Abstract
The cannabinoid system is known to interact with a variety of neuromodulators in the central nervous system and impacts diverse behaviors. Previous studies have demonstrated that limbic norepinephrine is a critical determinant in the behavioral expression of cannabinoid-induced aversion. The present study was carried out to define the adrenergic receptor subtype involved in mediating cannabinoid-induced behavioral responses. An acute microinjection of the β1-adrenergic receptor blocker, betaxolol, directly into the nucleus accumbens (Acb), was able to prevent WIN 55,212-2-induced aversion, but not lithium-induced aversion, as measured in a place conditioning paradigm. These results suggest that noradrenergic transmission in the Acb is important for cannabinoid-induced aversion and that beta-adrenergic antagonists may be effective in counteracting negative side effects of cannabinoid-based agents.
Keywords: Cannabinoids, lithium, adrenergic receptors, place conditioning, aversion
INTRODUCTION
Previous studies have shown an anatomical and functional interaction between the cannabinoid and noradrenergic systems in the brain. The cannabinoid receptor type 1 (CB1r) has been found in noradrenergic perikarya in the locus coeruleus (LC) [19] and the nucleus of the solitary tract (NTS) [3] as well as in its efferent projections including the prefrontal cortex (PFC) [12] and nucleus accumbens (Acb) [3]. Functionally, administration of the CB1r agonist WIN 55,212-2 increases norepinephrine (NE) release in the PFC and stimulates c-fos expression in the LC and is accompanied by an increase in anxiety-like behaviors [13,14]. Cannabinoids are known to differentially impact animal behaviors with low doses typically inducing reward-like responses and anxiolytic effects while higher doses are associated with aversive and anxiety-like behaviors [6,11]. In a previous study, we demonstrated that limbic NE is a critical determinant of cannabinoid-induced aversion but not cannabinoid-induced anxiety [4]. Although the study provided evidence for an important role of NE in the aversion induced by a cannabinoid agent, it did not establish the adrenergic receptor (AR) subtype involved and whether this effect was specific to cannabinoid-induced aversion or to other aversive stimuli. The present study was carried out using a combined pharmacological and behavioral approach to determine the role of the β1-AR in cannabinoid and lithium-induced aversion. It has been shown that acute and chronic injection of WIN 55,212-2 (3.0mg/kg) leads to a decrease in β1-AR protein expression in the Acb [3], possibly reflecting a role of this receptor in WIN 55,212-2-induced aversion. The experimental design involved conditioning rats to the CB1r agonist, WIN 55,212-2 or lithium, in a place conditioning paradigm and antagonizing the β1-AR using an intra-accumbal microinjection of a β1-AR blocker, betaxolol, prior to testing the animals for aversion.
METHODS
Subjects
Thirty two male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 220–250g were housed separately in a controlled environment (12-hour light schedule (lights on 7:00), temperature at 20°C, humidity at 55%). Food and water were provided ad libitum. The care and use of animals were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and were conducted in accordance with the NIH Guide for the care and use of laboratory animals. All efforts were made to minimize animal suffering and reduce the number of animals used.
Cannulae Implantation and Intracerebral Microinjections
Rats were anesthetized with an intraperitoneal (i.p.) injection of a saline solution containing a cocktail of Ketamine HCl (100mg/kg; Phoenix Pharmaceutical, Inc. St. Joseph, MO) and Xyla-Ject (2mg/kg; Phoenix Pharmaceutical, Inc.) and subsequently placed in a stereotaxic surgical frame (Stoelting Corp., Wood Dale, IL). The anesthesia was maintained by administration of isoflurane (Webster Veterinary Supply, Inc., Sterling, MA) through a nose cone. Bilateral cannulae (22 gauge, 8 mm long, from PlasticOne) were implanted into the Acb (AP: 1.5mm rostral to bregma, ML: +/− 0.9mm, DV: −6.4mm), according to Rat Brain Atlas of Paxinos and Watson [16] coordinates. Cannulae were affixed to the skull using acrylic cement and double stylets were placed in the cannulae to prevent blockage. Animals were given a week to recover from surgery before behavioral testing. For intracerebral microinjections, the obturators were removed and 28 gauge injector cannulae were lowered to the final site (1 mm past the guide). Infusions of 0.5 μL per side were made manually using a Hamilton syringe over a period of 30 sec, as previously described [1].
Drug preparation and administration
Cannabinoid effects on behavior vary depending on dose [6,11]. In the present study, we used a dose that has been shown (e.g. 3.0mg/kg) by our group as well as by others to exert anxiogenic and aversive–like behaviors [4,15]. WIN 55,212-2 (Sigma-Aldrich, St. Louis, MO) was dissolved in 5% dimethyl sulfoxide (DMSO)(Fisher Scientific, Fair Lawn, NJ) in saline and injected i.p. (3.0mg/kg) in a volume of 1ml/kg body weight. Vehicle injections consisted of 5% DMSO in saline. Lithium chloride (LiCl; Sigma-Aldrich) was dissolved in saline and was given intraperitoneally (IP, 1ml/kg body weight) in a dosage of 125 mg/kg. This dosage has been reported to be effective in producing a reliable place aversion [7,20]. Vehicle injections consisted of saline. Drugs were freshly prepared before each treatment trial. Betaxolol (Sigma-Aldrich) was dissolved in saline (1nmol/0.5μl); betaxolol or saline were microinjected in a volume of 0.5 μl per side (as previously described [1]).
Place conditioning
WIN 55,212-2-induced place aversion
The protocol used to induce WIN 55,212-2 aversion follows our previous report [4]. An unbiased place conditioning procedure was used, so that the side of the apparatus used to conditioned animals was counterbalanced in all the groups. The paradigm consisted of three phases: pre-test, conditioning and test. On pre-test day (day 1), animals were placed in the apparatus and allowed to freely explore both sides of the apparatus for 20 min. The time spent in each side was recorded by an investigator. During the conditioning phase (days 2–6), the rats were injected twice daily as follows: in the morning, animals were injected with vehicle and confined to one side of the apparatus for 45 min; in the afternoon, animals were injected with WIN 55,212-2 (3.0mg/kg) and confined to the opposite side for 45 min. On the test day (day 7), animals received a microinjection of betaxolol in the Acb five minutes before being place in the apparatus and allowed to explored both sides for 20 min. Control animals received a microinjection of saline in the Acb. The time spent in each side was measured by an investigator. No WIN 55,212-2 or vehicle injection was given to the animals on the test day.
Lithium-induced place aversion
A different set of animals was conditioned to LiCl. LiCl aversion was achieved as for WIN 55,212-2 with the following modifications. Animals were allowed to explore the apparatus for 15 min in the pre-test and test sessions [7,20]. Conditioning phase lasted 4 consecutive days and animals were confined to one side of the apparatus for 30 min.
Verification of cannula placement
At the conclusion of testing, animals were anesthetized with isoflurane (Isoflurane, USP, Webster Veterinary, Sterling, MA) and decapitated. Brains were removed and placed in 10% buffered formalin (Fisher Scientific) for about two hours and then immersed in O.C.T. Embedding Compound (Electron Microscopy Sciences, Hatfield, PA) and frozen in dry ice. Coronal sections of the forebrain (35um) were cut using a Microm HM550 cryostat (Richard-Allan Scientific, Kalamazoo, MI) and every other section was collected on slide. Slides were allowed to dry and then stained with neutral red. Slides were visualizes using a Leica DMRBE microscope (Wetzlar, Germany), and images were acquired using SPOT Advanced software (Diagnostics Instruments, Inc., Sterling Heights, MI). Figures were then assembled and adjusted for brightness and contrast in Adobe Photoshop CS2.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software. Behavioral data were analyzed using a repeated measures multivariate analysis of variance with “time of testing” as the within-subject factor and “treatment” as the between-subject factor. Post-hoc analyses included paired and independent t-tests. Significance was set at p < 0.05.
RESULTS
Verification of cannulae placement
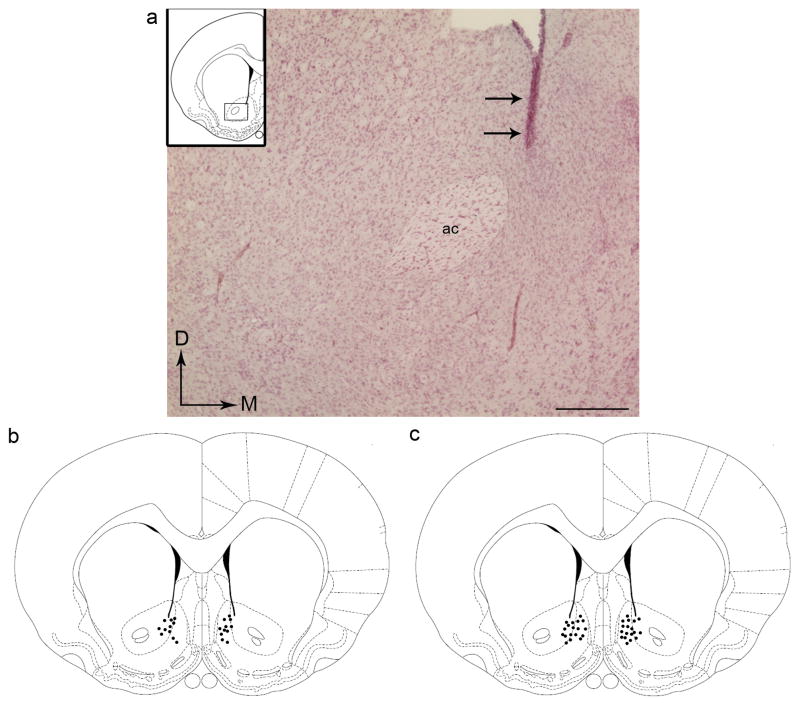
Coronal sections from the forebrain (ranging from plates 20–22 of the rat brain atlas of Paxinos and Watson [16]) were visualized using light microscopy for accuracy of cannulae placement. Of the thirty two subjects, twenty nine exhibited cannulae placements that were restricted to the Acb. Specifically, these did not significantly encroach on surrounding areas (e.g. PFC, BNST, lateral septum, dorsal striatum, ventral pallidum). Figure 1a shows a photomicrograph of a representative cannula placement. For simplicity, Figure 1b shows a schematic representation of all cannulae placements for the eleven animals included in the behavioral analysis for WIN 55,212-2-induced aversion (plate 13 of the brain atlas [16]). Figure 1c shows a schematic representation of all cannulae placements for the eighteen animals includes in the behavioral analysis for LiCl-induced aversion. Placements were localized primarily within the medial aspect of the Acb. Most of the cannulae placements were in the shell subregion but some extended into the core subregion as well.
Figure 1.
Anatomical placement of cannulae in the Acb. a) Photomicrograph of the Acb showing the placement of a cannula (arrows). b) Schematic diagram of a coronal section through the rostral forebrain adapted from the rat brain atlas of Paxinos and Watson [16] showing sites of bilateral cannulae placements into the Acb of animals used for WIN 55,212-2-induced aversion (n=11). c) Schematic diagram showing sites of bilateral cannulae placements into the Acb of animals used for lithium-induced aversion (n=18). Dots represent the tip of the cannulae. Scale bar, 25 μm.
Intra-accumbal injection of betaxolol prevents WIN 55,212-2-induced aversion
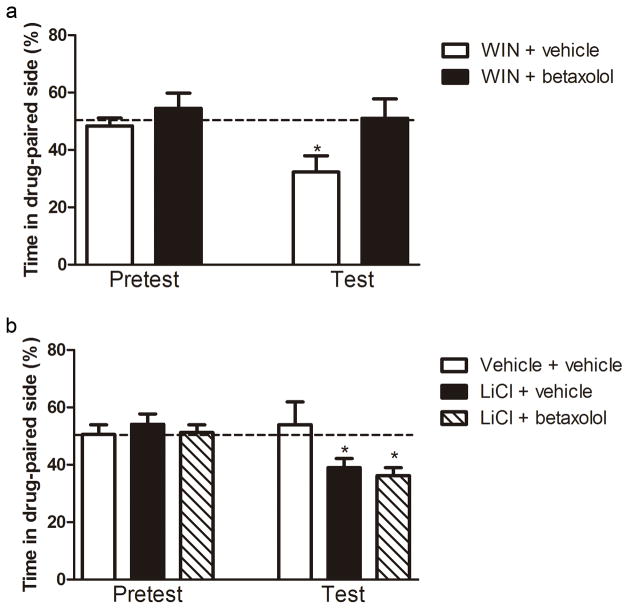
Betaxolol is a selective β1-AR blocker used in the treatment of hypertension and glaucoma. Its selective properties allowed parsing out the involvement of β1-AR in aversion. The place conditioning paradigm was used to assess the aversive effects of WIN 55,212-2 administered at a dose of 3.0 mg/kg [4]. All animals were conditioned to WIN 55,212-2 during the conditioning phase. Animals were assigned to two groups: animals that received betaxolol (n=6) or saline (n=5) prior to the test. Repeated measures analysis revealed that there was an overall effect of time of testing (F(1,9)=10.79, p=0.009), suggesting that the conditioning phase affected the performance of the animals on the test day (Figure 2a). The analysis also showed an interaction between the treatment and time (F(1,9)=6.04, p=0.036). Further analysis showed that the animals that were given saline prior to the test spent significantly less time in the side paired with WIN 55,212-2 in the test day when compared to the pre-test (paired t-test, t(4)=4.64, p=0.01), showing WIN 55,212-2-induced aversion. In contrast, the time spent on the side paired with WIN 55,212-2 on the test day did not differ from the pre-test in the animals that were given betaxolol (paired t-test, t(5)=0.55, p>0.05), suggesting that betaxolol injection prevents WIN 55,212-2-induced aversion. Moreover, the animals given saline spent less time spent in the side paired with WIN 55,212-2 in the test day than the animals that were given betaxolol (independent t-test, t(9)=-2.67, p=0.026). This suggests that β1-ARs in the Acb are important for the development of aversion to WIN 55,212-2.
Figure 2.
a) Effect of betaxolol on the development of WIN 55,212-2-induced place aversion. Animals that received a vehicle injection in the Acb prior to testing (n=5) developed place aversion to WIN 55,212-2 (* p<0.01 compared to saline in pre-test day), as seen by decreased time spent in the drug-paired chamber. Conversely, microinjection of betaxolol (n=6) prevented the development of aversion (+ p<0.05 compared to saline in test day). b) Effect of betaxolol on the development of lithium-induced place aversion. Animals that received a vehicle injection in the Acb prior to testing (LiCL + vehicle, n=7) developed place aversion to lithium (* p<0.05 comparing to pre-test day), as seen by decreased time spent in the drug-paired chamber. Similarly, microinjection of betaxolol (LiCl + betaxolol, n=6) did not affect the development of aversion (p<0.05 comparing to pre-test day).
Lithium-induced aversion is not blocked by intra-accumbal injection of betaxolol
The place conditioning paradigm was used to assess the aversive effects of LiCl administered at a dose of 125 mg/kg [7,20]. Animals were assigned to three groups: animals that received vehicle in both chambers of the apparatus during conditioning phase and intraaccumbal injection of vehicle prior to the test (vehicle + vehicle group, n=5); animals that were conditioned to LiCl and received intraaccumbal injection of vehicle prior to the test (LiCl + vehicle group, n=7) and animals that were conditioned to LiCl but received intraacumbal injection of betaxolol prior to the test (LiCl + betaxolol, n=6). Repeated measures analysis revealed that there was an overall effect of time of testing (F(1,15)=11.04, p=0.05), suggesting that the conditioning phase affected the performance of the animals on the test day (Figure 2b). The analysis also showed an interaction between the treatment and time (F(2,15)=4.81, p=0.024). Further analysis showed that the animals that were conditioned to LiCl and given vehicle prior to the test spent significantly less time in the side paired with LiCl in the test day when compared to the pre-test (paired t-test, t(6)=3.13, p<0.05), showing LiCl-induced aversion. Moreover, intraaccumbal injection of betaxolol prior to the test did not affect the aversion to LiCl (paired t-test, t(5)=4.78, p<0.005). As expected, the control group (vehicle + vehicle) did not show any change in preference in the test day compared with pre-test (paired t-test, t(4)=0.59, p>0.05). This suggests that β1-ARs in the Acb are not critical for the expression of aversion to LiCl.
DISCUSSION
In this study using a place conditioning paradigm, antagonism of β1-ARs in the Acb prior to testing abolished aversion to systemic WIN 55,212-2 administration but not aversion to systemic LiCl. We have previously shown that WIN 55,2121-2-induced aversion was abrogated by depletion of accumbal NE [4]. In that study, depletion of accumbal NE was achieved using an immunotoxin approach, allowing us to deplete NE specifically in the Acb. Thus, animals lacked accumbal NE during the entire conditioning protocol. The present study adds to these previous results by identifying the β1-AR as a target involved in NE signaling and by exploring the specificity of this effect to WIN 55,212-2-induced aversion. Moreover, we have shown that an acute injection of betoxolol in the Acb prior to testing was sufficient to inhibit the expression of aversion to WIN 55,212-2. However, this study did not explored whether the effect of betaxolol is long-lasting.
Methodological considerations
Although the anatomical localization of the injection sites is straightforward, the exact area affected by the injectate is not as precise. In any intracerebral injection, there is the possibility of affecting surrounding areas to the region of interest. This will depend on the amount of volume injected, diffusion properties of the drug and possibly on the area being targeted for analysis. The amount of betaxolol injected (0.5μl/side) has been used in other studies, for different areas such as bed nucleus of the stria terminalis and prefrontal cortex [1,10,18]. Although it is feasible that some dispersion of the drug occurred in surrounding areas, the present results present are consistent with our previous report where depletion of NE, specifically in the Acb, abolished WIN 55,212-2-induced aversion [4]. In recent years, cannabinoid based agents have been explored as potential therapeutic for several disorders ranging from pain to neurodegenerative diseases to psychiatric disorders [5,8]. However, due to the widespread distribution of the endocannabinoid system [17], unwanted side effects often occur. For this reason, it is important to understand targets of the cannabinoid system and determine how these interactions regulate functional endpoints. Our previous and present studies identify the noradrenergic system, specifically limbic NE, as a critical determinant in the expression of cannabinoid-induced aversion. We provide further evidence that an acute microinjection of betaxolol directly into the Acb, after conditioning, blocks the expression of cannabinoid-induced aversion. These data indicate a prominent role of the noradrenergic system in negative effects of cannabinoid exposure. Moreover, in an effort to clarify the specificity of the effect, injection of betaxolol in the Acb had no effect on the aversive properties of LiCl, a substance known to induce aversion, suggesting that alteration of noradrenergic transmission may be specific to cannabinoid-based agents. In addition, the lack of effect of betaxolol in LiCl-induced aversion suggests that betaxolol is not affecting learning per se but rather blocking the expression of WIN 55,212-2-induced aversion.
β1-AR is a G-protein coupled receptor that stimulates Gs, and whose activation can increase glutamate-mediated excitation of medium spiny neurons (MSN) in the Acb [9]. It is hypothesized that activation of MSN can trigger the development of aversive responses while inactivation of MSN can trigger reward responses [2]. Accordingly, inactivation of β1-AR by betaxolol may inhibit WIN 55,212-2-induced activation of Acb neuronal activity resulting in decreased expression of aversion-like behaviors, as seen in the present study. On the other hand, blocking β1-AR does not affect LiCl-induced aversion suggesting that candidate receptors and/or neurotransmitters are mediating LiCl-induced aversion. Altogether, these results suggest that beta-adrenergic antagonists may be effective in counteracting negative side effects of cannabinoid-based agents without affecting responses to other stimuli.
Research Highlights.
The synthetic cannabinoid agonist WIN 55,212-2 induces conditioned place aversion
Betaxolol in the nucleus accumbens blocks WIN 55,212-2-induced aversion
Potential use of beta blockers to block cannabinoid side effects
Acknowledgments
This work was supported by PHS grant DA 020129. Ana Franky Carvalho was supported by the Portuguese Foundation for Science and Technology (SFRH/BD/33236/2007).
Footnotes
Interest Statement
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 2.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci. 2010;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology (Berl) 2010;211:479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crippa JA, Zuardi AW, Hallak JE. Therapeutical use of the cannabinoids in psychiatry. Rev Bras Psiquiatr. 2010;32(Suppl 1):S56–66. [PubMed] [Google Scholar]
- 6.Degroot A. Role of cannabinoid receptors in anxiety disorders. In: Köfalvi A, editor. Cannabinoids and the Brain. Springer; USA: 2008. pp. 559–572. [Google Scholar]
- 7.Frisch C, Hasenohrl RU, Mattern CM, Hacker R, Huston JP. Blockade of lithium chloride-induced conditioned place aversion as a test for antiemetic agents: comparison of metoclopramide with combined extracts of Zingiber officinale and Ginkgo biloba. Pharmacol Biochem Behav. 1995;52(2):321–327. doi: 10.1016/0091-3057(95)00073-6. [DOI] [PubMed] [Google Scholar]
- 8.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 9.Kombian SB, Ananthalakshmi KV, Edafiogho IO. Enaminones and norepinephrine employ convergent mechanisms to depress excitatory synaptic transmission in the rat nucleus accumbens in vitro. Eur J Neurosci. 2006;24(10):2781–2788. doi: 10.1111/j.1460-9568.2006.05152.x. [DOI] [PubMed] [Google Scholar]
- 10.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22(13):5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray JE, Bevins RA. Cannabinoid conditioned reward and aversion: behavioral and neural processes. ACS Chem Neurosci. 2010;1(4):265–278. doi: 10.1021/cn100005p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127(1):36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046(1–2):45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86(1):162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandolfo P, Vendruscolo LF, Sordi R, Takahashi RN. Cannabinoid-induced conditioned place preference in the spontaneously hypertensive rat-an animal model of attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2009;205(2):319–326. doi: 10.1007/s00213-009-1542-3. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- 17.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 18.Ramos BP, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AF. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58(11):894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenk CM, Kavaliers M, Ossenkopp KP. Dose response effects of lithium chloride on conditioned place aversions and locomotor activity in rats. Eur J Pharmacol. 2005;515(1–3):117–127. doi: 10.1016/j.ejphar.2005.04.007. [DOI] [PubMed] [Google Scholar]