Abstract
Traditional lipid profiles often fail to fully explain the elevated cardiovascular risk of individuals with diabetes mellitus. Advanced lipoprotein testing offers a novel means to evaluate dyslipidemia and refine risk estimation. Numerous observational studies have demonstrated a characteristic pattern of elevated levels of small, dense LDL particles, out of proportion to traditional lipid levels, in patients with both diabetes mellitus and the metabolic syndrome. Commonly used glucose and lipid-lowering agents have varied effects in patients with diabetes on both LDL and HDL subfractions. The exact role of advanced lipoprotein testing in patients with diabetes mellitus and the metabolic syndrome remains unclear but may offer improved assessment of cardiovascular risk compared with traditional lipid measurements.
Keywords: diabetes, lipoprotein, metabolic syndrome, particles
The prevalence of diabetes mellitus continues to rise, with the WHO estimating that the number of patients with diabetes worldwide will increase from 171 million in 2000 to 360 million in 2030. In addition, the demographic expected to grow most significantly are those patients with diabetes aged 65 years and over [1]. As the number of individuals with diabetes mellitus continues to grow and their life expectancy increases, healthcare providers will be faced with the resultant increase in the incidence of the microvascular and macrovascular sequelae of diabetes. Though chronic hyperglycemia contributes to dysfunction in various organ systems, cardiovascular disease (CVD) remains the largest contributor to morbidity and mortality among those with diabetes [2] and remains the leading cause of premature death in this high-risk population [3,4], being responsible for approximately 65% of total deaths [5]. Both the American Diabetes Association [6] and American Heart Association [7] have designated diabetic patients as high risk for CVD, even in the absence of prior coronary artery disease. Furthermore, an analysis of data from the Framingham Heart Study demonstrated that the proportion of heart disease attributable to diabetes mellitus has increased over time [8].
The mechanism by which diabetes confers increased cardiovascular risk remains unclear. However, similar to patients without diabetes, a large body of evidence suggests a central role for lipids contributing to CVD in those with diabetes, a pattern termed diabetic dyslipidemia. In addition, there is a proinflammatory milieu at the level of the vascular endothelium due to the accumulation of advanced glycation end products [9] that stimulates accelerated atherosclerosis. Considerable work has demonstrated that the diabetic lipid profile possesses an intrinsic atherogenicity that is not present in those without diabetes.
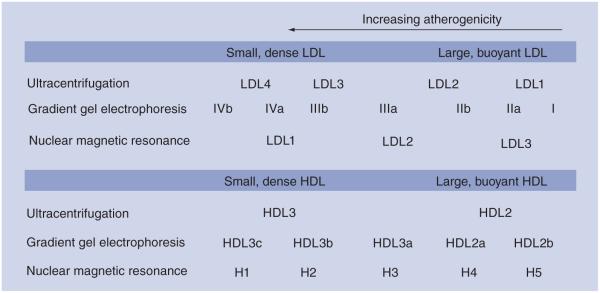
As diagnostic modalities have advanced, the ability to characterize lipid abnormalities that confer increased cardiovascular risk has also improved. Most clinicians are familiar with traditional cholesterol measurements, namely total cholesterol, LDL-C, HDL-C and triglycerides (TGs). However, analysis of the heterogeneous particles that comprise each of these cholesterol categories offers the possibility of discovering novel diagnostic and therapeutic modalities in lipid management. A variety of methodologies exist for the analysis of lipid subfractions. Density gradient ultracentrifugation is the oldest of the lipid subfraction technologies [10], which functions by exploiting the size and density differences that characterize various lipid fractions. Gradient gel electrophoresis (GGE) was described by Krauss and Burke in 1982 as a means to isolate LDL lipid subfractions by charge and size and has been refined extensively in subsequent years [11]. Nuclear magnetic resonance (NMR) spectroscopy, the most recent of the subfraction technologies, provides a simultaneous quantification of the size and concentration of lipoprotein particles (Table 1 & Figure 1) [12].
Table 1.
The most common lipid subfraction methods.
| Lipoprotein subfraction method | Commercial assay | Reported lipid parameters | Ref. |
|---|---|---|---|
| Density gradient ultracentrifugation | |||
| Ultracentrifugation is the oldest of the lipid subfraction technologies, initially described the 1950s and 1960s as a method to fractionate lipoproteins. Particles are separated according to size and density properties. |
Vertical Auto Profile (Atherotech, Birmingham, AL, USA) |
VLDL1,2 = buoyant VLDL3 = dense IDL LDL1,2 = large/buoyant LDL3,4 = small/dense HDL2 = large/buoyant HDL3 = small/dense apoB apoA1 Lp(a) |
[10,82] |
| Gradient gel electrophoresis | |||
| This technology is commonly used in various commercial and academic applications. It was described by Krauss and Burke in 1982 and is used extensively in a variety of laboratories globally. Size and charge variability are the key determinants of particle differentiation. |
Berkeley GGE (Berkeley Heart Lab, South San Francisco, CA, USA) |
LDL I, IIa, IIb = large/buoyant LDL IIIa, IIIb, IVa, IVb = small/dense HDL2b = large/buoyant HDL2a, 3a, 3b, 3c = small/dense apoB Lp(a) In addition to lipids, Berkeley HeartLab reports: Homocysteine CRP apoE genotype |
[11,83] |
| NMR spectroscopy | |||
| NMR as a lipid fractionation technique was described by Otvos, which separates lipoprotein particles based on differing methyl group NMR signals. |
LipoScience NMR (LipoScience, Raleigh, NC, USA) |
VLDL V1–V6, V1 = small/dense LDL1 = small/dense LDL2 = intermediate LDL3 = large/buoyant H1 = small/dense H5 = large/buoyant LDL size HDL size VLDL size LP-IR |
[12] |
apoA1: Apolipoprotein A1; apoB: Apolipoprotein B; CRP: C-reactive protein; Lp(a): Lipoprotein A; LP-IR: Lipoprotein insulin resistance; NMR: Nuclear magnetic resonance.
Figure 1. LDL and HDL subfractions reported by the common commercial lipid subfraction methodologies.
While both LDL-C concentrations are equivalent, the distribution of LDL-C differs depending on LDL particle concentrations. An increased percentage of small, dense LDL particles is described as the Pattern B phenotype and has been associated with an increased risk of coronary artery disease. Ultracentrifugation subfraction parameters are from the Vertical Auto Profile (Atherotech, Birmingham, AL, USA), gradient gel electrophoresis parameters are from Berkeley Heart Lab (South San Francisco, CA, USA), and nuclear magnetic resonance spectroscopy parameters are from LipoScience (Raleigh, NC, USA).
Although these various lipid subfraction methodologies differ in technique, no one technique has taken precedence over the others, and even within the various techniques, there is still disagreement about the boundaries that precisely define each lipid subfraction [13]. This lack of standardization raises the possibility of ambiguity within the literature utilizing subfraction technology. A review by Chung et al. of nine head to head analyses of these methodologies revealed significant hetero geneity in the results obtained from each of these techniques [14]. Concordance between subfraction data from technologies varied considerably in the study, and others have suggested that a consensus reference standard should be established to facilitate harmonization of this emerging field [15].
Lipid synthesis initiates at the liver and from intestinal absorption with production of VLDL and chylomicrons, respectively. Each contains apolipoproteins as well, with VLDL containing apolipoprotein B-100 and chylomicrons possessing apolipoprotein B-48. Both particles are also enriched with abundant TGs and cholesterol. As TGs are removed from VLDL particles through the action of lipases, the VLDL composition is transformed to an intermediate-density lipoprotein (IDL). Further lipolysis of IDL produces LDL particles. On the surface of all the atherogenic lipoproteins, including VLDL, IDL, and LDL, resides apolipoprotein B (apoB), in contrast to HDL, which contains apolipoprotein A. Measurement of circulating levels of apoB thus provides an accurate assessment of the number of these atherogenic lipid particles as each of those particles possesses one molecule of apoB. Alternatively, non-HDL-C (nHDL-C) provides similar assessment of the total atherogenic lipid pool and is recommended by the National Cholesterol Education Program as a secondary lipid goal after achieving LDL-C goals, particularly in those patients with hypertriglyceridemia [16]. nHDL-C has been demonstrated to be a stronger predictor for CVD risk than traditional LDL-C [17], and elevated levels were still associated with an increased risk of coronary heart disease with LDL levels below 100 mg/dl [18]. However, many individuals who reach their respective nHDL-C targets nevertheless still possess an atherogenic lipid profile with residual risk [19]. Recent studies demonstrated that apoB may be superior to both nHDL-C and LDL-C as a predictor of CVD [20].
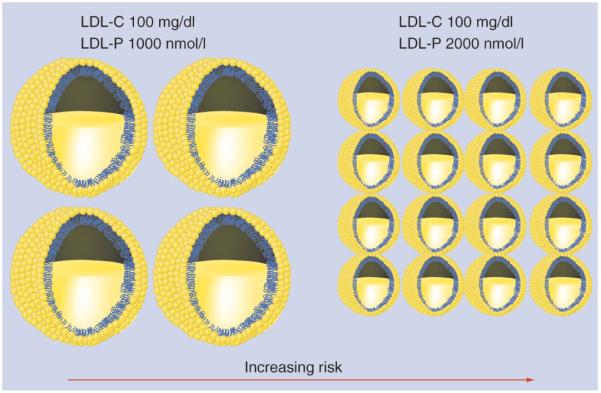
Standard lipid panels only provide evaluation of the total amount of cholesterol in each major circulating lipid fraction but no information about the individual particles that carry that particular type of cholesterol. Two individuals can possess the same total LDL-C, but disparate LDL particle (LDL-P) compositions (Figure 2).
Figure 2. Variability in LDL particle composition despite equivalent LDL-C levels.
Austin et al. utilized GGE to dichotomize these LDL phenotypes into Pattern A characterized by larger, buoyant LDL particles, and Pattern B, characterized by a smaller, dense LDL pattern [21]. Analysis of lipid subfractions by GGE in the Quebec Cardiovascular Study found that a preponderance of small, dense LDL was associated with a greater than threefold increase in the risk of ischemic heart disease [22].
It has also been demonstrated that small, dense LDL particles preferentially undergo endocytosis by macrophages and also are more susceptible to oxidation relative to larger, more buoyant particles. Both processes stimulate foam cell production in coronary atheromas and are possible mechanisms of the increased atherogenicity associated with pattern B. Though less well studied, HDL has similarly been deconstructed into various subfractions but with inconsistent findings. Some have found that the smaller HDL subfractions are associated with increased risk of coronary artery disease [23,24], while more recent analyses have noted that those small subfractions may protect against CVD by acting as antioxidants [25].
The atherogenic lipid profile in insulin resistant states
Diabetic dyslipidemia is classically characterized by an increase in VLDL-C, decreased production of HDL and a preponderance of small, dense LDL particles [26]. The relationship between insulin resistance and the Pattern B phenotype of LDL has been demonstrated in several observational studies across various diabetic populations (Table 2). Siegel et al. analyzed the nature of lipoprotein abnormalities using GGE in patients from the third examination of Framingham Offspring Study conducted from 1984 to 1987 [27]. A total of 174 men and women with diabetes mellitus were compared with 3757 control subjects. The prevalence of the Pattern B phenotype was significantly higher in both diabetic men (49 vs 33%; p < 0.05) and women (40 vs 11%; p < 0.001) compared with subjects without diabetes mellitus. Similar findings have been observed in smaller case-control studies [28,29]. Interestingly, a gender interaction was observed in a case-control study by Haffner et al. using ultracentrifugation [30]. They observed significantly lower LDL particle size in both men and women with diabetes (p = 0.007). However, the difference remained only in women when corrections were made for both TGs and HDL-C. A consistent and important observation in these observational studies was that glycemic control, whether measured as fasting blood glucose or hemoglobin A1c, was not significantly different in patients with Pattern A or B LDL phenotypes.
Table 2.
Summary of observational studies of lipid subfractions in diabetes mellitus and the metabolic syndrome.
| Study (year) |
Patients (n) | Country | Subfraction method |
Results† | Ref. |
|---|---|---|---|---|---|
| Feingold et al. (1992) |
29 DM2, 87 control | USA | Gradient gel electrophoresis |
LDL phenotype comparison: less Pattern A in DM2 (47 vs 28%, p = 0.025), increased Pattern B in DM2 (24 vs 52%, p < 0.025) |
[28] |
| Selby et al. (1993) |
704 female twins | USA (Kaiser Permanente Women Twins Study) |
Gradient gel electrophoresis |
↑ TG, waist:hip ratio, fasting insulin, 2 h postload insulin, 2 h postload glucose with increased probability of Pattern B, ↓ HDL associated with increased likelihood of Pattern B in female twins |
[32] |
| Haffner et al. (1994) |
95 DM2, 371 nondiabetic controls |
USA (San Antonio Heart Study) |
Analytical ultracentrifugation |
LDL size ↓ in both male and female patients with DM2, relationship explained by HDL and TG in males |
[30] |
| Syvanne et al. (1995) |
50 consecutive patients with DM2 and CAD |
Finland | Gradient gel electrophoresis |
HDL2a ↑ in DM2 patients, other subfractions NS difference |
[36] |
| Siegel et al. (1996) |
1878 male controls (120 male DM2); 1879 female controls (54 female DM2) |
USA (Framingham Heart Study) |
Gradient gel electrophoresis |
Pattern B in males with DM2: 49.2 vs 32.5% (p < 0.05), Pattern B in females with DM2: 10.7 vs 40.3% (p < 0.001), apoB signifcantly ↑ in females with DM2 |
[27] |
| Friedlander et al. (2000) |
200 male, 190 female without DM2 |
Israel | Gradient gel electrophoresis |
↑ TG, fasting insulin, 2 h postload insulin positively related to LDL particle size, HDL negatively related to LDL particle size |
[31] |
| Garvey et al. (2003) |
46 DM2, 56 insulin sensitive, 46 insulin resistant |
USA | NMR | ↑ Total LDL, large LDL, intermediate LDL and small LDL in patients with DM |
[84] |
| Tan et al. (2001) |
93 consecutive DM2 male |
Singapore | Gradient gel electrophoresis |
DM2 patients with ↑ small LDL and ↓ large LDL even with mean A1c of 6.6% |
[29] |
| Kathiresan et al. (2006) |
376 males and 265 females with MetSyn against 1028 male and 1324 female controls |
USA (Framingham Heart Study) |
NMR | MetSyn ↑ total LDL-P, small LDL-P, apoB ↓ LDL size, HDL size, large LDL, large HDL |
[46] |
| Festa et al. (2005) |
830 nondiabetic patients at baseline, 130 converted to DM2 |
USA (Insulin Resistance Atherosclerosis Study) |
NMR | ↑ Total LDL particles, small LDL, small HDL, VLDL size and ↓ LDL/HDL particle size in patients who became diabetic |
[39] |
| Gazi et al. (2006) |
105 with MetSyn, 70 controls |
Greece | Polyacrylamide gel electrophoresis |
MetSyn ↑ cholesterol concentration in dense LDL and VLDL ↓ LDL mean particle size and peak particle diameter |
[47] |
| Rizzo et al. (2009) |
124 with MetSyn, 102 controls |
Italy | Gradient gel electrophoresis |
LDL particle size, low HDL-C predictors of cardio/cerebrovascular risk at 2 years |
[48] |
| Otvos et al. (2011) |
6814 from community-based cohort |
USA (Multi-Ethnic Study of Atherosclerosis) |
NMR | ↑ Prevalence of DM2 and MetSyn in discordant LDL-P > LDL-C; both LDL-P and LDL-C associated with incident CVD in concordant, only LDL-P associated with CVD in discordant |
[50] |
All changes noted are statistically signifcant unless marked NS.
CVD: Cardiovascular disease; DM2: Type 2 diabetes mellitus; LDL-P: LDL particle; MetSyn: Metabolic syndrome; NMR: Nuclear magnetic resonance; NS: Not signifcant; TG: Triglyceride.
Several predictors of LDL phenotype were assessed by Friedlander et al. from the second examination (1994–1996) of the Jerusalem Diabetes Prevalence Study [31]. In almost 400 diabetic men and women, a variety of parameters including elevated TGs (p < 0.001), low HDL-C (p < 0.001), elevated fasting insulin (p = 0.005), and 2 h postload insulin (p = 0.02) were associated with smaller LDL particle size as measured by GGE. Selby et al. similarly analyzed predictors of the Pattern B phenotype in twin comparisons from the Kaiser Permanente Women Twins Study conducted from 1989 to 1990 [32]. Hypertriglyceridemia (p < 0.0001), low HDL-C (p < 0.0001), elevated waist:hip ratio (p < 0.0001), and increased fasting insulin (p < 0.0001) were associated with the Pattern B phenotype assessed by GGE. Of note, a sub-group analysis of 25 pairs of nondiabetic, monozygotic twins discordant for LDL phenotype revealed elevated BMI and waist:hip ratio in the twin with a small, dense LDL pattern.
Isolated lipid abnormalities are rare in those with diabetes mellitus. More commonly observed is the ‘lipid triad’ of hypertriglyceridemia, small LDL particles, and low HDL, which together confer an increased risk of CVD [33]. An excess of TG-rich lipoproteins and concomitant elevated serum TGs results in a pattern shift to smaller LDL particles along with lower overall HDL particle concentrations. Each of these characteristic lipid abnormalities are related to the underlying insulin resistance, characteristic of both the metabolic syndrome (MetSyn) and overt diabetes mellitus. Drexel et al. found that a small LDL particle diameter (p = 0.002), low HDL particle concentration (p = 0.001), low apoA-I (p = 0.003), and elevated TGs (p = 0.007) predicted vascular events in a population of 491 Caucasian patients with angiography-proven coronary artery disease on statin therapy [34]. This relationship persisted in the 116 patients with diabetes mellitus in the analysis. Similarly Berneis et al., in a study of 38 Swiss individuals with diabetes mellitus demonstrated that LDL particle size was the strongest predictor of the presence of coronary artery disease (p = 0.002) and carotid intima-media thickness (p = 0.03) relative to other traditional lipid parameters such as total cholesterol, LDL-C, HDL-C, apoB, apoA-I and apoA-III [35].
As mentioned before, the prognostic importance of HDL subfractions in patients with diabetes has been less well studied. Syvanne et al. studied HDL subfractions using GGE in 50 consecutive patients with diabetes undergoing elective coronary catheterization [36]. Patients with both diabetes mellitus and significant coronary artery disease found at cardiac catheterization had smaller HDL particle size relative to patients with neither coronary artery disease nor diabetes or just one of those conditions (p = 0.04). Furthermore, an analysis by Williams et al. of 116 male and 78 female patients found that the proportion of HDL particles in the HDL 3b subfractions correlated positively (p < 0.01) with small LDL, TGs, and apolipoprotein B particle concentrations [37]. HDL particles are also intimately involved in mediating oxidation throughout the body. A recent study by Stefanovic et al. of 114 individuals with diabetes mellitus demonstrated an increased prevalence of the smaller HD3 particles relative to controls (28.3 vs 4.4%; p < 0.001) [38]. Those with the predominant HDL3 phenotype, the majority of which possessed diabetes mellitus, had elevated levels of superoxide anions and increased activity of superoxide dismutase. These findings suggest an adaptation to a molecular environment in diabetes mellitus of oxidative stress and provide a physiologic explanation for varying particle sizes in individuals with diabetes mellitus.
The lipoprotein subfraction abnormalities of diabetic dyslipidemia are observed even in prediabetic subjects. The Insulin Resistance Atherosclerosis Study analyzed lipid subfractions by NMR in a cohort of 830 individuals without diabetes at baseline [39]. There was no significant difference in baseline LDL-C noted in those that developed diabetes mellitus (n = 130) over the mean follow-up of 5.2 years relative to those who remained free from diabetes. However, they did manifest a discordant phenotype with significantly smaller baseline LDL and HDL particle size (p = 0.0004 and p < 0.0001, respectively) and significantly increased baseline levels of total LDL-P, small LDL-P, small HDL-P, and VLDL size. These lipid abnormalities suggest that healthy prediabetic individuals possess an atherogenic lipid profile prior to development of overt diabetes mellitus. Furthermore, several studies using a variety of lipid subfraction technologies have demonstrated that a preponderance of small LDL [40], small HDL, and VLDL particles [41] can predict the future development of diabetes mellitus.
Not only do atherogenic lipoprotein subfraction patterns predict prevalence and incidence of diabetes, but atherogenic LDL particles also directly promote vascular dysfunction in diabetic patients. Lu et al. isolated various electronegative LDL subfractions by means of fast protein liquid chromatography in six diabetic patients [42]. The L5 electronegative subfraction, initially observed in hyperlipidemic nondiabetic patients [43], was also found in those with diabetes mellitus. This subfraction dysregulates FGF-2 function and potentiates subsequent endothelial cell apoptosis. Another study using density gradient ultracentrifugation determined that LDL isolated from diabetic subjects had a significant effect in decreasing the ability of animal endothelium to appropriately relax in the setting of cholinergic administration [44]. Finally, decreasing LDL particle size has been correlated with impaired endothelial flow mediated vasodilation in patients with and without diabetes mellitus (r = 0.48, p < 0.05; r = 0.39, p < 0.0005, respectively) [45]. Together, these studies suggest that the LDLs of diabetic patients may intrinsically confer cardiovascular risk by disrupting autoregulation at the microvascular level.
The MetSyn, characterized by dyslipidemia and central adiposity, is on an earlier spectrum of insulin resistance ending with diabetes. Analysis of 641 patients (376 men, 265 women) with MetSyn and 2352 controls from the Framingham Heart Study found that both men and women with MetSyn had a comparatively increased number of small LDL particles (p < 0.0001) and significantly less large LDL particles (p < 0.0001) as determined by NMR spectroscopy [46]. The number of small LDL particles increased in a step-wise fashion with increasing components of the MetSyn. A smaller study of 105 individuals with the MetSyn versus 70 age and sex-matched controls using GGE found a significant increase in cholesterol concentration within dense LDL, a decrease in mean particle size, and a decrease in LDL peak particle size (all parameters with p < 0.05) in those with MetSyn [47]. Rizzo et al. demonstrated in an analysis of 124 Italian patients with MetSyn that an elevated concentration of small, dense LDL was associated (p = 0.004) with an increased incidence of cardiovascular and cerebro vascular events at 2 years of follow-up [48]. The increasing epidemic of MetSyn [49] and obesity will likely lead to an increasing number of diabetic patients in the future and subsequent CVD, with a significant number characterized by abnormal LDL subfraction patterns out of proportion to their LDL-C levels.
Otvos et al. recently reported data from the Multi-Ethnic Study of Atherosclerosis (MESA) study to compare LDL-P derived from NMR spectroscopy and LDL-C as a predictor of incident CVD in a community based cohort of 6814 ethnically diverse individuals [50]. Specifically, the study focused on prognostic utility of LDL-P in individuals with discordant levels of LDL-P and LDL-C (defined as a difference of ≥12 percentile units). While both LDL-P (hazard ratio (HR) per standard deviation (SD) 1.32, 95% CI: 1.19–1.47; p < 0.0001) and LDL-C (HR per SD 1.20, 95% CI 1.08–1.34, p = 0.0009) independently predicted incident CVD in the overall population, only LDL-P retained its prognostic significance (HR per SD 1.45, 95% CI: 1.19–1.78, p = 0.0003) when the LDL-P and LDL-C values were discordant. Though LDL-C and LDL-P were highly correlated (r = 0.75), discordance was markedly higher in those with diabetes mellitus and MetSyn. In particular, discordance characterized by LDL-P greater than LDL-C suggests the presence of numerous, cholesterol-poor LDL particles representative of the Pattern B phenotype. Compared to individuals with concordant LDL-P and LDL-C, the 1407 subjects with discordant LDL-P > LDL-C in the MESA study had markedly increased prevalence of diabetes mellitus (19%) and MetSyn (54%; p < 0.0001 for both comparisons). This large population-based study thus suggests an increasing role for measuring LDL subfractions in those with insulin resistance as a prognostic index of coronary artery disease beyond the traditional lipid profile (Tables 3 & 4).
Table 3.
Discordant LDL-C and LDL-P and cardiovascular disease from the Multi-Ethnic Study of Atherosclerosis.
| LDL-P > LDL-C (n = 1407)† |
LDL-P = LDL-C (n = 2775) |
LDL-P < LDL-C (n = 1416)† |
|
|---|---|---|---|
| DM (%) | 19 | 14 | 9 |
| MetSyn (%) | 54 | 33 | 16 |
| LDL-P (nmol/l) | 1372 ± 240 | 1249 ± 395 | 1117 ± 201 |
| LDL-C (mg/dl) | 104 ± 20 | 117 ± 37 | 130 ± 23 |
| LDL size (nm) | 20.3 ± 0.5 | 20.7 ± 0.5 | 21.1 ± 0.4 |
| HOMA-IR | 2.4 ± 2.8 | 1.8 ± 1.8 | 1.5 ± 1.8 |
Signifcant difference compared with the concordant subgroup, p < 0.0001. HOMA-IR is a measure of insulin resistance. LDL size was measured with nuclear magnetic resonance spectroscopy. Adapted from [50].
Table 4.
Discordant LDL-C and LDL-P and cardiovascular disease from the Multi-Ethnic Study of Atherosclerosis.
| Predictor of incident cardiovascular events | Hazard ratio (95% CI) |
|---|---|
| LDL-P & LDL-C concordant | |
| LDL-C | 1.27 (1.12–1.44); p = 0.0003 |
| LDL-P | 1.27 (1.12–1.44); p = 0.0002 |
| LDL-P & LDL-C discordant | |
| LDL-C | 1.07 (0.88–1.30); p = 0.52 |
| LDL-P | 1.45 (1.19–1.78); p = 0.0003 |
Adapted from [50].
Therapies & lipid subfraction changes
Therapies used in patients with diabetes targeted at both hyperglycemia and diabetic dyslipidemia have potential in altering the lipoprotein subfraction composition to a more favorable phenotype (Table 5 & Figure 3). Generally, therapies which reduce TG concentration have been demonstrated to have the most potent effect in reducing the proportion of small, dense LDL. However, the vast majority of literature to date in this area has comprised relatively small cohorts. Additionally, the correlation of changes in these lipid subfractions to actual cardio vascular outcome measures in patients with diabetes has yet to be clarified.
Table 5.
Summary of trials of lipid subfraction changes in response to various lipid- and glucose-lowering therapies in diabetes mellitus.
| Author | Patients, country |
Intervention | Study type | Subfraction method |
Results† | Ref. |
|---|---|---|---|---|---|---|
| Therapeutic lifestyle changes | ||||||
| Halle et al. (1998) |
34 DM2, Germany |
2200 kcal/week exercise; 1000 kcal/day diet |
Prospective, longitudinal |
Density gradient ultracentrifugation |
TLC ↑ large LDL, ↓ small LDL, ↓ apoB |
[51] |
| Wagner et al. (2003) |
33 poorly controlled DM2, Spain |
TLC, exercise, oral hypoglycemic agents |
Prospective, longitudinal |
Gradient gel electrophoresis |
↓ LDL, ↑ HDL, ↓ apoB, ↓ Pattern B, ↑ LDL particle size in Pattern B, no LDL particle size change in Pattern A |
[52] |
| Insulin | ||||||
| Caixas et al. (1997) |
37 DM1, 33 DM2, poorly controlled, 33 control, Spain |
Intensive insulin therapy |
Prospective, longitudinal |
Ultracentrifugation | ↓ Pattern B after glycemic control in NIDDM, larger ↓ in HgbA1c in patients that converted Pattern B ⇒ A |
[53] |
| Rivellese et al. (2000) |
9 DM2, Italy | Insulin vs glibenclamide |
Randomized | Density gradient ultracentrifugation |
Insulin ↓ amount/proportion of small LDL and ↑ large LDL |
[54] |
| MET | ||||||
| Ohira et al. (2007) |
28 DM2, Japan | MET 500 mg BID | Prospective, longitudinal |
Polyacrylamide gel disc electrophoresis |
↑ LDL particle size independent of improvement in Hgb A1c and fasting blood glucose |
[55] |
| TZD | ||||||
| Bavirti et al. (2003) |
10 DM2, USA | Troglitazone | Prospective, longitudinal |
NMR | ↓ small LDL (NS) , large HDL ↑ LDL particle size, large LDL, small HDL |
[56] |
| Lawrence et al. (2004) |
67 DM2, UK | MET 500 mg BID vs PIO 30 daily vs gliclazide 80 mg daily |
Randomized, open label, parallel |
Density gradient ultracentrifugation |
MET ↓ small LDL, apoB PIO ↓ small LDL ↑ HDL2, large LDL. SU ⇒ NS change |
[57] |
| Goldberg et al. (2005) |
GLAI, 369 PIO, 366 ROS, USA/ Puerto Rico/ Mexico/ Colombia |
PIO 45 mg vs ROS 4 mg BID |
Randomized, open label, parallel |
NMR | PIO ↓ LDL particle conc, PIO with greater increase in particle size than ROS, ROS ↑ LDL particle concentration |
[59] |
| Deeg et al. (2007) |
GLAI, 369 PIO, 366 ROS, USA/ Puerto Rico/ Mexico/ Colombia |
PIO 30 mg vs ROS 4 mg BID |
Randomized, open label, parallel |
NMR | PIO ↑ large HDL ↓ small LDL to greater extent than ROS, both ↑ large LDL |
[60] |
| Nakano et al. (2010) |
50 DM2, newly diagnosed, Japan |
MET 500 mg/ 1000 mg BID vs PIO 15 mg daily |
Randomized, open label, parallel |
HPLC | PIO ↓ very small LDL, large VLDL, MET ↓ very small LDL |
[58] |
| Incretin | ||||||
| Horton et al. (2010) |
6280 exenatide, 3861 sitagliptin, 3239 insulin, USA |
Exenatide vs sitagliptin vs insulin |
Retrospective | None | Exenatide ↓ LDL, exenatide/ sitagliptin ↓ TG, Insulin (NS)/ exenatide (NS) ↓ HDL |
[62] |
| Statin | ||||||
| Soedamah- Muthu et al. (2003) |
122 DM2, UK/Ireland |
ATORVA 10 mg vs placebo |
Randomized, double blind |
NMR | ATORVA ↓ large/medium LDL, small LDL (NS), LDL/VLDL particle number ↑ large HDL |
[64] |
| Lee et al. (2003) |
CRE, 90 DM2, USA |
PRAVA 40 mg vs placebo |
Randomized, prospective |
Ultracentrifugation | PRAVA ↓ IDL + LDL CIII+ | [67] |
| Charlton- Menys et al. (2009) |
2350 DM2, UK/Ireland |
ATORVA 10 mg vs placebo |
Randomized, double blind |
NMR | ATORVA ↓ apoB at all LDL levels | [65] |
| Kappelle et al. (2010) |
DALI, 185 DM2, Netherlands |
Placebo vs 10 mg ATORVA vs 80 mg ATORVA |
Randomized, prospective |
Density gradient ultracentrifugation |
ATORVA ↓ cholesterol in all LDL subfractions, no change mean particle size, dose dependent ↓ apoB |
[66] |
| Bile acid binding resin | ||||||
| Rosenson et al. (2006) |
65 DM2, USA | COLE 3.75 g/day vs placebo |
Randomized, prospective |
NMR | COLE ↓ total LDL particle conc., small LDL (p = 0.054) |
[68] |
| Fish oil | ||||||
| Patti et al. (1999) |
16 DM2, Italy | Fish oil vs placebo | Randomized | Density gradient ultracentrifugation |
Fish oil tended ↓ all VLDL subfractions, no signifcant change LDL subfractions or LDL particle size |
[75] |
| Mostad et al. (2008) |
27 DM2, Norway |
Corn oil vs fsh oil | Randomized, prospective |
NMR | Fish oil tended ↑ small/very small LDL (NS), ↓ small HDL |
[74] |
| Niacin | ||||||
| Pan et al. (2002) |
65 DM2, USA | Niacin 100 mg TID vs placebo |
Retrospective | Polyacrylamide gel electrophoresis |
Niacin ↑ HDL2, LDL particle size ↓ small LDL |
[79] |
| FENO | ||||||
| Vakkilainen et al. (2003) |
418 DM2, Finland/ Sweden/France/ Canada |
FENO 200 mg daily vs placebo |
Randomized, prospective |
Polyacrylamide gel electrophoresis |
FENO ↑ LDL particle size ↓ apoB | [73] |
All changes noted are statistically signifcant unless marked NS.
ATORVA: Atorvastatin; BID: Twice daily; CIII: Apolipoprotein-CIII; COLE: Colesevelam; Conc.: Concentration; DM1: Type 1 diabetes mellitus; DM2: Type 2 diabetes mellitus; FENO: Fenofbrate; HgbA1c: Hemoglobin A1c; MET: Metformin; NIDDM: Noninsulin-dependent diabetes mellitus; NMR: Nuclear magnetic resonance; NS: Not signifcant; PIO: Pioglitazone; PRAVA: Pravastatin; ROS: Rosiglitazone; SU: Sulfonylurea; TLC: Therapeutic lifestyle changes; TZD: Thiazolidinediones.
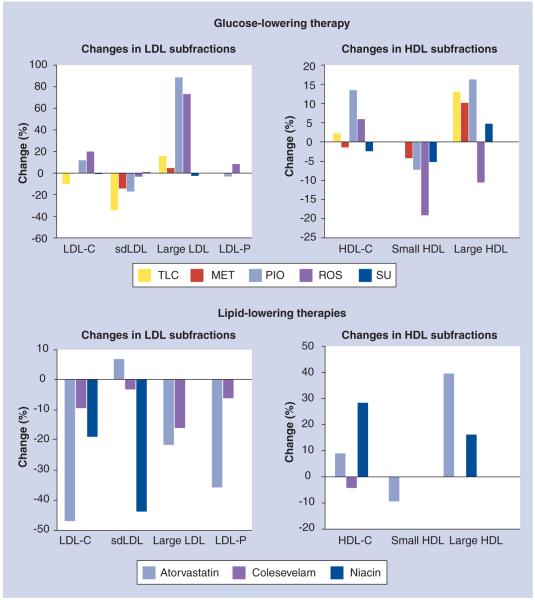
Figure 3. Changes in lipid subfractions by lipid- and glucose-lowering therapies in patients with diabetes.
Mean percentage changes are reported when multiple studies for any particular therapy were available. Data for all lipid parameters was not available for each therapy. apoB: Apolipoprotein B; MET: Metformin; PIO: Pioglitazone; ROS: Rosiglitazone; sdLDL: Small, dense LDL; SU: Sulfonylurea; TLC: Therapeutic lifestyle changes.
Primary glucose-lowering therapies
Lifestyle changes
In one lifestyle study assessing the effects of weight reduction and dietary modifications in 34 German patients with Type 2 diabetes mellitus [51], an exercise regimen of 2200 kcal/week and a concomitant diet of 1000 kcal/day caused a significant reduction in small LDL particles (1.42–0.93 mg/dl; p = 0.001) relative to baseline measured by density gradient ultracentrifugation. Wagner et al. analyzed the effects of aggressive control of hyperglycemia by means of therapeutic lifestyle changes in combination with initiation of oral hypoglycemic or insulin therapy in a prospective trial of 33 patients with poorly controlled diabetes mellitus [52]. A significant reduction in A1c (10.5–7.0%; p < 0.0005) was achieved and, while apoB and LDL-C decreased, no change in LDL particle size was observed by means of GGE among all patients. However, when considering just those 14 of 33 patients with the Pattern B phenotype at baseline, a significant increase in LDL particle size was noted (from 25.1 to 25.6 nm; p < 0.005). However, another prospective analysis that assessed the impact of improved glycemic control in 70 individuals with poorly controlled diabetes mellitus found that those patients that transitioned from the Pattern B to Pattern A phenotype detected by ultracentrifugation also had larger decreases in hemoglobin A1c (4.9 vs 3.1%; p < 0.05) [53]. Finally, Rivellese et al. studied insulin versus sulfonylurea administration in nine patients with diabetes mellitus and determined that insulin use was significantly associated with a decrease in small and increase in large LDL particle subfractions as measured by ultracentrifugation (p < 0.03) [54].
Metformin
The different classes of oral hypoglycemic agents also have characteristic effects on the lipid profile of patients with diabetes. Metformin use was evaluated in a prospective study of 28 Japanese patients who were on prior sulfonylurea monotherapy [55]. LDL relative mobility (LDL-Rm), a measurement of LDL movement with polyacrylamide gel disc electrophoresis that is inversely related to particle size, was utilized to quantify LDL particle size. Use of metformin resulted in an increase in LDL particle size (LDL:Rm ratio 0.3521:0.3339; p < 0.05) compared with baseline, a particle size change that was independent of the improvement in glycemic control.
Thiazolidinediones
The effects of thiazolidinediones (TZD) on diabetic dyslipidemia have been more extensively studied. In a small study of eight diabetic patients assessing the effects of troglitazone [56], LDL particle size determined by NMR spectroscopy increased from 20.5 to 21.3 nm (p < 0.05) over 8 weeks of therapy, a change that was driven mainly by an increase in large LDL particle concentration (from 53 to 86 mg/dl; p < 0.05). The LDL particle size subsequently decreased to baseline after troglitazone therapy had been discontinued for 4 weeks. Lawrence and associates also compared both pioglitazone and metformin administration versus sulfonylurea utilization [57]. A total of 60 diabetic patients were randomized to gliclazide, pioglitazone, or metformin therapy. Glycemic control was equivalent across the three treatment groups, yet disparate effects on lipid subfractions were observed. Significant decreases in small LDL were observed in patients treated with metformin (from 42.7 to 31.5 mg/dl) as well as pioglitazone (from 36.2 to 28.0 mg/dl) but not with sulfonyureas (p < 0.05 for both pioglitazone and metformin). Only those randomized to pioglitazone had significant changes in other lipid subfractions, including increases in large LDL particles (from 62.1 to 75.5 mg/dl, p < 0.001) as well as increases in the larger HDL2 fraction (from 11.5 to 15.7 mg/dl, p < 0.05). In addition, a study of 50 newly diagnosed patients with diabetes treated with either pioglitazone or metformin determined that pioglitazone significantly reduced the concentration of small LDL-C, but the inter-group difference relative to metformin was not statistically significant [58].
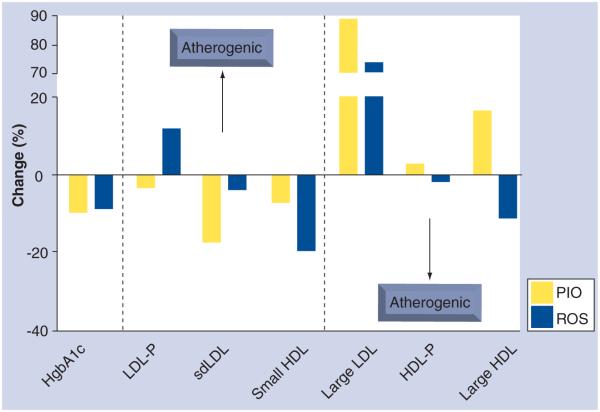
Goldberg and associates randomized 802 diabetic patients in the GLAI study to either pioglitazone (n = 400) or rosiglitazone (n = 402) in order to analyze differential effects on a variety of lipid parameters using NMR spectroscopy (Figure 4) [59]. The pioglitazone treatment group demonstrated a decrease in LDL particle concentration (from 1394 to 1341 nmol/l; p = 0.02) while the rosiglitazone group demonstrated a significant increase (from 1368 to 1488 mmol/l; p < 0.001) in the same parameter. While both treatment groups were associated with increases in LDL particle size, the increased particle size was significantly greater in those treated with pioglitazone (p = 0.005). In addition, the patients treated with rosiglitazone developed increases in apoB, TG, and nHDL-C, all trends toward a more atherogenic lipoprotein profile. Importantly, there was no significant difference between the two groups in terms of glycemic control as measured by HgbA1c. In a subgroup analysis from the GLAI study of the patients with complete lipoprotein subfractions performed at baseline and at trial completion, 333 patients with diabetes treated with pioglitazone were compared against 325 treated with rosiglitazone therapy [60]. Pioglitazone-treated patients again demonstrated favorable changes to their lipid profile assessed by NMR spectroscopy with a larger decrease in small LDL (least square mean between treatment groups −162.2 nmol/l; p < 0.001) and a larger increase in large HDL particle concentration (least square mean between treatment groups 1.0 μmol/l; p < 0.001) relative to rosiglitazone administration. Large LDL particle concentrations increased significantly from baseline in both treatment groups. A small randomized study of nine diabetic patients determined that pioglitazone led to a larger decrease in postprandial small LDL concentration measured by GGE as well as a significant increase in fasting/postprandial large LDL particle concentration relative to rosiglitazone therapy [61].
Figure 4. Variable changes in lipid subfractions among subjects treated with pioglitazone and rosiglitazone despite equivalent glycemic control in the GLAI Study.
Despite no significant differences in glycemic control, considerable variability is observed in the lipid subfractions of patients receiving PIO versus ROS in the GLAI Study [58,59].
HgbA1c: Hemoglobin A1c; PIO: Pioglitazone; ROS: Rosiglitazone.
There is no evidence to date comparing the efficacy of glucagon-like peptide (GLP)-1 or dipeptidyl peptidase (DPP)-4 antagonists in altering lipid subfractions. However, a recent large retrospective analysis compared exenatide, sitagliptin, and insulin therapy and found differential effects on traditional lipid parameters [62]. Specifically, the only therapy to significantly decrease total LDL concentration was exenatide, although all three decreased total cholesterol. In animal studies, augmentation of the incretin response was found to decrease fasting VLDL levels and also to dampen postprandial apolipoprotein-B48 secretion involved in chylomicron formation [63].
Primary lipid-lowering therapies
Statins
Statin therapy has also been proven in a number of studies to have a variety of beneficial effects on the atherogenic diabetic lipid profile (Table 2 & Figure 3). The Collaborative Atorvastatin Diabetes Study (CARDS) was a large multicenter randomized trial which compared atorvastatin 10 mg daily to placebo in patients with diabetes mellitus from the UK and Ireland. Data from the secondary prevention arm was used to compare NMR lipid subfractions in 69 diabetic patients on statin therapy to 53 control patients [64]. Statin therapy was associated with a significant decrease in total LDL particle concentration compared with baseline (from 1572 to 1065 nmol/l; p < 0.001). However, this decrease was driven by a decrease in large and medium LDL particles as a non-significant increase in small LDL concentration was observed among those treated with atorvastatin. The patients treated with atorvastatin also demonstrated a significant increase in their large HDL particle concentration (from 3.8 to 5.3 μmol/l; p = 0.02) that was not observed in the placebo group. Data from the primary prevention arm of CARDS, which randomized 2350 patients with diabetes to atorvastatin or placebo, determined that apoB levels were lower at any given LDL level among the atorvastatin group [65].
The Diabetes Atorvastatin Lipid Intervention cohort, a prospective multicenter Dutch study, randomized diabetic patients to 10 or 80 mg of atorvastatin versus placebo [66]. Both low and high doses of atorvastatin had no significant effect on LDL particle diameter measured by density gradient ultracentrifugation relative to placebo. However, a dose dependent decrease in apoB was described and the amount of cholesterol in all LDL subfractions was decreased in patients treated with atorvastatin. Data from 45 diabetic age/gender matched pairs from the Cholesterol and Recurrent Events trial, a double blind randomized trial of pravastatin 40 mg versus placebo [67], determined that pravastatin decreased the concentration of apoCIII+ lipoproteins determined by ultracentrifugation, a group of apolipoproteins thought to represent atherogenic remnants of VLDL degradation.
Bile acid binding resins
Bile acid binding resins have been demonstrated to produce increases in LDL particle size in nondiabetic patients independent of changes in serum TGs [68]. In a study of 65 diabetic patients randomized to colesevelam or placebo on top of baseline oral hypoglycemic therapy, a significant reduction in total LDL particle concentration by NMR spectroscopy was seen in the group treated with colesevelam (from 1669 to 1556 nmol/l; p = 0.006), a change that was driven mainly by a nonsignificant reduction in small LDL particles (from 1186 to 1145 nmol/l; p = 0.054) [69].
Ezetimibe
Ezetimibe, an intestinal cholesterol absorption inhibitor, was studied in a single center randomized trial of healthy, nondiabetic patients both as monotherapy and in combination with simvastatin and was found to increase small, dense LDL particle concentrations and decrease the concentration of large, buoyant LDL particles [70]. However, others analyses have demonstrated ezetemibe to either impart no change in LDL particle size [71] or to decrease the proportion of small, dense LDL [72]. Ezetimibe’s effect on lipoprotein subfractions in patients with diabetes has not been reported.
Fibrates
The Diabetes Atherosclerosis Intervention Study (DAIS) demonstrated in a randomized trial of 418 patients with diabetes mellitus that patients given 200 mg of fenofibrate daily had significantly larger LDL particle size by electrophoresis (from 25.66 to 25.07 nm; p < 0.001) as well as lower apoB relative to individuals given placebo [73]. In addition, smaller LDL particle size was associated with angiographic worsening on repeat cardiac catheterization performed at the conclusion of the study, with a mean time to repeat catheterization of 39.6 months. Small LDL size was associated with progression of coronary artery disease among the combined study population of fenofibrate and placebo treated patients (Pearson correlation coefficient = −0.16, p < 0.01). However, unlike statins and LDL-C, changes in lipid subfractions with fibrate therapy have not been consistently correlated with improved hard outcomes.
Fish oils
N-3 marine fatty acids are commonly administered to patients with dyslipidemia as they effectively lower TG and nHDL-C levels. Their effects on lipoprotein subclasses have been inconsistent. In a randomized, placebo controlled study of 27 diabetic patients in Norway, those assigned to the fish oil group demonstrated a trend toward increased levels of small (p = 0.06) and very small LDL (p = 0.055) particle concentrations measured by NMR spectroscopy compared with placebo [74]. In addition, patients treated with fish oil also demonstrated a significant decrease in small HDL concentration (p =0.004). By contrast, a trial of fish oil versus placebo in 16 noninsulin dependent diabetic patients using density gradient ultracentrifugation found no change in LDL size pattern over the course of 6 months while diet and hypoglycemic therapy were held constant [75]. The results of both of these studies are in contrast to an earlier study of patients without diabetes mellitus by Baumstark [76] which demonstrated a significant decrease in the small, dense LDL fraction, a reciprocal increase in the large/buoyant LDL fraction and an increase in the protective HDL2b subfraction in seven patients with type IIb hyperlipoproteinemia. It is possible that the effects of fish oil on lipoprotein subfractions in patients with diabetes are disparate from those in nondiabetic patients and will need to be investigated further.
Niacin
Niacin has several beneficial effects on several lipid parameters, including large increases in HDL-C and decreases in TG and nHDL-C, particularly when added to other lipid-lowering agents in many nondiabetic populations. Atorvastatin 10 mg was tested against 3000 mg of immediate release niacin in patients without diabetes mellitus with both interventions significantly reducing small LDL and VLDL particle concentration [77]. Niacin therapy possessed additional advantages including an increase in large LDL particles, large HDL particles, and an overall increase in HDL particle size. Patients treated with 1 g of extended release niacin in a placebo controlled randomized trial of 54 patients in which 12 were diabetic demonstrated significantly lower TG, decreased proportion of small LDL, increased large HDL proportion, and decreased small HDL particle size relative to placebo [78]. Pan et al. analyzed the lipid subfractions of 42 diabetic patients during and after niacin therapy by means of electrophoresis, finding a significant decrease in small, dense LDL particle mass (25 mg/dl to 11 mg/dl, p < 0.01), an increase in LDL peak diameter (252 angstroms to 262 angstroms, p < 0.05), an increase in HDL-C (from 39 to 50 mg/dl; p < 0.0001) as well as a corresponding increase in the proportion of cardioprotective HDL2 particles (from 5 to 11 mg/dl; p < 0.0001). As observed in studies of niacin in nondiabetic populations, the effects of niacin in diabetic patients are diminished by the high rate of intolerance to the drug [79]. However, there are emerging therapeutic modalities, such as laropiprant which may aid in minimizing the flushing associated with this therapy [80].
Conclusion
Abnormalities in the traditional lipid profile provide some insight into the increased CVD burden of patients with diabetes mellitus but do not fully explain their high risk status. Several studies have demonstrated variability in lipoprotein particle composition even in patients with grossly similar total cholesterol measurements, suggesting that the lipid manifestations of diabetes mellitus may best be observed by analyzing lipoprotein subfractions [21,22]. Interestingly, the atherogenic subfraction profile (Pattern B) seems to manifest even prior to the development of diabetes mellitus which suggests an abnormality in lipid function and composition that is independent of hyperglycemia. Advanced lipoprotein testing can thus provide a means to explain the incremental CVD risk of diabetes mellitus, as evidenced by the recent landmark MESA study highlighting the marked increase in discordant lipid fractions in patients with MetSyn and diabetes and failure of LDL-C to predict incident cardiovascular events in the discordant population.
Whether measurement of lipoprotein subfractions in patients with diabetes or MetSyn will improve clinical utility in prognosis or assessing response to therapies remains to be seen. Based on current guidelines, LDL-C and nHDL-C remain the primary targets in reducing lipid-based risk in patients with diabetes or MetSyn [16]. Measuring apoB and lipid subfractions may be useful once LDL-C and nHDL-C targets are achieved given the marked increase in discordant lipid fractions in these populations. Statins remain an integral and invaluable component of the pharmacologic management of dyslipidemia, and large randomized trials such as the Heart Protection Study [81] have demonstrated a reduction in major cardiovascular events in those patients with diabetes mellitus with and without established CVD. Several studies analyzed in this review found a favorable change in the lipid subfractions of diabetic patients treated with relatively low doses of statins which suggest benefits of statin therapy beyond simple LDL-C reduction. However, there are a number of other therapeutic interventions which are relatively underused and can also elicit favorable changes in lipid subfractions in patients with diabetes mellitus. Niacin and fenofibrate, for example, have been demonstrated to significantly improve LDL particle size in this population. Furthermore, agents designed to achieve glucose control in patients with diabetes such as pioglitazone and metformin also achieved beneficial changes within the lipid profile. These pleiotropic effects, which frequently occurred independently of glycemic control, could warrant further utilization of these agents in patients with diabetes mellitus or MetSyn, particularly those at markedly higher risk for CVD. It remains to be seen whether these beneficial changes in lipid subfractions associated with nonstatin pharmacologic therapies actually translate into improved clinical outcomes.
The precise clinical role of advanced lipoprotein testing in the general population is presently unclear. The lack of standardization and the absence of a ‘gold standard’ technique by which to compare the various technologies have slowed its adaptation and utilization. Advanced lipoprotein testing has provided great insight into a potential means by which diabetic patients develop additional risk for CVD. However, the clinical applicability of this technology remains controversial. Analysis of lipid subfractions may offer a means of risk stratification and identification of patients that may benefit from multidrug pharmacotherapy. Development of efficient and low-cost testing for small dense LDL (Pattern B) will facilitate further research and clinical use. The ultimate test of the applicability of advanced lipoprotein testing is whether it will promote the initiation of beneficial therapeutic changes in patients. To date, no large, randomized outcome studies have been performed to answer this question.
Future perspective
The prevalence of diabetes mellitus and the MetSyn will only continue to increase over the coming years. The rapid growth of this high risk population will require aggressive risk factor modification. Diabetic dyslipidemia, while partially explained by characteristic cholesterol abnormalities, also manifests as abnormalities of lipoprotein particles out of proportion to traditional cholesterol measurements. Measurement of these advanced lipoprotein parameters have allowed for a more nuanced assessment of lipid abnormalities in diabetes mellitus. As our knowledge regarding diabetic dyslipidemia grows, possibilities of novel therapeutic interventions and more efficient risk stratification will likely emerge. The lack of consistency across and within these technologies nevertheless is the most prominent limiting factor in its broader utilization. There is a need for additional comparative studies of the lipid subfraction methodologies that will facilitate standardization in this emerging field as well as validation for improved clinical utility in this high risk population.
Executive summary.
-
■
The prevalence of diabetes mellitus and the metabolic syndrome is increasing.
-
■
Both diabetes mellitus and the metabolic syndrome carry an increased risk of cardiovascular disease.
-
■
Novel technologies allow for advanced lipoprotein analysis such as nuclear magnetic resonance, electrophoresis and ultracentrifugation, though there is a lack of standardization.
-
■
Small, dense LDL (Pattern B phenotype) and elevated apolipoprotein B (apoB) have been associated with an increased risk of cardiovascular disease.
The atherogenic lipid profile in insulin resistant states
-
■
The diabetic lipid profile possesses characteristic abnormalities including a prominence of small, dense LDL and small HDL particles as well as an elevation in apoB and triglycerides.
-
■
These lipoprotein particle abnormalities persist even in patients with normal LDL-C and HDL-C.
-
■
The MESA study determined that LDL particle (LDL-P) concentration is a better predictor of incident cardiovascular disease when LDL-P and LDL-C concentrations are discordant (LDL-P > LDL-C).
Therapies & lipid subfraction changes in patients with diabetes
-
■Glycemic control
-
–Enhanced glycemic control with exercise and diet control improves atherogenic profile in diabetes mellitus and increases the probability of changing from a Pattern B to a Pattern A (large, buoyant LDL) phenotype.
-
–Ability to change lipoprotein particle size is greatest in those with a Pattern B phenotype at baseline.
-
–Pioglitazone produces favorable changes in the lipid abnormalities of diabetes mellitus relative to rosiglitazone and troglitazone.
-
–Incretin augmentation has not been studied in this context.
-
–
-
■Lipid lowering
-
–Statins have been extensively studied in this context and have significantly decreased atherogenic lipoproteins in diabetes mellitus, with atorvastatin being the most studied statin.
-
–Colesevelam and fenofibrate increase LDL particle size. The effect of ezetimibe on lipid subfractions has not been assessed in patients with diabetes mellitus.
-
–Niacin, though it was only studied in one trial, significantly decreased small LDL-P concentration.
-
–
Conclusion
-
■
Advanced lipoprotein parameters can be utilized after LDL-C and non-HDL cholesterol targets are achieved in patients with the metabolic syndrome and diabetes mellitus.
-
■
The clinical applicability of this technology is unclear, but the recent MESA study indicates that LDL-P concentration may be more useful than LDL-C in metabolic syndrome and diabetes mellitus as a predictor of cardiovascular disease risk.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21(7):1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 3.Merz CN, Buse JB, Tuncer D, Twillman GB. Physician attitudes and practices and patient awareness of the cardiovascular complications of diabetes. J. Am. Coll. Cardiol. 2002;40(10):1877–1881. doi: 10.1016/s0735-1097(02)02529-9. [DOI] [PubMed] [Google Scholar]
- 4.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl. 2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes – 2006. Diabetes Care. 2006;29(Suppl. 1):S4–S42. [PubMed] [Google Scholar]
- 7.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115(12):1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 9.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 10.Lossow WJ, Lindgren FT, Murchio JC, Stevens GR, Jensen LC. Particle size and protein content of six fractions of the Sf 20 plasma lipoproteins isolated by density gradient centrifugation. J. Lipid Res. 1969;10(1):68–76. [PubMed] [Google Scholar]
- 11.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J. Lipid Res. 1982;23(1):97–104. [PubMed] [Google Scholar]
- 12.Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin. Chem. 1992;38(9):1632–1638. [PubMed] [Google Scholar]
- 13.Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann. Intern. Med. 2009;150(7):474–484. doi: 10.7326/0003-4819-150-7-200904070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung M, Lichtenstein AH, Ip S, Lau J, Balk EM. Comparability of methods for LDL subfraction determination: a systematic review. Atherosclerosis. 2009;205(2):342–348. doi: 10.1016/j.atherosclerosis.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Rosenson RS, Brewer HB, Jr, Chapman MJ, et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011;57(3):392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 17.Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch. Intern. Med. 2001;161(11):1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 18.Arsenault BJ, Rana JS, Stroes ES, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J. Am. Coll. Cardiol. 2009;55(1):35–41. doi: 10.1016/j.jacc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 19.Brook RD, Kansal M, Bard RL, Eagle K, Rubenfire M. Usefulness of low-density lipoprotein particle size measurement in cardiovascular disease prevention. Clin. Cardiol. 2005;28(11):534–537. doi: 10.1002/clc.4960281109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112(22):3375–3383. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 21.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82(2):495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 22.Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95(1):69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 23.Griffin BA, Skinner ER, Maughan RJ. Plasma high density lipoprotein subfractions in subjects with different coronary risk indices as assessed by plasma lipoprotein concentrations. Atherosclerosis. 1988;70(1–2):165–169. doi: 10.1016/0021-9150(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 24.Cheung MC, Brown BG, Wolf AC, Albers JJ. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. J. Lipid Res. 1991;32(3):383–394. [PubMed] [Google Scholar]
- 25.Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23(10):1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg IJ. Clinical review 124: Diabetic dyslipidemia: causes and consequences. J. Clin. Endocrinol. Metab. 2001;86(3):965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 27.Siegel RD, Cupples A, Schaefer EJ, Wilson PW. Lipoproteins, apolipoproteins, and low-density lipoprotein size among diabetics in the Framingham offspring study. Metabolism. 1996;45(10):1267–1272. doi: 10.1016/s0026-0495(96)90246-2. [DOI] [PubMed] [Google Scholar]
- 28.Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler. Thromb. 1992;12(12):1496–1502. doi: 10.1161/01.atv.12.12.1496. [DOI] [PubMed] [Google Scholar]
- 29.Tan CE, Chew LS, Chio LF, et al. Cardiovascular risk factors and LDL subfraction profile in Type 2 diabetes mellitus subjects with good glycaemic control. Diabetes Res. Clin. Pract. 2001;51(2):107–114. doi: 10.1016/s0168-8227(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 30.Haffner SM, Mykkanen L, Stern MP, Paidi M, Howard BV. Greater effect of diabetes on LDL size in women than in men. Diabetes Care. 1994;17(10):1164–1171. doi: 10.2337/diacare.17.10.1164. [DOI] [PubMed] [Google Scholar]
- 31.Friedlander Y, Kidron M, Caslake M, Lamb T, Mcconnell M, Bar-On H. Low density lipoprotein particle size and risk factors of insulin resistance syndrome. Atherosclerosis. 2000;148(1):141–149. doi: 10.1016/s0021-9150(99)00215-4. [DOI] [PubMed] [Google Scholar]
- 32.Selby JV, Austin MA, Newman B, et al. LDL subclass phenotypes and the insulin resistance syndrome in women. Circulation. 1993;88(2):381–387. doi: 10.1161/01.cir.88.2.381. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am. J. Cardiol. 1998;81(4A):18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 34.Drexel H, Aczel S, Marte T, Vonbank A, Saely CH. Factors predicting cardiovascular events in statin-treated diabetic and non-diabetic patients with coronary atherosclerosis. Atherosclerosis. 2010;208(2):484–489. doi: 10.1016/j.atherosclerosis.2009.08.026. ■ Demonstrates similar changes in the lipid profile of those with prediabetes compared to those with overt diabetes mellitus.
- 35.Berneis K, Jeanneret C, Muser J, Felix B, Miserez AR. Low-density lipoprotein size and subclasses are markers of clinically apparent and non-apparent atherosclerosis in Type 2 diabetes. Metabolism. 2005;54(2):227–234. doi: 10.1016/j.metabol.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Syvanne M, Ahola M, Lahdenpera S, et al. High density lipoprotein subfractions in non-insulin-dependent diabetes mellitus and coronary artery disease. J. Lipid Res. 1995;36(3):573–582. [PubMed] [Google Scholar]
- 37.Williams PT, Krauss RM, Vranizan KM, Stefanick ML, Wood PD, Lindgren FT. Associations of lipoproteins and apolipoproteins with gradient gel electrophoresis estimates of high density lipoprotein subfractions in men and women. Arterioscler. Thromb. 1992;12(3):332–340. doi: 10.1161/01.atv.12.3.332. [DOI] [PubMed] [Google Scholar]
- 38.Stefanovic A, Kotur-Stevuljevic J, Spasic S, et al. HDL 2 particles are associated with hyperglycaemia, lower PON1 activity and oxidative stress in Type 2 diabetes mellitus patients. Clin. Biochem. 2010;43(15):1230–1235. doi: 10.1016/j.clinbiochem.2010.08.005. ■■ Demonstrates that the prevalence of diabetes mellitus and the metabolic syndrome was higher among those with discordant phenotypes. In these patients, LDL-P is a more reliable predictor of cardiovascular events.
- 39.Festa A, Williams K, Hanley AJ, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111(25):3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. ■ Shows similar changes in the lipid profile of those with prediabetes compared to those with overt diabetes mellitus.
- 40.Austin MA, Mykkanen L, Kuusisto J, et al. Prospective study of small LDLs as a risk factor for non-insulin dependent diabetes mellitus in elderly men and women. Circulation. 1995;92(7):1770–1778. doi: 10.1161/01.cir.92.7.1770. [DOI] [PubMed] [Google Scholar]
- 41.Hodge AM, Jenkins AJ, English DR, O’dea K, Giles GG. NMR-determined lipoprotein subclass profile predicts Type 2 diabetes. Diabetes Res. Clin. Pract. 2009;83(1):132–139. doi: 10.1016/j.diabres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Lu J, Jiang W, Yang JH, et al. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes. 2008;57(1):158–166. doi: 10.2337/db07-1287. [DOI] [PubMed] [Google Scholar]
- 43.Chen CH, Jiang T, Yang JH, et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation. 2003;107(16):2102–2108. doi: 10.1161/01.CIR.0000065220.70220.F7. ■■ Reports significant differences in lipid subfractions in data extracted from the Framingham Heart Study in the metabolic syndrome.
- 44.Mcneill KL, Fontana L, Russell-Jones DL, Rajman I, Ritter JM, Chowienczyk PJ. Inhibitory effects of low-density lipoproteins from men with type II diabetes on endothelium-dependent relaxation. J. Am. Coll. Cardiol. 2000;35(6):1622–1627. doi: 10.1016/s0735-1097(00)00607-0. [DOI] [PubMed] [Google Scholar]
- 45.Skyrme-Jones RA, O’brien RC, Luo M, Meredith IT. Endothelial vasodilator function is related to low-density lipoprotein particle size and low-density lipoprotein vitamin E content in Type 1 diabetes. J. Am. Coll. Cardiol. 2000;35(2):292–299. doi: 10.1016/s0735-1097(99)00547-1. [DOI] [PubMed] [Google Scholar]
- 46.Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113(1):20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. ■■ Reports significant differences in lipid subfractions in data extracted from the Framingham Heart Study in the metabolic syndrome.
- 47.Gazi I, Tsimihodimos V, Filippatos T, Bairaktari E, Tselepis AD, Elisaf M. Concentration and relative distribution of low-density lipoprotein subfractions in patients with metabolic syndrome defined according to the National Cholesterol Education Program criteria. Metabolism. 2006;55(7):885–891. doi: 10.1016/j.metabol.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Rizzo M, Pernice V, Frasheri A, et al. Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebro-vascular events in subjects with the metabolic syndrome. Clin Endocrinol. (Oxf.) 2009;70(6):870–875. doi: 10.1111/j.1365-2265.2008.03407.x. [DOI] [PubMed] [Google Scholar]
- 49.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. adults. Diabetes Care. 2004;27(10):2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 50.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J. Clin. Lipidol. 2011;5(2):105–113. doi: 10.1016/j.jacl.2011.02.001. ■■ Demonstrates that the prevalence of diabetes mellitus and the metabolic syndrome was higher among those with discordant phenotypes. In these patients, LDL-P is a more reliable predictor of cardiovascular events.
- 51.Halle M, Berg A, Garwers U, et al. Influence of 4 weeks’ intervention by exercise and diet on low-density lipoprotein subfractions in obese men with Type 2 diabetes. Metabolism. 1999;48(5):641–644. doi: 10.1016/s0026-0495(99)90064-1. [DOI] [PubMed] [Google Scholar]
- 52.Wagner AM, Jorba O, Rigla M, et al. Effect of improving glycemic control on low-density lipoprotein particle size in Type 2 diabetes. Metabolism. 2003;52(12):1576–1578. doi: 10.1016/s0026-0495(03)00326-3. [DOI] [PubMed] [Google Scholar]
- 53.Caixas A, Ordonez-Llanos J, De Leiva A, Payes A, Homs R, Perez A. Optimization of glycemic control by insulin therapy decreases the proportion of small dense LDL particles in diabetic patients. Diabetes. 1997;46(7):1207–1213. doi: 10.2337/diab.46.7.1207. [DOI] [PubMed] [Google Scholar]
- 54.Rivellese AA, Patti L, Romano G, et al. Effect of insulin and sulfonylurea therapy, at the same level of blood glucose control, on low density lipoprotein subfractions in Type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2000;85(11):4188–4192. doi: 10.1210/jcem.85.11.6956. [DOI] [PubMed] [Google Scholar]
- 55.Ohira M, Miyashita Y, Ebisuno M, et al. Effect of metformin on serum lipoprotein lipase mass levels and LDL particle size in Type 2 diabetes mellitus patients. Diabetes Res. Clin. Pract. 2007;78(1):34–41. doi: 10.1016/j.diabres.2007.02.012. ■ Indicates that metformin therapy leads to favorable changes in lipid subfractions independent of improved glycemic control.
- 56.Bavirti S, Ghanaat F, Tayek JA. Peroxisome proliferator-activated receptor-gamma agonist increases both low-density lipoprotein cholesterol particle size and small high-density lipoprotein cholesterol in patients with Type 2 diabetes independent of diabetic control. Endocr. Pract. 2003;9(6):487–493. doi: 10.4158/EP.9.6.487. [DOI] [PubMed] [Google Scholar]
- 57.Lawrence JM, Reid J, Taylor GJ, Stirling C, Reckless JP. Favorable effects of pioglitazone and metformin compared with gliclazide on lipoprotein subfractions in overweight patients with early Type 2 diabetes. Diabetes Care. 2004;27(1):41–46. doi: 10.2337/diacare.27.1.41. [DOI] [PubMed] [Google Scholar]
- 58.Nakano K, Hasegawa G, Fukui M, et al. Effect of pioglitazone on various parameters of insulin resistance including lipoprotein subclass according to particle size by a gel-permeation high-performance liquid chromatography in newly diagnosed patients with Type 2 diabetes. Endocr. J. 2010;57(5):423–430. doi: 10.1507/endocrj.k10e-006. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with Type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28(7):1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 60.Deeg MA, Buse JB, Goldberg RB, et al. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with Type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30(10):2458–2464. doi: 10.2337/dc06-1903. ■ Demonstrates that pioglitazone has a larger effect on improving lipid subfractions in patients compared to rosiglitazone therapy in a large randomized trial.
- 61.Berneis K, Rizzo M, Stettler C, et al. Comparative effects of rosiglitazone and pioglitazone on fasting and postprandial low-density lipoprotein size and subclasses in patients with Type 2 diabetes. Expert Opin. Pharmacother. 2008;9(3):343–349. doi: 10.1517/14656566.9.3.343. [DOI] [PubMed] [Google Scholar]
- 62.Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with Type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33(8):1759–1765. doi: 10.2337/dc09-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh J, Longuet C, Baker CL, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53(3):552–561. doi: 10.1007/s00125-009-1611-5. [DOI] [PubMed] [Google Scholar]
- 64.Soedamah-Muthu SS, Colhoun HM, Thomason MJ, et al. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in Type 2 diabetic patients with ischaemic heart disease. Atherosclerosis. 2003;167(2):243–255. doi: 10.1016/s0021-9150(02)00428-8. [DOI] [PubMed] [Google Scholar]
- 65.Charlton-Menys V, Betteridge DJ, Colhoun H, et al. Targets of statin therapy: LDL cholesterol, non-HDL cholesterol, and apolipoprotein B in Type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) Clin. Chem. 2009;55(3):473–480. doi: 10.1373/clinchem.2008.111401. [DOI] [PubMed] [Google Scholar]
- 66.Kappelle PJ, Dallinga-Thie GM, Dullaart RP. Atorvastatin treatment lowers fasting remnant-like particle cholesterol and LDL subfraction cholesterol without affecting LDL size in Type 2 diabetes mellitus: relevance for non-HDL cholesterol and apolipoprotein B guideline targets. Biochim. Biophys. Acta. 2010;1801(1):89–94. doi: 10.1016/j.bbalip.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 67.Lee SJ, Sacks FM. Effect of pravastatin on intermediate-density and low-density lipoproteins containing apolipoprotein CIII in patients with diabetes mellitus. Am. J. Cardiol. 2003;92(2):121–124. doi: 10.1016/s0002-9149(03)00524-1. [DOI] [PubMed] [Google Scholar]
- 68.Rosenson R. Colesevelam HCl reduces LDL particle number and increases LDL size in hypercholesterolemia. Atherosclerosis. 2006;185(2):327–330. doi: 10.1016/j.atherosclerosis.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 69.Rosenson RS, Abby SL, Jones MR. Colesevelam HCl effects on atherogenic lipoprotein subclasses in subjects with Type 2 diabetes. Atherosclerosis. 2009;204(2):342–344. doi: 10.1016/j.atherosclerosis.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 70.Berneis K, Rizzo M, Berthold HK, Spinas GA, Krone W, Gouni-Berthold I. Ezetimibe alone or in combination with simvastatin increases small dense low-density lipoproteins in healthy men: a randomized trial. Eur. Heart J. 2010;31(13):1633–1639. doi: 10.1093/eurheartj/ehq181. [DOI] [PubMed] [Google Scholar]
- 71.Ose L, Reyes R, Johnson-Levonas AO, Sapre A, Tribble DL, Musliner T. Effects of ezetimibe/simvastatin on lipoprotein subfractions in patients with primary hypercholesterolemia: an exploratory analysis of archived samples using two commercially available techniques. Clin. Ther. 2007;29(11):2419–2432. doi: 10.1016/j.clinthera.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Nakou ES, Filippatos TD, Agouridis AP, Kostara C, Bairaktari ET, Elisaf MS. The effects of ezetimibe and/or orlistat on triglyceride-rich lipoprotein metabolism in obese hypercholesterolemic patients. Lipids. 2010;45(5):445–450. doi: 10.1007/s11745-010-3409-0. ■ Demonstrates particular benefits in HDL subfractions in patients with diabetes mellitus with niacin therapy and is of interest.
- 73.Vakkilainen J, Steiner G, Ansquer JC, et al. Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS) Circulation. 2003;107(13):1733–1737. doi: 10.1161/01.CIR.0000057982.50167.6E. [DOI] [PubMed] [Google Scholar]
- 74.Mostad IL, Bjerve KS, Lydersen S, Grill V. Effects of marine n-3 fatty acid supplementation on lipoprotein subclasses measured by nuclear magnetic resonance in subjects with Type II diabetes. Eur. J. Clin. Nutr. 2008;62(3):419–429. doi: 10.1038/sj.ejcn.1602703. [DOI] [PubMed] [Google Scholar]
- 75.Patti L, Maffettone A, Iovine C, et al. Long-term effects of fish oil on lipoprotein subfractions and low density lipoprotein size in non-insulin-dependent diabetic patients with hypertriglyceridemia. Atherosclerosis. 1999;146(2):361–367. doi: 10.1016/s0021-9150(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 76.Baumstark MW, Frey I, Berg A, Keul J. Influence of n-3 fatty acids from fish oils on concentration of high- and low-density lipoprotein subfractions and their lipid and apolipoprotein composition. Clin. Biochem. 1992;25(5):338–340. doi: 10.1016/0009-9120(92)80012-6. [DOI] [PubMed] [Google Scholar]
- 77.Mckenney JM, McCormick LS, Schaefer EJ, Black DM, Watkins ML. Effect of niacin and atorvastatin on lipoprotein subclasses in patients with atherogenic dyslipidemia. Am. J. Cardiol. 2001;88(3):270–274. doi: 10.1016/s0002-9149(01)01639-3. [DOI] [PubMed] [Google Scholar]
- 78.Kuvin JT, Dave DM, Sliney KA, et al. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am. J. Cardiol. 2006;98(6):743–745. doi: 10.1016/j.amjcard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Pan J, Lin M, Kesala RL, Van J, Charles MA. Niacin treatment of the atherogenic lipid profile and Lp(a) in diabetes. Diabetes Obes. Metab. 2002;4(4):255–261. doi: 10.1046/j.1463-1326.2002.00205.x. ■ Demonstrates particular benefits in HDL subfractions in patients with diabetes mellitus with niacin therapy.
- 80.Lai E, De Lepeleire I, Crumley TM, et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin. Pharmacol. Ther. 2007;81(6):849–857. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- 81.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 82.Chung BH, Segrest JP, Ray MJ, et al. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- 83.Warnick GR, Mcnamara JR, Boggess CN, Clendenen F, Williams PT, Landolt CC. Polyacrylamide gradient gel electrophoresis of lipoprotein subclasses. Clin. Lab. Med. 2006;26(4):803–846. doi: 10.1016/j.cll.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and Type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]