTR-D to create donor plasmid pENTR-D-PDD1. LR recombination of pENTR-D-PDD1 with pICY-GTW using LR clonase II (Invitrogen) created pICY-PDD1, which was then introduced into cells by conjugative electroporation (24).
For Pdd1p-YFP localization in DRB2 mic knockout strains, pENTR-D-PDD1 was recombined with pBS2-ICY-GTW by using LR clonase II (Invitrogen) to create pBS2-ICY-PDD1. BclI- and SalI-digested pBS-ICY-PDD1 was transformed into starved, homozygous micronuclear DRB2 knockout strains (B*VIΔD2/ΔD2 1 and 6 and B*VIIΔD2/ΔD2 1 and 2) by using the PDS-1000/He particle bombardment system (Bio-Rad) as previously described (6, 8). Transformants were identified by their resistance to 25 μg/ml cycloheximide.
To visualize localization, starved transformed cells were mixed to begin conjugation in 0.08 μg/ml CdCl2 to induce expression of the fusion protein. Live cells were harvested by low-speed centrifugation (1,000 × g) at 4 h, 10 h, and 14 h postmixing, stained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml) and immobilized in 5 μl 2% methylcellulose. DIC, CFP fluorescence, YFP fluorescence, and DAPI fluorescence images were captured using a Qimaging RetigaEX charge-coupled-device camera (Burnaby, British Columbia, Canada) and Openlab software (PerkinElmer). Images were cropped and their brightness and contrast uniformly adjusted using Adobe Photoshop CS3.
Generation of DRB1, DRB2, and PDD1 knockout strains.
Genomic sequences upstream and downstream of each gene's coding region were amplified by PCR and recombined into pDONR-P4-P1R (upstream) and pDONR-P2R-P3 (downstream) by using BP Clonase (Invitrogen) (Table 1). The resulting donor plasmids containing up- and downstream regions were mixed with equal amounts of pENTR-D-MTT1/NEO3 and the multisite destination vector pDEST-R4-R3, along with LR Clonase Plus II (Invitrogen) to create the DRB knockout plasmids pDEST-B4-DRB1Up-B1-MTT1/NEO3-B2-DRB1Down-B3 and pDEST-B4-DRB2UpN1-B1-MTT1/NEO3-B2-DRB2Down-B3. DRB1 and DRB2 knockout constructs were linearized by digestion with KpnI and transformed into conjugating wild-type cells (CU428 × B2086) between 2 and 3 h after mixing by using a PDS-1000/He particle bombardment system (Bio-Rad) as previously described (6, 8). Heterozygous micronuclear transformants were identified by their resistance to 80 μg/ml paromomycin with 1 μg/ml CdCl2 and 15 μg/ml 6-methylpurine. Heterozygous micronuclear transformants were verified through matings with CU427 by monitoring segregation of paromomycin resistance conferred by the MTT1-neomycin (NEO3) paromomycin resistance cassette among cycloheximide-resistant progeny (61), as well as through PCR screening of T. thermophila crude cell lysates (Table 1 provides sequences of the primers) (10). Homozygous micronuclear knockout heterokaryons were generated by crossing heterozygous micronuclear transformants with B*VI or B*VII star strains. Homozygous micronuclear knockout heterokaryons were identified by paromomycin/CdCl2 sensitivity and verified through crosses with CU427, which produced progeny resistant to 100 μg/ml paromomycin with 1 μg/ml CdCl2 and 25 μg/ml cycloheximide. Complete (micro- and macronuclear) DRB1 knockout strains were generated by crossing homozygous micronuclear knockouts of compatible mating types, screening for progeny resistant to paromomycin/CdCl2, and verifying by PCR detection of the knockout allele (10).
To generate ΔPDD1 strains, the NEO3 cassette (61), cloned between genomic sequences upstream and downstream of the PDD1 coding region (obtained from Yifan Liu, University of Michigan), was introduced into conjugating B2086 and CU428 cells by biolistic transformation (6, 8). Homologous recombination of this cassette into the genome removed 1,714 bp, including the entire PDD1 coding sequence (nucleotides 76,907 to 78,620 of scaffold CH445650.1; accession gi∣62422296∣gb∣ CH445650.1). Germ line (micronuclear) knockouts were identified by their resistance to both paromomycin/CdCl2 and 6-methylpurine (resistance gene from the micronucleus of CU428). Genomic exclusion crosses of heterozygous germ line transformants with star strains B*VI or B*VII generated homozygous mutants that were subsequently crossed to produce complete PDD1 knockouts ΔPDD1 39.1 and ΔPDD1 W3.3, missing all copies of the gene from both the micro- and macronucleus.
Southern blotting and PCR analyses.
T. thermophila genomic DNA was isolated using a Wizard genomic DNA purification kit (Promega). Gel electrophoresis, blotting, and hybridization were performed as previously described, except blots were washed with 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate–1% SDS after hybridization (42). The probe for DRB2 was a radiolabeled KpnI and BsrGI restriction fragment of pDONR-R2-DRB2Down-L3. The DRB1 probe was a labeled BsrGI and XmnI restriction fragment from pDONR-R2-DRB1Down-L3. Genomic DNA from heterozygous, homozygous, and homozygous micronuclear DRB1 knockouts was digested with XmnI and separated on a 1.0% agarose gel. Genomic DNA from heterozygous DRB2 knockouts was digested with ClaI and SacI and fractionated on a 0.8% agarose gel prior to blotting.
Chromosome breakage was assayed in DRB2 mic knockouts by using genomic DNA from CU428 × B2086, ΔPDD1 39.1 × ΔPDD1 W3.3, ΔDCL1 1.8.6 × ΔDCL1 4.2.4, and B*VIΔD2/ΔD2 1 × B*VIIΔD2/ΔD2 1 30 h after mixing and then digestion with EcoRI and separated on a 0.8% agarose gel. The Southern blotting probe for chromosome breakage was created using a 0.8-kbp probe fragment that spans the EcoRI site at position 335013 of chromosomal scaffold CH445662 (GenBank accession number gi62422284). DNA rearrangement of IES B and the M IES was assayed by PCR using CU428 × B2086, ΔDCL1 1.8.6 × ΔDCL1 4.2.4, B*VIΔD2/ΔD2 1 × B*VIIΔD2/ΔD2 1, and B*VIΔD2/ΔD2 6 × B*VIIΔD2/ΔD2 2 30-h genomic DNA and primers flanking each IES (Table 1).
Analysis of nuclear morphology postconjugation.
Wild-type or the indicated knockout cells 30 h into conjugation were harvested by low-speed centrifugation (1,000 × g), DAPI stained (1 μg/ml), and immobilized in 5 μl 2% methylcellulose. Differential interference contrast (DIC) and DAPI fluorescence images were captured using a Qimaging RetigaEX charge-coupled-device camera (Burnaby, British Columbia, Canada) and Openlab software (PerkinElmer). The conjugation stage of each mating at 30 h was determined by comparison of images with previously described wild-type stages of conjugation (44). Images were cropped and their brightness and contrast uniformly adjusted using Adobe Photoshop CS3.
RESULTS
The T. thermophila macronuclear genome encodes two proteins with tandem DSRMs.
For optimal sRNA production and protein localization, Dicer and Drosha homologues in C. elegans, D. melanogaster, and H. sapiens require association with a tandem DSRM-containing protein (4, 12, 17, 23, 27, 37, 53, 57, 65). Bioinformatic analysis (BLAST, Pfam, and ClustalW) of the T. thermophila macronuclear genome identified two genes, DRB1 and DRB2, encoding tandem DSRM-containing proteins (Fig. 1A). Alignment of their putative DSRMs with other DSRM-containing proteins indicated conservation in the regions where key residues known to be important for DSRM structure and function are located. The homologies of these proteins with other tandem DSRM proteins did not extend beyond these domains (Fig. 1A and data not shown). However, alignment of full-length DRB1 and DRB2 revealed additional regions of similarity outside the DSRMs; one in the N-terminal region (NTR) and two in the C-terminal regions (CT1 and CT2) of each protein (data not shown). In the ciliate Paramecium, only DRB1 homologues are evident, which suggests that the duplication and diversification of these proteins occurred after these two ciliates diverged.
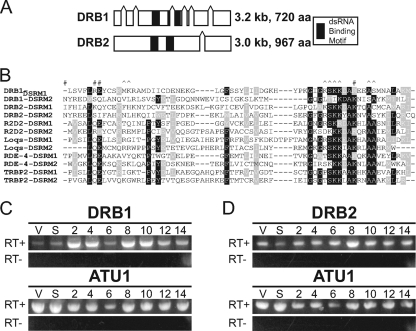
Fig. 1.
T. thermophila contains two predicted tandem double-stranded RNA binding motif proteins. (A)Genomic locus, conserved motifs, and length of the putative tandem double-stranded RNA binding motif proteins Drb1p and Drb2p. Splice sites are indicated by small connected gaps in the gene. aa, amino acids. (B) ClustalW alignment of DSRMs from Dicer family tandem DSRM-containing partner proteins. #, sites determined to be essential for structure and function of DSRMs; ^, sites that have been mutated in DSRM-containing proteins and shown to cause loss of RNA binding. Conserved aa (white text) are shaded in black (identical aa) or gray (similar aa). (C and D) RT-PCR analysis of DRB1 and DRB2 expression relative to α-tubulin (ATU1). RNA samples were isolated from CU428 cells growing vegetatively (V), after an 18-h starvation (S), and from CU428 × B2086 conjugating cells at 2-h intervals and were used to monitor the expression of each gene.
RT-PCR and Northern blot analysis demonstrated that DRB1 and DRB2 are both expressed throughout much of the T. thermophila life cycle (Fig. 1C and D and data not shown). DRB1 mRNA levels are low in growing and starved cells but increase significantly during meiosis (2 to 4 h into conjugation, when scnRNA production occurs) and again after the appearance of the zygotic macronuclei (8 h), a pattern that parallels DCL1 expression (Fig. 1C) (42, 49). Its decrease in expression at 6 h coincides with the drop in ATU1 RNA levels, which may simply reflect the switch between parental and zygotic expression. DRB2 expression is higher during vegetative growth but also shows less dramatic induction during conjugation relative to DRB1. After decreased expression during starvation, DRB2 is induced starting at 2 h of conjugation and peaks at 8 h, shortly after the appearance of the zygotic macronuclei (Fig. 1D). This profile suggests possible roles for Drb2p during both growth and development.
DRB1 and DRB2 encode nuclear proteins that localize to distinct structures.
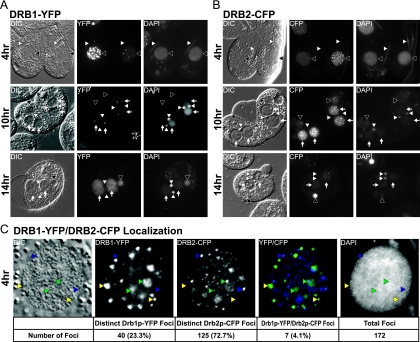
Ectopic expression of Drb1p and Drb2p tagged with YFP or CFP, respectively, on their C termini showed that both are nuclear proteins visible in small foci throughout the macronucleus during vegetative growth (data not shown), whereas green fluorescent protein (GFP) alone expressed in cells is uniformly distributed (42, 43). During early conjugation, both proteins localize to the parental macronucleus in distinct foci (Fig. 2). Later, at the beginning of zygotic macronuclear differentiation (10 h), all Drb1p-YFP and most Drb2p-CFP disappeared from the parental macronucleus and then appeared in zygotic macronuclei (Fig. 2A and B, middle rows). Whether the foci seen in the parental macronucleus are functionally related to those observed in zygotic macronuclei could not be determined (Fig. 2A and B, compare top and middle rows). Near completion of zygotic macronuclear development (14 h into conjugation), Drb1p-YFP localization was primarily diffuse (Fig. 2A, bottom row). In contrast, the small Drb2p-CFP foci coalesced into larger foci, although low-level diffuse localization remained throughout the zygotic macronucleus as well (Fig. 2B, bottom row).
Fig. 2.
Nuclear localization of Drb1p and Drb2p during conjugation. (A and B) Nuclear localization of Drb1p-YFP (A) and Drb2p-CFP (B) at 4, 10, and 14 h into conjugation. White arrowheads, micronuclei; black arrowheads, parental macronuclei; white arrows, zygotic macronuclei. (C) Simultaneous localization of Drb1p-YFP and Drb2p-CFP in the parental macronucleus 4 h into conjugation. (Top) Drb1p-YFP and Drb2p-CFP foci are predominantly distinct in the macronucleus early during conjugation. Yellow arrowheads, Drb1p-YFP foci only; blue arrowheads, Drb2p-CFP foci only; green arrowheads, Drb1p-YFP and Drb2p-CFP foci. (Bottom) The number of Drb1p-YFP foci, Drb2p-CFP foci, and Drb1p-YFP/Drb2p-CPF colocalization foci, and the total number of foci in the parental macronucleus above.
Upon initial inspection, the size and number of nuclear foci of Drb1p and Drb2p in parental macronuclei appeared rather different. To better compare their localizations, Drb1p-YFP and Drb2p-CFP were coexpressed and visualized 4 h into conjugation. Their nuclear foci were distinct, with only a small degree of overlapping localization (Fig. 2C). DRB1 and DRB2 were best reciprocal hits in a BLASTp analysis of the CT2 regions, which could explain the small overlap in localization through partially redundant protein function. Despite this, it seems that both Drb1p and Drb2p have distinct primary functions based on their localizations and divergent protein sequences outside their DSRMs and CT2.
In addition to its abundant macronuclear localization, Drb1p-YFP also localized to the micronucleus just prior to and during crescent formation (prophase meiosis I) (Fig. 2A, top row, and data not shown). Drb1p-YFP was observed specifically at the poles of these nuclei, at either one or both ends depending on the developmental stage. This micronuclear localization pattern is quite distinct from that of Dcl1p, which is found throughout the nucleoplasm of the crescent micronucleus, and suggests that Drb1p may not be a critical Dcl1p protein partner (42, 49). Point localization of Drb1p-YFP was seen early in conjugation once the micronucleus began to elongate at one end, and later, after the crescent micronucleus fully elongated, it was seen at both ends of the micronucleus (Fig. 2A, top row, and data not shown). Upon anaphase of meiosis I, Drb1p-YFP micronuclear localization is lost. While it is likely that DRB1 and DRB2 arose from an ancient gene duplication, differential localization and expression patterns indicate that each DSRM-containing protein has specific cellular roles.
DRB2, but not DRB1, is essential for growth and development.
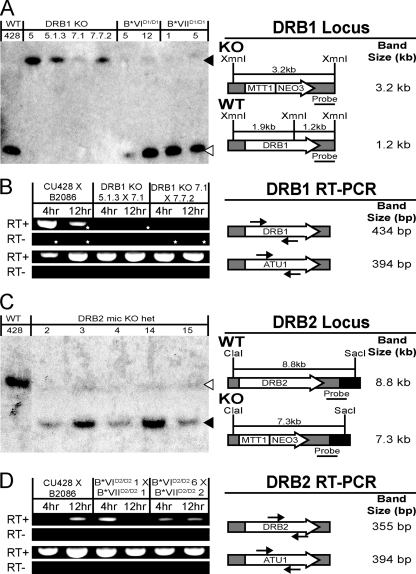
We created strains lacking each gene to establish whether and when each protein functions during the T. thermophila life cycle. Constructs containing the NEO3 selectable marker, flanked by up- and downstream homology regions to either DRB1 or DRB2, were biolistically transformed into cells during conjugation to generate heterozygous micronuclear/macronuclear knockout strains. By taking advantage of the random assortment of alleles during amitotic macronuclear division, we obtained strains for which all wild-type DRB1 gene copies in the macronucleus were replaced with the knockout allele, which revealed that Drb1p is not required for vegetative growth (Fig. 3A and B and data not shown).
Fig. 3.
Generation of DRB1 complete and DRB2 mic knockout strains, based on Southern blot analysis of knockout strain genomic DNA. (A) Genomic DNA isolated from wild-type (WT) CU428, four DRB1 macronuclear/micronuclear knockout strains (DRB1 KO), and four micronuclear strains (B*VI and B*VIIΔD1/ΔD1) was digested with XmnI prior to gel electrophoresis. (C) Genomic DNA from DRB2 mic knockout heterozygous strains (DRB2 mic KO het) was digested with ClaI and SacI prior to analysis. The diagram of WT (DRB1/2) and KO (MTT1/NEO3) alleles is shown on the right of each panel. Black arrowhead, band expected for the knockout allele; white arrowhead, band expected for the wild-type fragment. (B and D) RT-PCR expression analysis of DRB KO strain matings. RNA isolated 4 h (parental expression) and 12 h (zygotic expression) into conjugation was converted to cDNA (RT+), and PCR using gene-specific primers was used to assess loss/reduction of expression. A control reaction with reverse transcriptase omitted (RT-) is also shown. Primers specific to the α-tubulin gene (ATU1) provided a normalization control between samples. In panel B, the star marks a nonspecific RT-PCR band detected with DRB1 primers. Diagrams of the DRB1/2 and ATU1 loci; the relative locations of forward and reverse PCR primers (black arrows) are shown on the right.
To further verify that DRB1 is not essential, homozygous micronuclear knockout strains were crossed to produce complete DRB1 knockout cell lines. Southern blot analysis of genomic DNA isolated from these strains detected only the DRB1 knockout allele (Fig. 3A). RT-PCR of the DRB1 knockout strains during conjugation confirmed loss of all DRB1 expression (Fig. 3B). While these complete DRB1 knockout strains showed no growth defects, matings between two DRB1 knockout strains generated progeny at a reduced rate relative to crosses of wild-type strains (Table 2). The DRB1 knockout cells that were able to complete conjugation arrested with two new macronuclei and a single micronucleus, as do wild-type conjugants, until they were returned to growth medium and started vegetative growth (Fig. 4). The observation that only a fraction of mated DRB1 knockout cells progressed to zygotic development suggests that Drb1p is important, but not essential, for prezygotic development. The lack of Drb1p during this stage(s) of early conjugation resulted in substantial premature abortion of conjugation (data not shown).
Table 2.
Progeny production of DRB1 knockouts in wild-type and knockout matings
| Cross | % pair survival (S/N)a | % progeny production (P/S)b |
|---|---|---|
| CU427 × DRB1 KO 5.1.3 | 97.2 (171/176) | 96.5 (165/171) |
| CU427 × DRB1 KO 6.1.6 | 98.8 (87/88) | 98.9 (86/87) |
| CU427 × DRB1 KO 6.1.12.1 | 96.0 (169/176) | 98.2 (166/169) |
| CU427 × DRB1 KO 6.1.12.2 | 98.9 (174/176) | 97.1 (169/174) |
| CU427 × DRB1 KO 7.1 | 97.7 (129/132) | 98.4 (127/129) |
| CU427 × DRB1 KO 7.7.2 | 99.2 (131/132) | 94.7 (124/131) |
| DRB1 KO 5.1.3 × 6.1.12.1 | 93.5 (247/264) | 33.3 (6/18) |
| DRB1 KO 5.1.3 × 6.1.12.2 | 94.7 (250/264) | 51.5 (35/68) |
The pair survival is the percentage of pairs alive (S) of the total pairs (N) isolated.
Progeny production is the percentage of surviving pairs (S) that successfully completed conjugation and made new macronuclei (P).
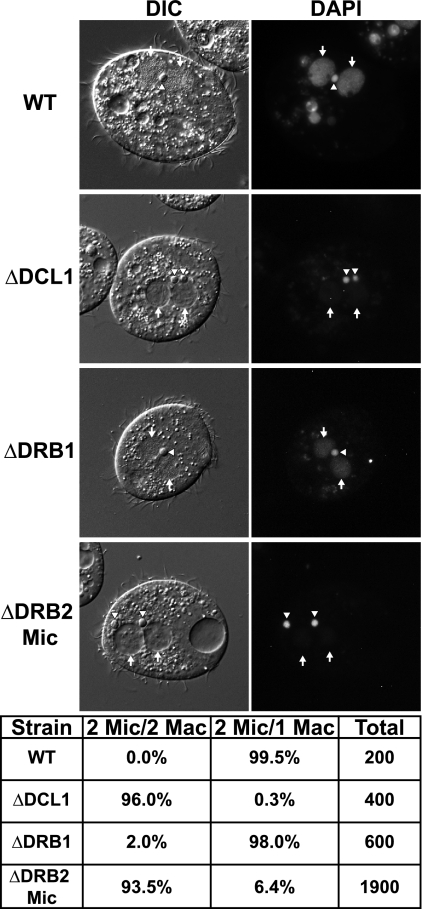
Fig. 4.
Zygotic expression of DRB2 is necessary for completion of conjugation. (Top) Terminal arrest phenotype of wild-type (WT), ΔDCL1, ΔDRB1, and ΔDRB2 mic cells 30 h into conjugation. WT, ΔDCL1, ΔDRB1, and ΔDRB2 mic cells were mated and harvested after 30 h into conjugation. Cells were then DAPI stained, and DIC (left) and DAPI (right) images were obtained. White arrowheads, micronuclei; white arrows, zygotic macronuclei. (Bottom) Cells with the indicated terminal arrest phenotype of WT, ΔDCL1, ΔDRB1, and ΔDRB2 mic 30 h into conjugation.
Unlike our experience with DRB1, we were unable to identify strains in which all macronuclear copies of DRB2 were disrupted, which indicates that vegetative DRB2 expression is essential (Fig. 3C and D). To verify this, we first performed genomic exclusion crosses between the original heterozygous micronuclear knockout strains and “star” strains (B*VI and B*VII) to create strains homozygous for the knockout cassette in the micronucleus while maintaining wild-type copies of DRB2 in the macronucleus to support growth (see Materials and Methods for details). These homozygous micronuclear knockout strains were then crossed in an attempt to generate strains homozygous for the knockout cassette in both the micro- and macronucleus, thus eliminating all wild-type DRB2 gene copies. Despite each individual DRB2 micronuclear knockout strain being able to produce progeny when complemented by crossing to wild-type strains, when these lines were crossed to each other no viable progeny emerged (Table 3).
Table 3.
Progeny production of DRB2 mic knockouts in wild-type and knockout matings
| Cross | % pair survival (S/N)a | % progeny production (P/S)b |
|---|---|---|
| B*VII427 × B*VIΔD2/ΔD2 1 | 99.6 (263/264) | 98.8 (260/263) |
| B*VII427 × B*VIΔD2/ΔD2 6 | 99.2 (262/264) | 99.2 (260/262) |
| CU427 × B*VIIΔD2/ΔD2 1 | 95.1 (251/264) | 99.6 (250/251) |
| CU427 × B*VIIΔD2/ΔD2 2 | 97.3 (257/264) | 100 (257/257) |
| B*VIΔD2/ΔD2 1 × B*VIIΔD2/ΔD2 1 | 2.8 (5/176) | 0.0 (0/5) |
| B*VIΔD2/ΔD2 1 × B*VIIΔD2/ΔD2 2 | 1.7 (3/176) | 0.0 (0/3) |
| B*VIΔD2/ΔD2 6 × B*VIIΔD2/ΔD2 1 | 0.0 (0/176) | 0.0 (0/0) |
| B*VIΔD2/ΔD2 6 × B*VIIΔD2/ΔD2 2 | 1.1 (2/176) | 0.0 (0/2) |
Pair survival is the percentage of pairs alive (S) of the total pairs (N) isolated.
Progeny production is the percentage of surviving pairs (S) that successfully completed conjugation and made new macronuclei (P).
Further analysis revealed that DRB2 micronuclear knockout strains are unable to reach the terminal stage of conjugation with 2 macronuclei and 1 micronucleus even 30 h after pairing, but instead arrest with 2 macronuclei and 2 micronuclei (Fig. 4). Thus, not only is DRB2 expression necessary for vegetative growth, but zygotic DRB2 expression is essential for completion of conjugation as well (Fig. 3C and 4). As observed in other mutants that arrest at the 2-macronuclei, 2-micronuclei stage, conjugating DRB2 mic knockouts underamplified their macronuclear DNA relative to zygotic macronuclei of wild-type conjugants at their terminal stage prior to refeeding (15, 42, 47, 49). Although DRB2 mic knockout strains only lack zygotic expression of DRB2, the majority of conjugants arrest at the 2-macronuclei, 2-micronuclei stage, while the remainder arrest after elimination of one of the remaining micronuclei (Fig. 4, bottom). RT-PCR analysis of DRB2 mic knockout matings showed reduced, but not complete loss of, expression after 12 h of conjugation relative to wild-type cells, when zygotic DRB2 expression normally should predominate (Fig. 3D). Unmated cells as well as parentally expressed DRB2 mRNA in the DRB2 mic knockout mating population accounted for the DRB2 mRNA detected. The residual, parentally expressed DRB2 transcripts may enable a fraction of cells to proceed further into conjugation and eliminate one micronucleus.
DRB2 mic knockouts fail to remodel chromosomes late in conjugation.
The DRB2 conjugation arrest phenotype is commonly observed in knockouts of genes necessary for genome rearrangement in T. thermophila, including DCL1, TWI1, and PDD1 (15, 42, 47, 49). To determine whether the DRB2 mic knockout arrest is accompanied by failure of RNA-directed DNA elimination or due to some other perturbation during conjugation, we monitored the rearrangements of several IESs. Genomic DNA was isolated from mated cell populations 30 h after initiating conjugation, when all genome reorganization should be completed in wild-type cells. PCR using primers able to detect both the unrearranged (micronuclear form of the locus) and rearranged (macronuclear form) IESs allowed assessment of the level of excision. Whereas DNA from wild-type mating populations showed predominantly the rearranged locus for each IES, DRB2 mic knockout or control DCL1 knockout matings exhibited accumulation of the unrearranged form of both IES B and the M IES (Fig. 4 and data not shown). IES B is a 327-bp IES found within the LIA2 gene, and the M IES is a well-studied intergenic IES that undergoes alternative rearrangement that removes either 0.6 kb or the complete 0.9-kb IES (2, 22a). PCR analysis of IES B clearly showed that the 597-bp product indicative of the micronuclear locus was overrepresented in the DCL1 and DRB2 mic knockout matings relative to wild type (Fig. 5A). It is important to note that the cell populations monitored included some percentage of unmated cells, whose DNA likely contributed much of the template for the 270-bp product representing the rearranged form in the mutant cell lines. The PCR analysis of the M IES utilized three primers for PCR, which we have found provides a more quantitative assessment of its rearrangement. Two bands at 1,192 bp and 386 bp resulted from amplification of micronuclear DNA containing the IES, while two other bands at 592 bp and 292 bp were the products of removal of either 0.6 kb or 0.9 kb of the M IES locus. As observed for IES B, the unrearranged form of the M IES was overrepresented in the DCL1 and DRB2 mic knockout mating populations relative to wild-type matings (Fig. 5B). This difference was less apparent in DRB2 mic knockout matings than in the DCL1 mutant, which may have been due to persistence of parental Drb2p. Analysis of other IESs further demonstrated that these mutants exhibit substantial failure of RNA-directed DNA elimination (data not shown).
Fig. 5.
DNA rearrangements of IESs and chromosome breakage are impaired in DRB2 mic knockouts. (A and B) Rearrangements of IES B (A) and the M IES (B) were assessed by two- or three-primer PCR, respectively, in genomic DNA isolated from wild-type (WT), ΔDCL1, and ΔDRB2 mic cells postconjugation. White arrowheads, the unrearranged/micronuclear form; black arrowheads, the rearranged/macronuclear form; unlabeled bands, nonspecific products. Diagrams of each IES locus are shown below the gel image. IES, white and dark gray boxes; flanking DNA, gray boxes; PCR primers, black and gray arrows. The M IES undergoes alternative rearrangement through elimination of the 0.6-kb (white box) or the 0.9-kb (white and dark gray boxes) sequence. The expected PCR product size is provide beside each form. (C) Chromosome breakage fails in DRB2 mic knockouts. (Left) Southern blot hybridization of total genomic DNA isolated from WT or mutant cells postconjugation. White arrowhead, micronucleus-specific fragment; gray arrowhead, parental macronucleus-specific fragment; black arrowhead, zygotic macronucleus-specific fragment. (Right) Diagram of CBS near the LIA1 locus in the micro- and macronuclei. Southern blot band sizes are listed next to each locus diagram. White circle, CBS; white arrow, LIA1 gene; Tel, telomere.
Assessment of chromosome breakage near the LIA1 locus also showed that DRB2 mic knockout progeny fail to properly fragment chromosomes (Fig. 5C). Before the completion of conjugation, the chromosomes in the zygotic macronuclei, which contain 5 chromosomes amplified to between 4 and 8 copies, are fragmented at approximately 180 chromosome breakage sites (CBSs) to produce the shortened macronuclear chromosomes. In knockouts of genes essential for genome rearrangement, including DCL1 and TWI1, chromosome breakage fails, as does IES elimination (42, 47). In a Southern blot assay of wild-type progeny, chromosome breakage at the LIA1 locus resulted in a band of approximately 2.5 kb in the zygotic macronuclei. The copies of this chromosome from the parental macronucleus were visible as a 2.6-kb band, as they have longer telomeres relative to newly fragmented ends. Unbroken micronuclear chromosomes were detected as a 10.5-kb band. The probe also detected a 7.8-kb fragment present in all nuclei. Due to the increased copy number of the locus in the macronucleus in the progeny of wild-type crosses, the 2.5-kb and 2.6-kb fragments are more intense than the larger 10.5-kb micronucleus-specific fragment. As in the control matings of DCL1 knockout cells, the postconjugation populations of DRB2 mic knockout crosses have increased levels of the 10.5-kb unrearranged fragment and lack the 2.5-kb fragment indicative of de novo chromosome breakage (Fig. 4C). A previous report on chromosome breakage in a somatic knockout of PDD1 showed that chromosome fragmentation was able to occur (15). Here we report that crosses of homozygous PDD1 knockout strains showed failure of chromosome breakage, as observed with DCL1 and DRB2 mic knockouts, emphasizing the importance of zygotic expression of PDD1 and DRB2 in chromosome breakage (Fig. 5C).
DRB2 colocalizes with Pdd1p in DNA elimination bodies.
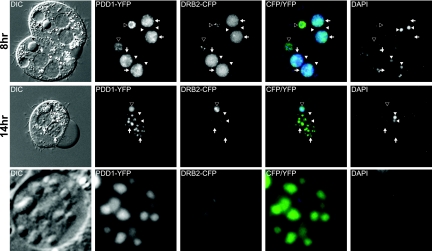
Failure of DNA elimination and chromosome breakage in DRB2 mic knockout strain matings indicated that the conjugation arrest phenotype described earlier was a result of failure to complete RNA-directed DNA elimination. The localization of Drb2p-CFP into large foci 14 h into conjugation, which is when DNA elimination normally occurs, prompted us to ascertain whether Drb2p-CFP was localized into DNA elimination bodies. These nuclear structures are enriched for the essential DNA elimination, chromodomain-containing protein Pdd1p and are the putative sites of IES removal. Strains expressing Drb2p-CFP or Pdd1p-YFP were mated, and localization of both proteins was monitored at 8 h into conjugation, very early in zygotic macronuclear differentiation, and later at 14 h into conjugation, when DNA elimination occurs (Fig. 6). As was previously reported, Pdd1-YFP was diffusely localized in the zygotic macronuclei at 8 h, and as conjugation proceeded toward DNA elimination around 14 h, Pdd1p-YFP localization gradually became unevenly dispersed, forming first small foci and then finally large foci (Fig. 6) (40, 41). Localization of Drb2p-CFP in the zygotic macronuclei at 8 h into conjugation was not markedly different from Pdd1p-YFP localization, with small Drb2p-CFP foci throughout the nucleus (Fig. 5). However, at 14 h into conjugation Drb2p-CFP foci aggregated into larger foci, which colocalized with the Pdd1p-YFP-containing DNA elimination bodies, indicating a possible interaction with each other in zygotic macronuclei.
Fig. 6.
Drb2p colocalizes with the essential conjugation chromodomain protein Pdd1p in DNA elimination bodies. Cells expressing Drb2p-CFP were mated with cells expressing Pdd1p-YFP. Both proteins localized to the developing zygotic macronucleus (8 h) and in DNA elimination bodies (14 h). The bottom panels show a magnified view of one zygotic macronucleus from the 14-h cell above. White arrowheads, micronuclei; black arrowheads, parental macronuclei; white arrows, zygotic macronuclei.
Localization of Pdd1-YFP and Drb2p-CFP is not exclusive to the zygotic macronuclei. Residual localization of both proteins was seen in the parental macronucleus as well. At 8 h into conjugation, both proteins formed strong, distinct foci in the parental macronucleus, with Pdd1p-YFP foci localized to the nuclear periphery and Drb2p-CFP foci found in the nuclear interior. During DNA elimination at 14 h into conjugation, remaining Pdd1p-YFP was found throughout the parental macronucleus but away from the interior, while Drb2p-CFP was still seen only in the interior. The significance of this late parental macronuclear localization remains to be explored.
Pdd1p fails to form DNA elimination bodies in DRB2 mic knockouts.
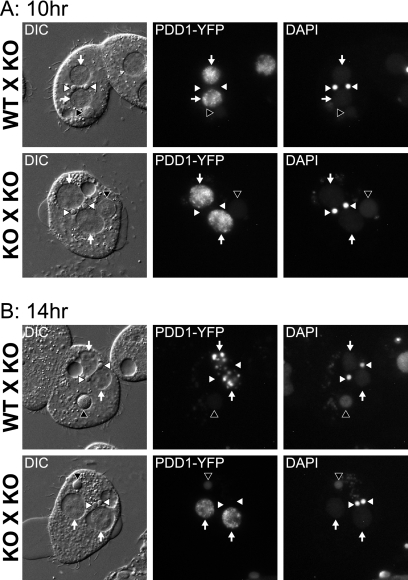
To understand if Pdd1p and Drb2p colocalization is relevant to the conjugation arrest phenotype and failure of DNA elimination in DRB2 mic knockouts, we sought to determine how Pdd1p localization was affected in DRB2 mic knockout strain matings. DRB2 mic knockout strains were transformed with an inducible Pdd1p-YFP expression construct, and the resulting transformants were mated and their Pdd1p-YFP localization was examined. At 10 h into conjugation during zygotic macronuclear differentiation, Pdd1p-YFP localization in both DRB2 mic knockouts crossed to wild-type strains, which rescues loss of DRB2 from the mating partner, and DRB2 mic knockout matings appeared mottled throughout the developing zygotic macronucleus without obvious defects (Fig. 7A). However, late in conjugation (14 h), Pdd1p-YFP failed to form DNA elimination bodies in zygotic macronuclei in DRB2 mic knockout matings (Fig. 7B). Thus, Pdd1p-YFP foci fail to mature into DNA elimination bodies without zygotic DRB2 expression. These data indicate that DRB2 participates in the maturation of DNA elimination bodies and implicates a possible role for uncharacterized dsRNAs in genome reorganization.
Fig. 7.
Failure of DNA elimination bodies to form in DRB2 mic knockouts late in conjugation. (A) Normal zygotic macronuclear localization of Pdd1p in DRB2 mic knockout matings midway through conjugation. DRB2 mic knockouts ectopically expressing Pdd1p-YFP were mated with wild-type or with DRB2 mic knockouts. Pdd1p-YFP localized to the developing zygotic macronucleus in both matings. White arrowheads, micronuclei; black arrowheads, parental macronuclei; white arrows, zygotic macronuclei. (B) Ectopically expressed Pdd1p fails to form DNA elimination bodies in DRB2 mic knockouts. DRB2 mic knockouts ectopically expressing Pdd1p-YFP were mated as described for panel A. When DRB2 mic knockouts were mated to DRB2 mic knockouts, Pdd1p-YFP failed to form DNA elimination bodies in the developing zygotic macronucleus. White arrowheads, micronuclei; black arrowheads, parental macronuclei; white arrows, zygotic macronuclei.
DISCUSSION
Our analyses of DRB1 and DRB2 have revealed that each has unique and important functions. While both are predominantly nuclear proteins, they localize into distinct subnuclear foci. Furthermore, disruption of the each gene showed that Drb2p has essential functions during both growth and development, while Drb1p appears to be important for prezygotic development. The similarities of these two proteins outside their predicted DSRMs suggest that they may have arisen from an ancestral gene duplication. If that is the case, they have significantly diverged in function since the duplication event.
Upon initial recognition that the T. thermophila genome encodes two DSRM-containing proteins, we looked for evidence that would connect them as protein partners for the Dicer homologues encoded by DCL1 and DCR2 (42, 49). Tandem DSRM-containing partner proteins for Dicer and Drosha family proteins, including R2D2, Loqs, and Pasha in D. melanogaster, RDE-4 in C. elegans, and TRBP2 and DGCR8 in H. sapiens and other mammals, play vital roles in RNAi by ensuring proper sRNA delivery and in many cases cleavage of sRNA precursors (12, 17, 23, 27, 37, 57, 65). Our analyses provided little support that Drb1p or Drb2p serve as major Dicer partners. Neither protein showed abundant localization in meiotic micronuclei, where Dcl1p acts (Fig. 2A and B, top rows) (42, 49). We also did not find defects in scnRNA accumulation in complete DRB1 knockouts (data not shown). As Drb2p is essential for growth, we were unable to generate full knockouts with which to examine scnRNA accumulation upon its loss. The T. thermophila Dicer protein, Dcr2p, is also essential for growth, but a previously published characterization of Dcr2p complexes did not find Drb2p to be an interacting protein (34, 35).
While we did not find evidence that these proteins act with Dcl1p, we uncovered a critical role for Drb2p in the RNAi-directed DNA elimination pathway. Loss of zygotic expression was sufficient to block DNA rearrangement; thus, Drb2p is needed well downstream of scnRNA biogenesis by Dcl1p (Fig. 5A and B). Colocalization of Drb2p with Pdd1p-containing DNA elimination bodies and loss of these DNA elimination bodies in DRB2 mic knockouts implicate zygotically expressed Drb2p in promoting development or stabilizing these large nucleoprotein structures (Fig. 6 and 7). This may indicate that Drb2p/RNA complexes mediate the formation of mature DNA elimination bodies through facilitating protein-RNA or protein-protein interactions within these structures. Although the exact mechanism of Drb2p action remains to be discovered, its importance in late stages of genome reorganization suggests an unrecognized role for dsRNA in RNAi-directed DNA elimination.
Drb2p is also required for vegetative growth, as we were unable to replace all wild-type DRB2 gene copies with the disrupted allele. We tried extensively to assort DRB2 out of the macronucleus without success (data not shown). Furthermore, when DRB2 partial knockout strains were grown in nonselective medium (without paromomycin), the remaining wild-type DRB2 copies rapidly replaced the DRB2 knockout allele (data not shown). As both Drb2p and Dcr2p are essential for growth, it remains possible that they act in the same pathway (34, 35). We cannot rule out the possibility that these proteins transiently interact, as do RDE-4 and DCR-1 in C. elegans (65). Further investigation of the function of Drb2p during growth may provide key insights into the role of this protein during both growth and genome reorganization.
While Drb1p is predominantly a macronuclear protein, it also localizes to one or both ends of the crescent micronucleus during the prophase of meiosis I (Fig. 2 and data not shown). Further investigation of this micronuclear point localization indicated that colocalization of Drb1p with cenH3, the centromeric histone H3 (unpublished data) (9, 16, 39). Knockouts of DRB1 were able to complete conjugation, yet a significant percentage of pairs aborted mating without forming new macronuclei. Together, the localization of Drb1p near centromeres and possibly with telomeres and the reduction in knockout cells completing prezygotic stages of development are consistent with a role for Drb1p in maintaining micronuclear chromosome structure (Fig. 2 and data not shown). Thus, the analysis of both of these DSRM-containing proteins strongly suggests that they perform critical chromosomal functions.
Although many tandem DSRM-containing proteins have been found to interact with Dicer and Drosha family proteins, this is by no means the only job that these proteins containing DSRMs undertake (12, 17, 23, 27, 37, 57, 65; reviewed in references 20 and 67). Roles for these proteins include cleavage of long noncoding RNAs into sRNAs by RNase III family members, RNA editing by the ADAR family, translation inhibition in response to viruses by PKR family members, and developmental RNA localization by the Staufen family (3, 4, 26, 28, 30, 36, 45, 50, 55, 62, 63). Besides the partner proteins for the Dicer and Drosha families, at least one other protein family, the NFAT family, also encodes tandem DSRMs. The NFAT family proteins, which contain a DZF protein domain in addition to tandem DSRMs, are putative nuclear, nucleotide transferases that participate in DNA repair and RNA transport (32, 59, 60, 73; reviewed in reference 31). Further study of DRB1 and DRB2 in T. thermophila may reveal new roles for tandem DSRM-containing proteins. The great evolutionary distance between ciliates and other eukaryotes could also facilitate understanding of how DSRM-containing proteins evolved within the eukaryotic lineage (54). Much remains to be gleaned about the roles of DSRM-containing proteins in eukaryotes, and we expect further investigation of Drb1p and Drb2p functions will provide greater understanding of RNAi-directed DNA elimination and roles for dsRNA in regulating chromosome structure.
ACKNOWLEDGMENT
This research was supported primarily by a grant from the National Science Foundation (NSF MCB-0642162) to D.L.C.
Footnotes
Published ahead of print on 21 October 2011.
REFERENCES
- 1. Altschuler M. I., Yao M. C. 1985. Macronuclear DNA of Tetrahymena thermophila exists as defined subchromosomal-sized molecules. Nucleic Acids Res. 13:5817–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Austerberry C. F., Allis C. D., Yao M. C. 1984. Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena. Proc. Natl. Acad. Sci. U. S. A. 81:7383–7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bass B. L., Weintraub H. 1988. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 55:1089–1098 [DOI] [PubMed] [Google Scholar]
- 4. Bernstein E., Caudy A. A., Hammond S. M., Hannon G. J. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366 [DOI] [PubMed] [Google Scholar]
- 5. Bondos S. E., et al. 2004. Hox transcription factor Ultrabithorax Ib physically and genetically interacts with disconnected interacting protein 1, a double-stranded RNA-binding protein. J. Biol. Chem. 279:26433–26444 [DOI] [PubMed] [Google Scholar]
- 6. Bruns P. J., Cassidy-Hanley D. 2000. Biolistic transformation of macro- and micronuclei. Methods Cell Biol. 62:501–512 [DOI] [PubMed] [Google Scholar]
- 7. Cassidy-Hanley D., et al. 2005. Genome-wide characterization of Tetrahymena thermophila chromosome breakage sites. II. Physical and genetic mapping. Genetics 170:1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cassidy-Hanley D., et al. 1997. Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics 146:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cervantes M. D., Xi X., Vermaak D., Yao M. C., Malik H. S. 2006. The CNA1 histone of the ciliate Tetrahymena thermophila is essential for chromosome segregation in the germline micronucleus. Mol. Biol. Cell 17:485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chalker D. L., Fuller P., Yao M. C. 2005. Communication between parental and developing genomes during Tetrahymena nuclear differentiation is likely mediated by homologous RNAs. Genetics 169:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chalker D. L., Yao M. C. 2001. Nongenic, bidirectional transcription precedes and may promote developmental DNA deletion in Tetrahymena thermophila. Genes Dev. 15:1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chendrimada T. P., et al. 2005. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436:740–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng C. Y., Vogt A., Mochizuki K., Yao M. C. 2010. A domesticated piggyBac transposase plays key roles in heterochromatin dynamics and DNA cleavage during programmed DNA deletion in Tetrahymena thermophila. Mol. Biol. Cell 21:1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conover R. K., Brunk C. F. 1986. Macronuclear DNA molecules of Tetrahymena thermophila. Mol. Cell. Biol. 6:900–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coyne R. S., Nikiforov M. A., Smothers J. F., Allis C. D., Yao M. C. 1999. Parental expression of the chromodomain protein Pdd1p is required for completion of programmed DNA elimination and nuclear differentiation. Mol. Cell 4:865–872 [DOI] [PubMed] [Google Scholar]
- 16. Cui B., Gorovsky M. A. 2006. Centromeric histone H3 is essential for vegetative cell division and for DNA elimination during conjugation in Tetrahymena thermophila. Mol. Cell. Biol. 26:4499–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Denli A. M., Tops B. B., Plasterk R. H., Ketting R. F., Hannon G. J. 2004. Processing of primary microRNAs by the Microprocessor complex. Nature 432:231–235 [DOI] [PubMed] [Google Scholar]
- 18. DeSousa D., et al. 2003. A novel double-stranded RNA-binding protein, Disco interacting protein 1 (DIP1), contributes to cell fate decisions during Drosophila development. J. Biol. Chem. 278:38040–38050 [DOI] [PubMed] [Google Scholar]
- 19. Doerder F. P., Deak J. C., Lief J. H. 1992. Rate of phenotypic assortment in Tetrahymena thermophila. Dev. Genet. 13:126–132 [DOI] [PubMed] [Google Scholar]
- 20. Doyle M., Jantsch M. F. 2002. New and old roles of the double-stranded RNA-binding domain. J. Struct. Biol. 140:147–153 [DOI] [PubMed] [Google Scholar]
- 21. Eisen J. A., et al. 2006. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 4:e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Q., Sweeney R., Yao M.-C. 1999. Creation and use of antisense ribosomes in Tetrahymena thermophila. Methods Cell Biol. 62:533–547 [DOI] [PubMed] [Google Scholar]
- 22a. Fass J. N., et al. 2011. Genome-scale analysis of programmed DNA elimination sites in Tetrahymena thermophila. G3 1:515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forstemann K., et al. 2005. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol. 3:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaertig J., Gu L., Hai B., Gorovsky M. A. 1994. High frequency vector-mediated transformation and gene replacement in Tetrahymena. Nucleic Acids Res. 22:5391–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. 1975. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 9:311–327 [DOI] [PubMed] [Google Scholar]
- 26. Green S. R., Mathews M. B. 1992. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase, DAI. Genes Dev. 6:2478–2490 [DOI] [PubMed] [Google Scholar]
- 27. Gregory R. I., et al. 2004. The Microprocessor complex mediates the genesis of microRNAs. Nature 432:235–240 [DOI] [PubMed] [Google Scholar]
- 28. Grishok A., et al. 2001. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106:23–34 [DOI] [PubMed] [Google Scholar]
- 29. Hamilton E., et al. 2005. Genome-wide characterization of Tetrahymena thermophila chromosome breakage sites. I. Cloning and identification of functional sites.. Genetics 170:1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim U., Wang Y., Sanford T., Zeng Y., Nishikura K. 1994. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc. Natl. Acad. Sci. U. S. A. 91:11457–11461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuchta K., Knizewski L., Wyrwicz L. S., Rychlewski L., Ginalski K. 2009. Comprehensive classification of nucleotidyltransferase fold proteins: identification of novel families and their representatives in human. Nucleic Acids Res. 37:7701–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larcher J. C., et al. 2004. Ilf3 and NF90 associate with the axonal targeting element of Tau mRNA. FASEB J. 18:1761–1763 [DOI] [PubMed] [Google Scholar]
- 33. Lee S. R., Collins K. 2006. Two classes of endogenous small RNAs in Tetrahymena thermophila. Genes Dev. 20:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee S. R., Collins K. 2007. Physical and functional coupling of RNA-dependent RNA polymerase and Dicer in the biogenesis of endogenous siRNAs. Nat. Struct. Mol. Biol. 14:604–610 [DOI] [PubMed] [Google Scholar]
- 35. Lee S. R., Talsky K. B., Collins K. 2009. A single RNA-dependent RNA polymerase assembles with mutually exclusive nucleotidyl transferase subunits to direct different pathways of small RNA biogenesis. RNA 15:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee Y., et al. 2003. The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419 [DOI] [PubMed] [Google Scholar]
- 37. Liu Q., et al. 2003. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science 301:1921–1925 [DOI] [PubMed] [Google Scholar]
- 38. Liu Y., et al. 2007. RNAi-dependent H3K27 methylation is required for heterochromatin formation and DNA elimination in Tetrahymena. Genes Dev. 21:1530–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loidl J., Scherthan H. 2004. Organization and pairing of meiotic chromosomes in the ciliate Tetrahymena thermophila. J. Cell Sci. 117:5791–5801 [DOI] [PubMed] [Google Scholar]
- 40. Madireddi M. T., et al. 1996. Pdd1p, a novel chromodomain-containing protein, links heterochromatin assembly and DNA elimination in Tetrahymena. Cell 87:75–84 [DOI] [PubMed] [Google Scholar]
- 41. Madireddi M. T., Davis M. C., Allis C. D. 1994. Identification of a novel polypeptide involved in the formation of DNA-containing vesicles during macronuclear development in Tetrahymena. Dev. Biol. 165:418–431 [DOI] [PubMed] [Google Scholar]
- 42. Malone C. D., Anderson A. M., Motl J. A., Rexer C. H., Chalker D. L. 2005. Germ line transcripts are processed by a Dicer-like protein that is essential for developmentally programmed genome rearrangements of Tetrahymena thermophila. Mol. Cell. Biol. 25:9151–9164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malone C. D., et al. 2008. Nucleus-specific importin alpha proteins and nucleoporins regulate protein import and nuclear division in the binucleate Tetrahymena thermophila. Eukaryot. Cell 7:1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martindale D. W., Allis C. D., Bruns P. 1982. Conjugation in Tetrahymena thermophila: a temporal analysis of cytological stages. Exp. Cell. Res. 140:227–236 [DOI] [PubMed] [Google Scholar]
- 45. Meurs E., et al. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379–390 [DOI] [PubMed] [Google Scholar]
- 46. Meyer E., Chalker D. L. 2007. Epigenetics of ciliates, p. 127–150.In Allis C. D., Jenuwein T., Reinberg D., Caparros M.-L. A. E. (ed.), Epigenetics. Cold Spring Harbor Press, Cold Spring Harbork, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mochizuki K., Fine N. A., Fujisawa T., Gorovsky M. A. 2002. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in Tetrahymena. Cell 110:689–699 [DOI] [PubMed] [Google Scholar]
- 48. Mochizuki K., Gorovsky M. A. 2004. Conjugation-specific small RNAs in Tetrahymena have predicted properties of scan (scn) RNAs involved in genome rearrangement. Genes Dev. 18:2068–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mochizuki K., Gorovsky M. A. 2005. A Dicer-like protein in Tetrahymena has distinct functions in genome rearrangement, chromosome segregation, and meiotic prophase. Genes Dev. 19:77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicholson R. H., Nicholson A. W. 2002. Molecular characterization of a mouse cDNA encoding Dicer, a ribonuclease III ortholog involved in RNA interference. Mamm. Genome 13:67–73 [DOI] [PubMed] [Google Scholar]
- 51. Nikiforov M. A., Gorovsky M. A., Allis C. D. 2000. A novel chromodomain protein, Pdd3p, associates with internal eliminated sequences during macronuclear development in Tetrahymena thermophila. Mol. Cell. Biol. 20:4128–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orias E., Hamilton E. P., Orias J. D. 2000. Tetrahymena as a laboratory organism: useful strains, cell culture, and cell line maintenance. Methods Cell Biol. 62:189–211 [DOI] [PubMed] [Google Scholar]
- 53. Parrish S., Fire A. 2001. Distinct roles for RDE-1 and RDE-4 during RNA interference in Caenorhabditis elegans. RNA 7:1397–1402 [PMC free article] [PubMed] [Google Scholar]
- 54. Philippe H., Germot A., Moreira D. 2000. The new phylogeny of eukaryotes. Curr. Opin. Genet. Dev. 10:596–601 [DOI] [PubMed] [Google Scholar]
- 55. Pires-daSilva A., et al. 2001. Mice deficient for spermatid perinuclear RNA-binding protein show neurologic, spermatogenic, and sperm morphological abnormalities. Dev. Biol. 233:319–328 [DOI] [PubMed] [Google Scholar]
- 56. Prescott D. M. 1994. The DNA of ciliated protozoa. Microbiol. Rev. 58:233–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saito K., Ishizuka A., Siomi H., Siomi M. C. 2005. Processing of pre-microRNAs by the Dicer 1-Loquacious complex in Drosophila cells. PLoS Biol. 3:e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saunders L. R., et al. 2001. Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 276:32300–32312 [DOI] [PubMed] [Google Scholar]
- 59. Schumacher J. M., Artzt K., Braun R. E. 1998. Spermatid perinuclear ribonucleic acid-binding protein binds microtubules in vitro and associates with abnormal manchettes in vivo in mice. Biol. Reprod. 59:69–76 [DOI] [PubMed] [Google Scholar]
- 60. Schumacher J. M., Lee K., Edelhoff S., Braun R. E. 1995. Spnr, a murine RNA-binding protein that is localized to cytoplasmic microtubules. J. Cell Biol. 129:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shang Y., et al. 2002. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc. Natl. Acad. Sci. U. S. A. 99:3734–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. St. Johnston D., Beuchle D., Nusslein-Volhard C. 1991. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell 66:51–63 [DOI] [PubMed] [Google Scholar]
- 63. St. Johnston D., Brown N. H., Gall J. G., Jantsch M. 1992. A conserved double-stranded RNA-binding domain. Proc. Natl. Acad. Sci. U. S. A. 89:10979–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stover N. A., et al. 2006. Tetrahymena Genome Database (TGD): a new genomic resource for Tetrahymena thermophila research. Nucleic Acids Res. 34:D500–D503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tabara H., Yigit E., Siomi H., Mello C. C. 2002. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 109:861–871 [DOI] [PubMed] [Google Scholar]
- 66. Taverna S. D., Coyne R. S., Allis C. D. 2002. Methylation of histone H3 at lysine 9 targets programmed DNA elimination in tetrahymena. Cell 110:701–711 [DOI] [PubMed] [Google Scholar]
- 67. Tian B., Bevilacqua P. C., Diegelman-Parente A., Mathews M. B. 2004. The double-stranded RNA-binding motif: interference and much more. Nat. Rev. Mol. Cell Biol. 5:1013–1023 [DOI] [PubMed] [Google Scholar]
- 68. Wickham L., Duchaine T., Luo M., Nabi I. R., DesGroseillers L. 1999. Mammalian Staufen is a double-stranded RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol. Cell. Biol. 19:2220–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woodard J., Kaneshiro E., Gorovsky M. A. 1972. Cytochemical studies on the problem of macronuclear subnuclei in tetrahymena. Genetics 70:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yao M. C., Gorovsky M. A. 1974. Comparison of the sequences of macro- and micronuclear DNA of Tetrahymena pyriformis. Chromosoma 48:1–18 [DOI] [PubMed] [Google Scholar]
- 71. Yao M. C., et al. 2007. Identification of novel chromatin-associated proteins involved in programmed genome rearrangements in Tetrahymena. J. Cell Sci. 120:1978–1989 [DOI] [PubMed] [Google Scholar]
- 72. Zdobnov E. M., Apweiler R. 2001. InterProScan: an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848 [DOI] [PubMed] [Google Scholar]
- 73. Zhao G., Shi L., Qiu D., Hu H., Kao P. N. 2005. NF45/ILF2 tissue expression, promoter analysis, and interleukin-2 transactivating function. Exp. Cell Res. 305:312–323 [DOI] [PubMed] [Google Scholar]