Abstract
Two-component signaling pathways based on phosphoryl group transfer between histidine kinase and response regulator proteins regulate environmental responses in bacteria, archaea, plants, slime molds, and fungi. Here we characterize a mutant form of DCC-1, a putative histidine kinase encoded by the NCU00939 gene of the filamentous fungus Neurospora crassa. We show that this protein participates in the regulation of processes such as conidiation, perithecial development, and, to a certain degree, carotenogenesis. Furthermore, DCC-1 is suggested to exert its effect by promoting cyclic AMP production, thereby placing this protein within the context of a signaling pathway.
TEXT
Signal transduction by phosphorylation and dephosphorylation of cellular proteins plays pivotal roles in regulating numerous cellular processes in both prokaryotes and eukaryotes. In prokaryotes, signaling by phosphoryl group transfer reactions relies on two-component signal transduction (TCS) pathways that depend on histidine and aspartyl residues as phosphoryl group donors and acceptors (24, 26, 39, 43). The prototypical two-component system comprises two protein components, a sensor histidine kinase (HK) and its cognate response regulator (RR), that contain a transmitter domain with an invariant histidine residue and a receiver domain with an invariant aspartate residue, respectively. Signal reception by the HK is believed to propagate conformational changes in the protein that stimulate an ATP-dependent autophosphorylation at the conserved histidine residue. The phosphokinase then donates the phosphoryl group to the conserved aspartate residue in the receiver domain of the cognate RR, thereby rendering it functional, in general as a transcriptional regulator (26, 39). Upon cessation of signaling, both the cognate RR and the HK undergo dephosphorylation, which results in silencing of the system (11). Many TCS pathways, however, are more elaborate and operate through a phosphorelay (7, 12, 23, 28, 40). In these systems, signal transmission involves, in addition to the two above-mentioned domains, an extra receiver domain and a histidine-containing phosphotransfer domain (HPT) that can be fused to the HK or can be present as independent proteins (12, 17, 20, 28, 40). Such TCS pathways have also been found in plants, slime molds, and fungi (8, 13, 28). It is noteworthy that most bacterial sensor kinases possess only one transmitter domain, whereas the sensor kinases present in eukaryotic microorganisms or plants are almost exclusively multipartite kinases (8, 13, 28, 33). Curiously, the architecture of filamentous fungal TCS pathways provides another notable difference. Prokaryotic TCS pathways are typically organized in protein pairs, whereas in filamentous fungi, multiple HKs appear to share a single HPT protein to transduce signals to two RRs. For instance, the Neurospora crassa genome predicts 11 hybrid HKs, one HPT protein, and two canonical RRs, in addition to the RR protein encoded by the NCU07378 gene and homologous to Saccharomyces cerevisiae Rim15p, which lacks the conserved aspartate residue of its receiver domain (5).
A number of studies have addressed the functions and phenotypes of various TCS proteins in N. crassa. For instance, it has been shown that RRG-1 is involved in the activation of a mitogen-activated protein kinase (MAPK) pathway that regulates female fertility and responses to osmotic and fungicide stresses (18). On the other hand, RRG-2 is implicated in cell responses against oxidative stress during vegetative growth (4). Moreover, the HPT-1 protein has been suggested to play a central role in N. crassa viability because attempts to delete the corresponding gene have been fruitless (4). Finally, the function of the OS-1, PHY-1, and PHY-2 HKs has also been addressed in N. crassa. OS-1 has been shown to participate in the activation of the above-mentioned MAPK pathway (1, 18, 36) and therefore has been placed upstream of RRG-1 in the signaling cascade responsive to osmotic and fungicide stresses (18). PHY-1 and PHY-2 show sequence similarity to bacteriophytochromes and plant phytochromes (5), and a bacterially expressed N-terminal fragment of PHY-2 was shown to covalently bind either biliverdin or phycocyanobilin in vitro, with the resulting holoprotein displaying red/far-red light photochromic absorption spectra and a photocycle. However, deletions of phy-1 and phy-2 cause no phenotypic defects (10), although they produce alterations in the expression of the light-responsive conidiation gene con-10 (25).
Here we describe the results of experiments demonstrating that the protein encoded by the NCU00939 gene, a putative histidine sensor kinase, participates in the regulation of asexual sporulation, perithecial development, and, to a certain degree, carotenogenesis. We therefore propose to coin the NCU00939-encoded protein DCC-1, for development and carotenogenesis control, and hereafter this protein will be referred to as DCC-1. Moreover, we provide experimental data indicating that DCC-1 most likely exerts its effect by promoting cyclic AMP (cAMP) production, thereby placing DCC-1 within the context of a signaling pathway that operates during conidiation and sexual development.
Probing for phenotypic differences in N. crassa HK mutants.
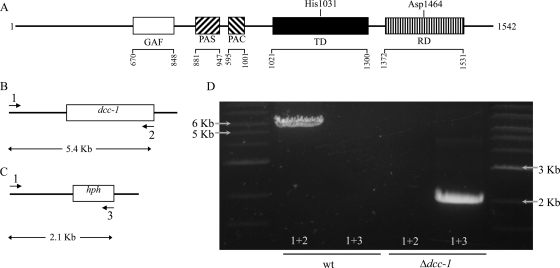
The N. crassa genome predicts 11 hybrid HKs, one HPT protein, and two canonical RRs (5). Although various studies have addressed the function of the RRs and HPT, only three HKs have been studied. To gain some insight into the TCS pathways and the functions of the various HK proteins, we pursued a phenotypic characterization of single HK deletion mutants. All strains were provided by the Fungal Genetics Stock Center (FGSC) (22), except the NCU04615 mutant, which was made in this study according to the previously published knockout procedure (9). All strains were confirmed by PCR (data not shown). For example, the dcc-1 mutant (FGSC catalog no. 13652) was verified by PCR using primers 5′-AGCCGCAAGTGGGAGCTACAT-3′ (placed upstream of the dcc-1 gene and indicated as primer 1 in Fig. 1B and C), 5′-CCAATGCATCTATGGCTCTCTATCGTAC-3′ (placed within the dcc-1 gene and indicated as primer 2 in Fig. 1B), and 5′-TTGGGCTTGGCTGGAGCTAGT-3′ (placed within the hph gene and indicated as primer 3 in Fig. 1C). As shown in Fig. 1, primers 1 and 2 amplified a band of the expected size (5.4 kb) in the wild-type strain but not in the mutant strain (Fig. 1D, lanes 2 and 4). On the other hand, primers 1 and 3 amplified a band of the expected size (2.1 kb) in the mutant strain but not in the wild-type strain (Fig. 1D, lanes 3 and 5), thereby confirming the replacement of the dcc-1 gene with hph.
Fig. 1.
Schematic representation of the NCU00939-encoded protein (hereafter referred to as DCC-1) and verification of NCU00939 gene deletion. (A) SMART analysis of the DCC-1 protein reveals the presence of GAF and PAS/PAC as sensing domains and transmitter (TD) and receiver (RD) domains with conserved histidine (H1031) and aspartate (D1464) residues. (B and C) Localization of the primers used for verification of the dcc-1 deletion and the sizes of the expected PCR products in the wild-type and mutant strains. (D) Products obtained by PCR using primers 1 and 2 and chromosomal DNA from the wild-type (wt) strain (lane 2) or the dcc-1 deletion mutant (lane 4) or primers 1 and 3 and DNA from the wild-type strain (lane 3) or the dcc-1 deletion mutant (lane 5).
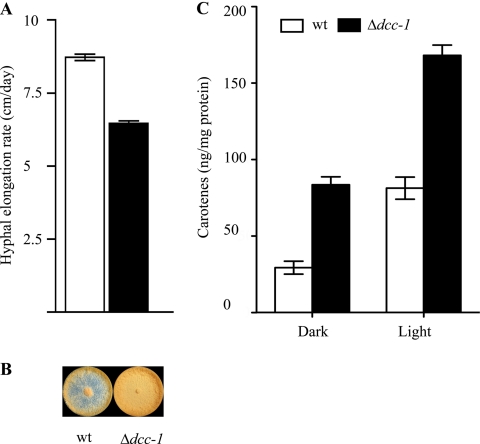
We observed that all mutants germinated and grew as well as the wild-type strain (FGSC catalog no. 2489) (data not shown), except for the Δdcc-1 mutant strain, which exhibited a slightly lower hyphal elongation rate (Fig. 2A). Moreover, although no defects in radial growth were observed in any of the mutants, a striking difference was noticeable in the dcc-1 deletion strain. Orange pigmentation, representative of carotenoid accumulation, was observed over the entire surface of the plate inoculated with the dcc-1 mutant strain, whereas in the case of the wild type, orange pigmentation was observed mainly at the center and near the edge of each plate (Fig. 2B). Because of these apparent differences, we decided to focus our efforts on the characterization of the dcc-1 mutant strain. The dcc-1 gene encodes a putative hybrid HK, which is conserved among Sordariomycetes but whose function is unknown. The SMART protein domain prediction program (21, 35) revealed the presence of GAF and PAS/PAC motifs as sensing or input domains and the conserved histidine and aspartate residues located at positions 1031 and 1464 in the transmitter and receiver domains, respectively (Fig. 1A).
Fig. 2.
Effect of DCC-1 on N. crassa vegetative growth and carotenoid accumulation. (A) The wild-type (wt) strain (open bars) and the dcc-1 mutant (closed bars) were grown on VSM agar in race tubes. The apical elongation rate was monitored for 5 days and is expressed as cm/day. The experiment was repeated three times in its entirety, and the standard deviation is depicted. (B) The wild-type (left) and dcc-1 mutant (right) strains were inoculated onto VGM plates. After 36 h of incubation in darkness, the plates were illuminated for 24 h. The images presented are representative of the growth of both strains in glucose-rich medium. (C) Duplicate cultures of the wild-type (open bars) and dcc-1 mutant (closed bars) strains were inoculated into liquid VSM supplemented with 0.2% Tween. After incubation for 36 h in darkness, one of the cultures was illuminated for 24 h while the other one was kept in darkness. Carotenoids were extracted from 0.1 g of mycelia, and the absorbance at 475 nm was determined. The data, expressed as ng carotenes/mg protein, represent the average of three independent experiments, and the standard deviation is depicted.
Carotenes accumulate in the dcc-1 mutant strain.
Carotenoids are widespread terpenoid pigments synthesized by all photosynthetic organisms (6, 16), some heterotrophic bacteria (2), and fungi (3, 32). In N. crassa, the characteristic orange pigmentation of mycelia is caused by the accumulation of the xanthophyll neurosporaxanthin and variable amounts of carotene precursors.
To test whether DCC-1 affects mycelial carotenoid accumulation, 1 × 106 wild-type or dcc-1 mutant spores were inoculated into liquid Vogel's sucrose medium (VSM) plates supplemented with 0.2% Tween 80 as a wetting agent to avoid conidiation (44). After 36 h of incubation in darkness, the cultures were divided into two groups; one was illuminated at 22°C with a fluorescent lamp (855 lx), and the other was maintained in darkness as a control. After 12 h of exposure to light, mycelia were collected, frozen in liquid nitrogen, and disrupted with a mortar and pestle. Acetone and hexane were used in consecutive extractions of total carotenoids from 0.1 g of frozen sample, and the maximal absorption at 475 nm was measured. It was found that after illumination, the dcc-1 mutant produced 2-fold more carotenes than the wild-type strain (Fig. 2C). More interestingly, the dcc-1 mutant accumulated significant amounts of carotenes even in darkness, in contrast to the wild-type strain, where no carotenoids were detected (Fig. 2C). It has to be noted that although the growth conditions were aimed to repress conidiation, a considerable amount of conidia was detected in the dcc-1 deletion strain (data not shown), and therefore, the total amount of mycelial and conidial carotenoids is presented for this strain. Although the experiments described here cannot differentiate between a direct effect of DCC-1 on carotene regulation and an indirect effect through the activation of conidiation, this result suggests that DCC-1 plays a significant role in the pathway that controls carotenogenesis and even asexual sporulation.
The DCC-1 HK regulates conidiation of N. crassa.
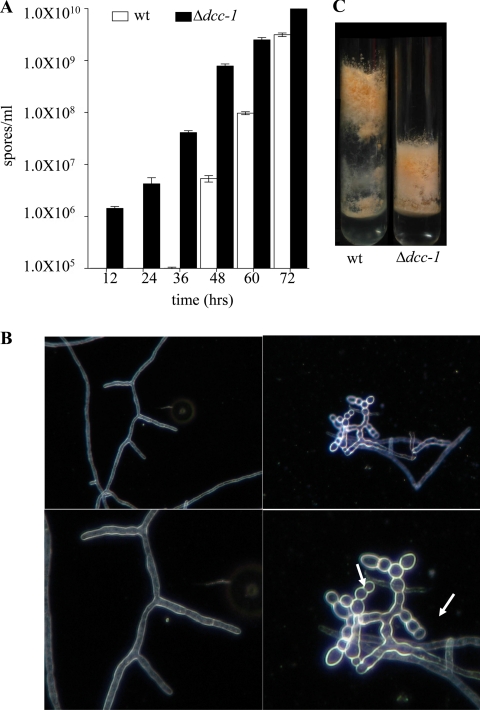
N. crassa is a filamentous fungus that has a complex life cycle due to its ability to produce both asexual (vegetative phase of growth) and sexual spores (38). During vegetative growth, N. crassa hyphae elongate, intertwine, and fuse, forming the mycelial network. Under carbon source deprivation and when a water-air interface exists, N. crassa enters its asexual developmental cycle, during which aerial hyphae differentiate from mycelia, generate chains of proconidia, and terminate with the septation of proconidia and the release of free mature conidia (macroconidia). We therefore argued that if the DCC-1 HK acts as a repressor of the process of conidiation, growing the dcc-1 mutant on glucose-rich medium and incubating the plates in complete darkness should result in augmented conidiation. This was tested by inoculating Vogel's glucose medium (VGM) plates with 1 × 103 wild-type or mutant conidia, incubating the plates at 30°C in complete darkness, and harvesting and counting the conidia every 12 h. It was found that the mutant strain produced conidia as early as 12 h after inoculation, unlike the wild-type strain, in which conidia were detected after 48 h. Remarkably, even at 72 h after inoculation, the mutant strain produced approximately 3.5 times more conidia than the wild type (Fig. 3A).
Fig. 3.
Effect of DCC-1 on conidiation of N. crassa. (A) The wild-type (wt; open bars) and dcc-1 deletion mutant (closed bars) strains were grown on VGM plates. Conidia were harvested and counted at the times indicated. The data, expressed as spores/ml, represent the average of three independent assays. (B) The wild type (left) and the dcc-1 mutant (right) were grown in liquid VSM at 30°C with agitation (200 rpm). After 16 h of incubation in darkness, an aliquot was withdrawn and examined by light microscopy. Arrows indicate conidia formed by the mutant strain. (C) Cultures of the wild-type (left) and dcc-1 mutant (right) strains were grown in standing liquid VSM cultures. After 3 days of incubation at 30°C in darkness, tubes were illuminated for 24 h and photographed.
As mentioned above, formation of aerial hyphae and subsequent conidiation require an air-water interface, and therefore conidiation does not occur in submerged cultures (37). Given the above-described phenotype of increased conidiation under growth-repressing conditions (glucose-rich medium and darkness), we asked whether the dcc-1 mutant also produces conidia in submerged cultures. We therefore inoculated wild-type and mutant strains into VSM at 1 × 106 spores/ml and incubated the cultures at 30°C with agitation (200 rpm) in complete darkness. After 16 h of incubation, a sample of each strain was withdrawn and examined by light microscopy. As expected, no conidia were observed in submerged cultures of the wild type. In contrast, the mutant strain produced a significant amount of conidia (Fig. 3B). Also, the hyphae of the mutant strain appeared to be shorter than those of the wild-type strain (Fig. 3B). This is to be expected if the hyphae of the mutant strain were to enter the program of conidiation prematurely. This was tested by comparing the growth of the wild-type and mutant strains in standing liquid VSM cultures. As shown in Fig. 3C, the wild-type strain conidiated at the tip of the elongated aerial hyphae, whereas dense premature conidiation and no elongated aerial hyphae were observed in the mutant strain (Fig. 3C). Premature conidiation could also provide a plausible explanation of the above-described slower lineal growth of the dcc-1 mutant (Fig. 2A). In view of the above results, it can be concluded that the N. crassa DCC-1 HK acts as a negative regulator of the asexual sporulation pathway, since its absence causes derepression of conidiation under nonsporulating conditions.
The DCC-1 HK is required for proper perithecial development in N. crassa.
Given the significant role of DCC-1 as a negative regulator of sporulation during vegetative growth, we asked whether it also participates in the regulation of sexual development. Under conditions of nitrogen limitation, N. crassa enters a sexual life cycle during which vegetative hyphae initiate a sexual differentiation pathway to form the female reproductive structure (protoperithecium) (27, 30). The protoperithecium consists of a multicellular hypha (or ascogonium) enclosed within an aggregate of knotted vegetative hyphae. A specialized hypha (trichogyne) extends from the protoperithecium and fuses with a fertilizing (male) cell. N. crassa has two distinct mating types (A and a), and fusion with the opposite mating type is required for fertilization of the protoperithecium. After fertilization, the protoperithecium enlarges and differentiates to form a fruiting body (or perithecium) consisting of several specialized tissues from which the meiotic products (ascospores) are eventually ejected into the air (14, 29).
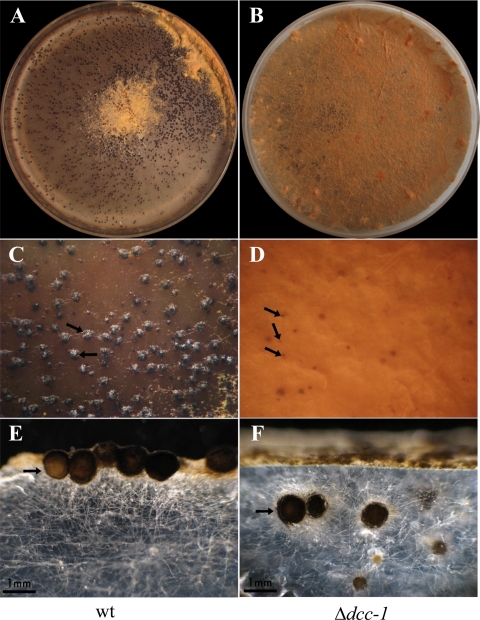
To test for possible effects of the DCC-1 HK on the processes of the N. crassa sexual cycle, the 74-ORS-6a (FGSC catalog no. 4200) wild-type and dcc-1 mutant strains were inoculated as females onto 10-cm plates under nitrogen-limiting conditions (Vogel's N modified medium [31]) and incubated at 25°C with constant light. At day 7 postinoculation, wild-type strain 74-OR23-1VA (FGSC catalog no. 2489) was inoculated as a male and the plates were incubated at 25°C with constant light. Seven days later, it was observed that, in contrast to the wild-type strain, which formed mature pigmented perithecia on the surface of the plate, only an insignificant number of small unpigmented mutant fruiting bodies were observed on the surface of the plate (Fig. 4A to D), suggesting that deletion of dcc-1 leads to sterility. However, after careful inspection of the plates inoculated with the mutant strain, it was observed that an almost equal number of mature perithecia were formed, although they were submerged in the agar (Fig. 4F). This phenotype should result in the ascospores, if they were formed, remaining enclosed in the perithecia or being ejected into the agar but unable to be released into the air. To test whether mature and viable ascospores are produced in the dcc-1 mutant, submerged perithecia were harvested from the agar and disrupted with a homogenizer to release the ascospores. Indeed, mature ascospores able to germinate after heat activation and form colonies at percentages similar to those of the wild type were obtained (data not shown). It can therefore be concluded that although the DCC-1 HK does not affect ascospore production and viability, it is required for proper localization of perithecia.
Fig. 4.
DCC-1 is required for proper perithecial development in N. crassa. The 74-ORS-6a (FGSC catalog no. 4200) wild-type (wt; A, C, and E) and dcc-1 mutant (B, D, and F) strains were inoculated as females onto Vogel's N modified agar plates. After 7 days of incubation at 25°C in the presence of light, the plates were inoculated with strain 74-OR23-1VA (FGSC catalog no. 2489) as the male, and 7 days later, the plates were examined. Representative pictures of the surface of the wild type (A, C) and the dcc-1 mutant (B, D) are presented. The cross sections of the plates with the wild-type (E) and Δdcc-1 mutant (F) strains are presented. Arrows indicate the localization of the perithecia formed.
Deletion of dcc-1 results in unmasking circadianly regulated conidiation of N. crassa.
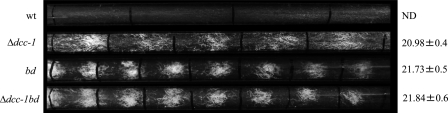
Circadian oscillators, which respond to environmental signals such as light and temperature, are known to control the expression of various genes whose products are associated with cellular processes, including development (42). The most obvious and prominent circadianly controlled rhythm in N. crassa is that of conidiation, which forms a characteristic conidial banding-pattern on plates or in long glass culture tubes (race tubes). Also, it has been previously reported that RRG-1 of N. crassa functions in an output pathway to regulate circadian rhythmicity, although the upstream HK was not identified (41). Because of the intimate association of the DCC-1 sensor HK with the process of conidiation and the involvement of a two-component system protein (RRG-1) in the regulation of rhythmicity (2), we asked whether DCC-1 also affects this process. To test this, we assayed the dcc-1 deletion strain for the formation of conidiation bands in race tubes with glucose-arginine medium. It has to be mentioned that the wild-type strain does not form the conidial banding pattern. This is because CO2 levels become elevated during vegetative growth, and as a consequence, conidiation becomes suppressed, thereby masking the rhythmic banding pattern. Therefore, a ras-1 mutant strain (FGSC catalog no. 1858), carrying a T79I substitution and referred to as a bd mutant (34), which is about 200-fold less sensitive to the CO2 masking effect, is used for circadian rhythm studies.
Exponentially grown mycelia of the wild-type, dcc-1, bd, and dcc-1 bd mutant strains were inoculated at one side of the race tubes and incubated with illumination at 25°C for 24 h to entrain the cultures before a shift to constant darkness. The edge of the colony was marked every 24 h under a red security light, and the period was determined. It was found that the dcc-1 deletion strain formed conidiation bands, although they were not as intense as those formed by the bd mutant strain (Fig. 5). This could be because the dcc-1 mutant conidiates throughout the race tube, which causes a partial masking of the conidiation bands (Fig. 5). The period of the dcc-1 mutant was found to be 20.98 ± 0.4 h, which is insignificantly shorter than the 21.73 ± 0.5- and 21.84 ± 0.6-h periods of the bd and the dcc-1 bd mutant strains, respectively (Fig. 5). Therefore, it can be concluded that the DCC-1 protein plays a role not in the regulation of circadian rhythmicity but rather in the masking process of circadianly regulated conidiation.
Fig. 5.
DCC-1 deletion unmasks circadianly regulated conidiation of N. crassa. Circadianly regulated conidiation patterns of the wild-type (wt) and dcc-1, bd, and dcc-1 bd mutant strains were tested in race tubes. To entrain the cultures, tubes were kept for 24 h in constant light. Tubes were then shifted to constant darkness, and growth was monitored every 24 h. The periods of the dcc-1, bd, and dcc-1 bd mutant strains are expressed in hours. The wild-type strain period was not determined (ND).
Exogenous cAMP reverses the dcc-1-dependent differentiation phenotypes.
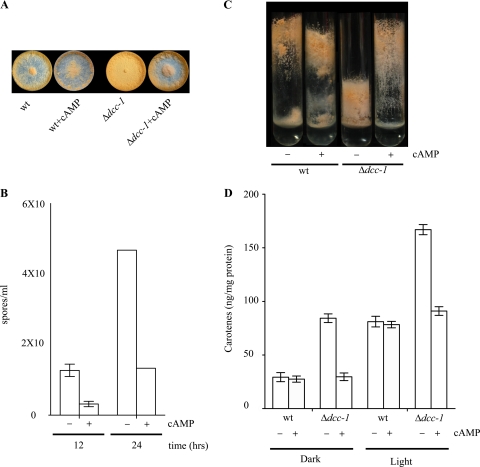
To gain some insight into the pathway(s) through which dcc-1 exerts its effect, we looked for mutant strains that exhibit phenotypes similar to the ones described for the dcc-1 mutant strain. Interestingly, similar phenotypes have been described for mutants encoding proteins that are implicated in cAMP metabolism. For example, a gna-3 (Gα3 subunit which, together with CR-1, participates in cAMP production) mutant shows dense premature conidiation and formation of short aerial hyphae, two phenotypes that are reversed by exogenous cAMP (19). Another phenotype due to Δgna-3 that is not reversed by exogenous cAMP is the formation of submerged perithecia (19). Considering the similar phenotypes of the gna-3 and dcc-1 mutants, the ability of cAMP to reverse the Δdcc-1-dependent phenotypes was tested. It was found that addition of cAMP to solid VGM and standing liquid cultures resulted in a drastic reduction of premature conidiation (Fig. 6A and B) and improved aerial hypha elongation of the Δdcc-1 mutant (Fig. 6C). It should be mentioned that, as has been reported for the Δgna-3 strain (19), cAMP addition to submerged cultures does not reverse the conidiation phenotype of the Δdcc-1 mutant strain (data not shown). Furthermore, carotenoid accumulation in the mutant strain was reduced to wild-type levels (Fig. 6D) whereas conidiation was decreased approximately 2-fold when the liquid VSM containing 0.2% Tween 80 as a wetting agent was supplemented with cAMP (data not shown). It may therefore be concluded that DCC-1 participates in the regulation of asexual sporulation and carotenogenesis most likely by affecting cAMP metabolism in a pathway that may include GNA-3 and CR-1.
Fig. 6.
Addition of cAMP restores the dcc-1 mutant phenotypes. (A) The wild-type (wt; left) and dcc-1 mutant (right) strains were inoculated into VGM with or without 2 mM cAMP. After 36 h of incubation in darkness, the plates were illuminated for 24 h. The images presented are representative of the growth of both strains in glucose-rich medium with or without cAMP. (B) The dcc-1 deletion mutant was grown in darkness on VGM plates with or without cAMP. Conidia were harvested and counted at the times indicated. The data, expressed as spores/ml, represent the average of three independent assays, and the standard deviation is depicted. (C) Cultures of the wild-type and dcc-1 mutant strains were grown in standing liquid VSM cultures with or without 2 mM cAMP. After 3 days of incubation at 30°C in darkness, tubes were illuminated for 24 h and photographed. (D) Duplicate cultures of the wild-type and dcc-1 mutant strains were inoculated into liquid VSM with or without 2 mM cAMP. After incubation for 36 h in darkness, one of the cultures was illuminated for 24 h while the other one was kept in darkness. Carotenoids were extracted from 0.1 g of mycelia, and the absorbance at 475 nm was determined. The data, expressed as ng carotenes/mg protein, represent the average of three independent experiments.
Finally, the effect of cAMP in circadianly regulated conidiation could not be tested because endogenous oscillations of the level of cAMP have been shown to be critical for clock-controlled conidiation (15). Also, as has been described for the Δgna-3 mutant (19), supplementation with cAMP did not restore the sexual defects (formation of submerged perithecia) of the Δdcc-1 mutant (data not shown).
Taken together, our findings strongly suggest that the DCC-1 protein of N. crassa plays a pivotal role in the pathways that regulate developmental processes such as conidiation and perithecial development and in the one responsible for masking circadianly regulated conidiation during vegetative growth and also, to a certain degree, affects carotenoid accumulation.
Conclusions.
The N. crassa NCU00939 gene encodes a putative hybrid HK consisting of GAF and PAS/PAC as input domains, a transmitter domain with a conserved histidine residue at position 1031, and a receiver domain with a conserved aspartate residue at position 1464. Phenotypic analyses of a DCC-1 mutant strain (FGSC catalog no. 13652) indicate that this putative sensor HK negatively regulates the processes of conidiation and, to a certain degree, carotenogenesis and also has pronounced effects on the development of perithecia during the sexual life cycle. Moreover, DCC-1 appears to exert its effect by affecting cAMP metabolism in a pathway that includes GNA-3 and CR-1, thereby placing DCC-1 within the context of a signaling pathway that operates during conidiation and sexual development. Although this study provides some insight into the processes regulated by DCC-1, the scope of its control remains to be clarified and the other components participating in the same signaling cascade(s) have yet to be determined.
Acknowledgments
We thank Jorge Nieto-Sotelo for critically reading the manuscript and Claudia Rodríguez Rangel for technical assistance.
This work was supported by grants 80684 from the Consejo Nacional de Ciencia y Tecnología (CONACyT); IN219709-3 from DGAPA, UNAM; and CRPMEX008-02 from ICGEB.
ADDENDUM IN PROOF
The observed phenotypes were found to segregate with the hygromycin B resistance gene (hph), indicating that they are the result of the deletion of the dcc-1 gene.
Footnotes
Published ahead of print on 4 November 2011.
REFERENCES
- 1. Alex L. A., Borkovich K. A., Simon M. I. 1996. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc. Natl. Acad. Sci. U. S. A. 93: 3416–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong G. 1997. Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu. Rev. Microbiol. 51: 629–659 [DOI] [PubMed] [Google Scholar]
- 3. Avalos J., Cerdá-Olmedo E. 2004. Fungal carotenoid production. Marcel Dekker, New York, NY [Google Scholar]
- 4. Banno S., et al. 2007. Roles of putative His-to-Asp signaling modules HPT-1 and RRG-2, on viability and sensitivity to osmotic and oxidative stresses in Neurospora crassa. Curr. Genet. 51: 197–208 [DOI] [PubMed] [Google Scholar]
- 5. Borkovich K. A., et al. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68: 1–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Britton G., Liaaen-Jensen S., Pfander H. 2004. Carotenoids: handbook. Birkhäuser, Boston, MA [Google Scholar]
- 7. Burbulys D., Trach K. A., Hoch J. A. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64: 545–552 [DOI] [PubMed] [Google Scholar]
- 8. Catlett N. L., Yoder O. C., Turgeon B. G. 2003. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot. Cell 2: 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colot H. V., et al. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U. S. A. 103: 10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Froehlich A. C., Noh B., Vierstra R. D., Loros J., Dunlap J. C. 2005. Genetic and molecular analysis of phytochromes from the filamentous fungus Neurospora crassa. Eukaryot. Cell 4: 2140–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Georgellis D., Kwon O., De Wulf P., Lin E. C. 1998. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem. 273: 32864–32869 [DOI] [PubMed] [Google Scholar]
- 12. Georgellis D., Lynch A. S., Lin E. C. 1997. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J. Bacteriol. 179: 5429–5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grefen C., Harter K. 2004. Plant two-component systems: principles, functions, complexity and cross talk. Planta 219: 733–742 [DOI] [PubMed] [Google Scholar]
- 14. Harris J. L., Howe H. B., Jr., Roth I. L. 1975. Scanning electron microscopy of surface and internal features of developing perithecia of Neurospora crassa. J. Bacteriol. 122: 1239–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasunuma K., Funadera K., Shinohara Y., Furukawa K., Watanabe M. 1987. Circadian oscillation and light-induced changes in the concentration of cyclic nucleotides in Neurospora. Curr. Genet. 12: 127–133 [Google Scholar]
- 16. Hirschberg J. 2001. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 4: 210–218 [DOI] [PubMed] [Google Scholar]
- 17. Hoch J. A. 1993. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu. Rev. Microbiol. 47: 441–465 [DOI] [PubMed] [Google Scholar]
- 18. Jones C. A., Greer-Phillips S. E., Borkovich K. A. 2007. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol. Biol. Cell 18: 2123–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kays A. M., Rowley P. S., Baasiri R. A., Borkovich K. A. 2000. Regulation of conidiation and adenylyl cyclase levels by the Galpha protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20: 7693–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwon O., Georgellis D., Lin E. C. 2000. Phosphorelay as the sole physiological route of signal transmission by the arc two-component system of Escherichia coli. J. Bacteriol. 182: 3858–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Letunic I., Doerks T., Bork P. 2009. SMART 6: recent updates and new developments. Nucleic Acids Res. 37: D229–D232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCluskey K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52: 245–262 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen A. N., Lee A., Place W., Shiozaki K. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11: 1169–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nixon B. T., Ronson C. W., Ausubel F. M. 1986. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc. Natl. Acad. Sci. U. S. A. 83: 7850–7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olmedo M., Ruger-Herreros C., Luque E. M., Corrochano L. M. 2010. A complex photoreceptor system mediates the regulation by light of the conidiation genes con-10 and con-6 in Neurospora crassa. Fungal Genet. Biol. 47: 352–363 [DOI] [PubMed] [Google Scholar]
- 26. Parkinson J. S., Kofoid E. C. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26: 71–112 [DOI] [PubMed] [Google Scholar]
- 27. Perkins D. D., Barry E. G. 1977. The cytogenetics of Neurospora. Adv. Genet. 19: 133–285 [DOI] [PubMed] [Google Scholar]
- 28. Posas F., et al. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86: 865–875 [DOI] [PubMed] [Google Scholar]
- 29. Raju N. 1980. Meiosis and ascospore genesis in Neurospora. Eur. J. Cell Biol. 23: 208–223 [PubMed] [Google Scholar]
- 30. Raju N. 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96: 241–262 [Google Scholar]
- 31. Russo V., Sommer T., Chambers J. A. A. 1985. A modified Vogel's medium for crossings, mating-type tests and the isolation of female-sterile mutants of Neurospora crassa. Neurospora Newsl. 32: 10–11 [Google Scholar]
- 32. Sandmann G., Misawa N. 2002. Fungal carotenoids. Springer-Verlag, Berlin, Germany [Google Scholar]
- 33. Santos J. L., Shiozaki K. 2001. Fungal histidine kinases. Sci. STKE 2001. (98): re1. [DOI] [PubMed] [Google Scholar]
- 34. Sargent M. L., Woodward D. O. 1969. Genetic determinants of circadian rhythmicity in Neurospora. J. Bacteriol. 97: 861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schultz J. R., Milpetz F., Bork P., Ponting C. P. 1998. SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. U. S. A. 95: 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schumacher M. M., Enderlin C. S., Selitrennikoff C. P. 1997. The Osmotic-1 locus of Neurospora crassa encodes a putative histidine kinase similar to osmosensors of bacteria and yeast. Curr. Microbiol. 34: 340–347 [DOI] [PubMed] [Google Scholar]
- 37. Springer M. L. 1993. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays 15: 365–374 [DOI] [PubMed] [Google Scholar]
- 38. Springer M. L., Yanofsky C. 1989. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 3: 559–571 [DOI] [PubMed] [Google Scholar]
- 39. Stock A. M., Robinson V. L., Goudreau P. N. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69: 183–215 [DOI] [PubMed] [Google Scholar]
- 40. Uhl M. A., Miller J. F. 1996. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 15: 1028–1036 [PMC free article] [PubMed] [Google Scholar]
- 41. Vitalini M. W., et al. 2007. Circadian rhythmicity mediated by temporal regulation of the activity of p38 MAPK. Proc. Natl. Acad. Sci. U. S. A. 104: 18223–18228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vitalini M. W., de Paula R. M., Park W. D., Bell-Pedersen D. 2006. The rhythms of life: circadian output pathways in Neurospora. J. Biol. Rhythms 21: 432–444 [DOI] [PubMed] [Google Scholar]
- 43. Weiss V., Magasanik B. 1988. Phosphorylation of nitrogen regulator I (NRI) of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 85: 8919–8923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zalokar M. 1954. Studies on biosynthesis of carotenoids in Neurospora crassa. Arch. Biochem. Biophys. 50: 71–80 [DOI] [PubMed] [Google Scholar]