Abstract
The in vivo efficacy of JNJ-Q2, a new broad-spectrum fluoroquinolone (FQ), was evaluated in a murine septicemia model with methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) and in a Streptococcus pneumoniae lower respiratory tract infection model. JNJ-Q2 and comparators were also evaluated in an acute murine skin infection model using a community-acquired MRSA strain and in an established skin infection (ESI) model using a hospital-acquired strain, for which the selection of resistant mutants was also determined. JNJ-Q2 demonstrated activity in the MSSA septicemia model that was comparable to that moxifloxacin (JNJ-Q2 50% effective dose [ED50], 0.2 mg/kg of body weight administered subcutaneously [s.c.] and 2 mg/kg administered orally [p.o.]) and activity in the MRSA septicemia model that was superior to that of vancomycin (JNJ-Q2 ED50, 1.6 mg/kg administered s.c.). In an S. pneumoniae lower respiratory tract infection model, JNJ-Q2 displayed activity (ED50, 1.9 mg/kg administered s.c. and 7.4 mg/kg administered p.o.) that was comparable to that of gemifloxacin and superior to that of moxifloxacin. In both MRSA skin infection models, treatment with JNJ-Q2 resulted in dose-dependent reductions in bacterial titers in the skin, with the response to JNJ-Q2 at each dose exceeding the responses of the comparators ciprofloxacin, moxifloxacin, linezolid, and vancomycin. Additionally, in the ESI model, JNJ-Q2 showed a low or nondetectable propensity for ciprofloxacin resistance selection, in contrast to the selection of ciprofloxacin-resistant mutants observed for both ciprofloxacin and moxifloxacin. JNJ-Q2 demonstrated activity that was comparable or superior to the activity of fluoroquinolone or antistaphylococcal comparators in several local and systemic skin infection models performed with both S. aureus and S. pneumoniae and is currently being evaluated in phase II human clinical trials.
INTRODUCTION
Successive improvements in the spectrum and antimicrobial potency of agents in the fluoroquinolone class have resulted in widespread clinical utility of these agents, and the activities of levofloxacin and moxifloxacin against Gram-positive pathogens, particularly Streptococcus pneumoniae, have contributed to the adoption of these agents for the empirical treatment of respiratory tract infections in the community setting. Although fluoroquinolone resistance in S. pneumoniae remains low, with the rate of levofloxacin resistance in U.S. isolates typically reported at less than 1% (12), the rate of fluoroquinolone resistance has, in selected populations or geographic regions, been reported to be greater than 10% (1). In association with the introduction of the seven-valent pneumococcal vaccine (PCV7), an increased prevalence in non-PCV7 serotypes has been observed (11, 12), including several predominant fluoroquinolone-resistant and multidrug-resistant clones (4, 10). Several of the marketed fluoroquinolone agents also display in vitro activity against Staphylococcus aureus isolates and have been used successfully to treat staphylococcal infections (31), although none of these marketed agents are approved for the treatment of methicillin-resistant S. aureus (MRSA) infections. MRSA has become an increasingly important pathogen in community infections (19), and the increased incidence of infection is associated with elevated resistance, with levofloxacin resistance observed in 70% of recent U.S. clinical MRSA isolates (18). Community staphylococcal isolates typically express elevated levels of several virulence determinants, which are associated with increased virulence in murine models of bacteremia and skin abscess infection (20). Efficacy in murine models of MRSA infection is a key attribute for new antibacterial agents targeted for the treatment of staphylococcal infections, including MRSA infections in the community setting. Several investigational fluoroquinolones active against MRSA (2, 5, 15, 17, 32) are reported to be the subject of ongoing clinical studies that are investigating their efficacy in the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by MRSA. The development of new fluoroquinolone agents retaining activity against multidrug-resistant S. pneumoniae isolates and displaying potent antistaphylococcal activities may prove valuable as a therapeutic option for the treatment of respiratory and skin infections.
JNJ-Q2 is a new broad-spectrum fluoroquinolone that displays potent in vitro activity against S. pneumoniae, including levofloxacin-resistant and multidrug resistant isolates, and S. aureus, including MRSA and ciprofloxacin-resistant MRSA isolates (24; D. J. Farrell, L. C. Liverman, D. J. Biedenbach, and R. N. Jones, JNJ-Q2: a new fluoroquinolone with potent in vitro activity against staphylococcus aureus, including methicillin- and fluoroquinolone-resistant strains; poster LB-3, presented at the 48th Annual Meeting of the Infectious Diseases Society of America, 2010) and is currently being evaluated in clinical studies for the treatment of ABSSSI and community-acquired bacterial pneumonia (CABP). JNJ-Q2 displayed lower MIC values than comparator fluoroquinolones against S. pneumoniae and S. aureus isolates, including MRSA (24), and also displayed a lower propensity for resistance selection with these Gram-positive pathogens (24). Here we report the in vivo activities of JNJ-Q2 in murine models of septicemia with MSSA and community-acquired MRSA (CA-MRSA) strains, S. pneumoniae lower respiratory tract infections, and MRSA acute and established skin infections. The selection of MRSA mutants with reduced susceptibility to test agents in the established skin infection model is also reported. In an accompanying article, the in vitro activities of JNJ-Q2 are presented, highlighting the inhibitory activity against purified target enzymes and in vitro biofilms, and in vitro resistance development in MRSA (25).
(Portions of this work were previously presented at the 50th Annual Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy, 2010 [13].)
MATERIALS AND METHODS
Antimicrobial agents.
JNJ-Q2 was synthesized at Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Moxifloxacin was obtained from Bayer AG, gemifloxacin from CB Research and Development, Inc. (New Castle, DE), and linezolid from Organix, Inc. (Wolburn, MA). Ciprofloxacin hydrochloride was obtained from Pentax-Bayer (Kankakee, IL). Vancomycin hydrochloride was purchased from MP Bio (Irvine, CA).
Microorganisms.
S. pneumoniae (ATCC 6301), S. aureus Smith (MSSA; ATCC 13709), and methicillin-resistant S. aureus MRSA (ATCC 43300) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). MRSA OC 8525, a community-acquired MRSA strain, was obtained from Barry Kreiswirth of the Public Health Research Institute, Newark, NJ.
Animals.
Female CF-1 mice (20 to 22 g) and female Crl:SKH1-hrBr hairless, immunocompetent mice (20 to 25 g) were purchased from Charles River Laboratories (Wilmington, MA), and female Swiss-Webster mice (20 to 22 g) were obtained from Taconic Farms, Inc. (Hudson, NY). Animals were allowed free access to food and water and were maintained on a 12 h light/dark cycle. Mice were allowed to acclimate for 5 days after receipt from the vendor. All animal studies were reviewed and approved by the Johnson & Johnson Pharmaceutical Research & Development Institutional Animal Care and Use Committee. Animal numbers were justified by a power analysis of the treatment group sample size necessary to detect a statistically significant decrease in bacterial CFU or mortality by use of Dunnett's multiple comparison test (33).
Inoculum Preparation.
For respiratory studies, S. pneumoniae was grown overnight on tryptic soy agar (TSA) containing 5% sheep blood at 35°C in a 5% CO2 atmosphere. Isolated colonies were inoculated into Trypticase soy broth with 5% heat-inactivated goat serum (Rockland Immunochemicals, Inc., Gilbertsville, PA) and incubated at 35°C until the mid-log growth phase, centrifuged, and then concentrated to approximately 1 × 108 CFU/animal for inoculation. For septicemia studies, overnight cultures of S. aureus Smith ATCC 13709 (MSSA) or S. aureus OC 8525 (CA-MRSA) were inoculated from frozen glycerol stocks into brain heart infusion (BHI) media and shaken for 18 h at 37°C, centrifuged, and diluted in 7.0% hog gastric mucin (Sigma-Aldrich Chemical Company, St. Louis, MO) in saline solution to approximately 3 × 105 CFU/animal (MSSA) or 1 × 107 CFU/animal (CA-MRSA). In skin studies, overnight cultures of S. aureus OC 8525 (CA-MRSA) or MRSA ATCC 43300 were centrifuged as described above and diluted in BHI media with Cytodex microcarrier dextrin beads (Sigma-Aldrich Chemical Company, St. Louis, MO) (131 to 220 μm in diameter) added at a final concentration of 0.1% (vol/vol) to the inoculating dose (7 × 106 CFU/mouse, CA-MRSA, acute skin infection model; 9 × 106 CFU/mouse, MRSA ATCC 43300, established skin infection model).
In vitro susceptibility.
MICs were determined by broth microdilution methods according to CLSI guidelines (9).
Mouse septicemia model.
To study the comparative efficacies of JNJ-Q2, ciprofloxacin, moxifloxacin, and gemifloxacin in the mouse septicemia model (14, 16), Swiss Webster mice were infected intraperitoneally (i.p.) with S. aureus Smith (MSSA) ATCC 13709 (approximately 3 × 105 CFU/mouse, 10× to 100× the 50% lethal dose [LD50]) or with CA-MRSA OC 8525 (approximately 1 × 107 CFU/mouse, 10× to 100× the LD50) and then treated either subcutaneously or orally for 1 h (JNJ-Q2) or 1 and 3 h (comparators) postinfection, and mortality was monitored for 3 days. The dosing regimen (once a day [QD] versus twice a day [BID]) was determined from pilot studies conducted in the mouse septicemia model (data not shown). The 50% effective dose (ED50) and the 90% effective dose (ED90), the doses at which 50% and 90% survival were observed, respectively, and 95% fiducial limits were calculated from the survival curves using a logistical regression. All statistical analyses were carried out in SAS version 5 or 9.1.3 (SAS Institute, Cary, NC).
Lower respiratory tract infection model.
To study the comparative efficacies of JNJ-Q2, moxifloxacin, and gemifloxacin in a murine lower respiratory tract infection model (14, 16), female CF-1 mice were briefly anesthetized with isoflurane and infected by placing 50 μl of S. pneumoniae ATCC 6301 (approximately 1 × 108 CFU/mouse) at the tip of the nares and allowing the mouse to inhale the inoculum. Mice were then treated subcutaneously or orally once at 24 h postinfection, and mortality was monitored over 2 days. The dose that provided 50% or 90% survival (ED50 or ED90) was calculated from the survival curves using a logistic regression.
Acute skin infection model.
To study the comparative efficacies of JNJ-Q2, linezolid, and vancomycin against S. aureus in an acute skin infection model (13, 14, 16), female SKH1 mice were anesthetized with isoflurane and given a 0.2-ml subcutaneous (s.c.) injection of a Cytodex bead inoculum containing S. aureus (CA-MRSA OC 8525) (approximately 7 × 106 CFU/mouse flank) on the left and right flanks. Animals received JNJ-Q2, linezolid, or vancomycin at 1, 3, 25, and 27 h postinfection at a dose of 1.6, 6.2, 25, or 100 mg/kg of body weight/day. All drugs were delivered orally except for vancomycin, which was given subcutaneously. Animals were euthanized by CO2 asphyxiation 48 h after infection, and the lesions on each flank were measured. A lesion volume score was calculated from the following equation; LV = (π/6) (L × W2), where LV represents the lesion volume, L represents the length of the lesion in millimeters, and W represents the width of the lesion in millimeters (7). For determination of CFU numbers per gram of skin, the skin from the infected areas was disinfected with chlorhexidine diacetate (Nolvasan; Fort Dodge Animal Health, Fort Dodge, IA), excised, weighed, and homogenized in 1 ml of saline solution (Omni Prep tissue homogenizer and Omni Tip disposable rotor stator generator probes; Omni International, Marietta, GA) (4°C and 35,000 rpm for 0.5 min). Serial 100-fold dilutions in saline solution (Remel, Lenexa, KS) (0.85% saline) were plated (Autoplate 4000; Spiral Biotech, Inc., Norwood, MA) on TSA plates. The plates were incubated for 18 h at 37°C and the CFU counted (Q-Count; Spiral Biotech, Inc., Norwood, MA).
Established skin infection model.
To study the comparative efficacies of JNJ-Q2, ciprofloxacin, and moxifloxacin against S. aureus in an established skin infection model (14), mice were anesthetized with isoflurane and given a 0.2-ml subcutaneous injection of the Cytodex bead inoculum containing S. aureus (MRSA ATCC 43300) (approximately 9 × 106 CFU/mouse flank) on the left and right flanks. Then, at 3 days postinfection, twice-daily treatment with JNJ-Q2, ciprofloxacin, or moxifloxacin (25 to 200 mg/kg/day) was initiated, and the treatment was administered for 3 days. All drugs were delivered orally except for ciprofloxacin, which was given intraperitoneally. At 24 h following the last dose (a time point chosen to minimize any drug carryover effect), the animals were euthanized, and skin tissue was processed and analyzed as described previously for the acute skin infection model.
To assess in vivo resistance selection, an undiluted skin sample was spiral plated on TSA plates containing ciprofloxacin (2 μg/ml). The plates were incubated for 48 h at 37°C, and the number of CFU was determined. Any colony that grew on a TSA plate containing 2 μg/ml of ciprofloxacin (4× the MIC) within 48 h was defined as exhibiting resistance.
Statistical methodology.
Preliminary evaluations using descriptive summary statistics suggested mean treatment differences between JNJ-Q2 and comparative treatment groups. Evaluation to determine whether these differences were significant was then performed using logistic regression (for septicemia, lower respiratory tract infection, and resistance selection) (29) or linear mixed-effects modeling (for the acute and established skin infection models) (21) to explain the response ratios as a function of log-transformed doses and treatment groups. The modeling was adjusted to account for overdispersion as appropriate.
Further comparisons of the effects of JNJ-Q2 seen with comparative treatments were assessed by using a linear-contrast argument within this model. Additionally, modeling diagnostics were provided for assessing goodness of fit. Differences were considered significant at the 0.05 level.
RESULTS
In vitro susceptibility.
The MICs for JNJ-Q2 and all comparators against S. pneumoniae ATCC 6301, S. aureus Smith (MSSA; ATCC 13709), and methicillin-resistant S. aureus (MRSA; OC 8525 and ATCC 43300) are shown in Table 1. Against S. pneumoniae, JNJ-Q2 was 2-, 16-, 16-, 32-, and 64-fold more potent than gemifloxacin, ciprofloxacin, moxifloxacin, vancomycin, and linezolid, respectively. Against MSSA, JNJ-Q2 and gemifloxacin were equipotent, and both were 8-, 32-, 128-, and 512-fold more potent than moxifloxacin, ciprofloxacin, vancomycin, and linezolid, respectively. Against MRSA, JNJ-Q2 was 4- to 128-fold more potent than the fluoroquinolone comparators and 64- to 512-fold more potent than the anti-MRSA comparators linezolid and vancomycin.
Table 1.
In vitro susceptibility of Gram-positive pathogens to JNJ-Q2 and comparators in the mouse infection models
| Drug | MIC (μg/ml) |
|||
|---|---|---|---|---|
| S. pneumoniae ATCC 6301 | MSSA ATCC 13709 | MRSA OC 8525 | MRSA ATCC 43300 | |
| JNJ-Q2 | 0.015 | 0.008 | 0.015 | 0.004 |
| Ciprofloxacin | 0.25 | 0.25 | 2 | 0.5 |
| Moxifloxacin | 0.25 | 0.06 | 0.25 | 0.03 |
| Gemifloxacin | 0.03 | 0.008 | 0.06 | 0.03 |
| Linezolid | 1 | 4 | 4 | 2 |
| Vancomycin | 0.5 | 1 | 1 | 1 |
Mouse septicemia model.
The activities (ED50s and ED90s) of JNJ-Q2, moxifloxacin, and gemifloxacin are summarized in Table 2. In the systemic infection model used with S. aureus Smith, JNJ-Q2 was 6- and 9-fold more potent than ciprofloxacin by the oral (P < 0.0021) and subcutaneous (P < 0.0001) routes of administration, respectively. JNJ-Q2 displayed ED50 values that were similar to moxifloxacin and gemifloxacin values by the subcutaneous route of administration. However, JNJ-Q2 was more potent (P < 0.0001) than gemifloxacin according to comparisons of the slopes of the dose-response profiles. When the compounds were administered orally, ED50 values for JNJ-Q2 were similar to moxifloxacin values (P > 0.85), but JNJ-Q2 was slightly less potent than gemifloxacin (P > 0.14).
Table 2.
In vivo efficacy of single doses of JNJ-Q2 and comparators in a murine septicemia model with S. aureus Smith (MSSA) or OC 8525 (CA-MRSA)
| Compound, organism category, and dosing route (na) | ED50 in mg/kg/dayb (95% fiducial limits) | ED90 in mg/kg/dayc (95% fiducial limits) | P valued |
|---|---|---|---|
| JNJ-Q2 | |||
| MSSA | |||
| p.o. (24) | 2.0 (1.5-2.5) | 4.0 (3.2-7.9) | NA |
| s.c. (16) | 0.15 (0.05-0.22) | 0.4 (0.3-0.8) | NA |
| CA-MRSA | |||
| p.o. (16) | 12 (9.1-20.4) | 33.7 (21.1-108.6) | NA |
| s.c. (16) | 1.6 (1.0-2.2) | 5.4 (3.4-10.6) | NA |
| Ciprofloxacin | |||
| MSSA | |||
| p.o. (16) | 11 (6.5-41) | 31.0 (NL) | <0.0021 |
| s.c. (16) | 1.4 (0.92-2.5) | 3.3 (NL) | <0.0001 |
| Moxifloxacin | |||
| MSSA | |||
| p.o. (13) | 1.5 (0.73-2.2) | 3.1 (2.2-7.6) | >0.85 |
| s.c. (13) | 0.4 (0.2-0.8) | 2.8 (1.5-9.7) | <0.0001 |
| Gemifloxacin | |||
| MSSA | |||
| p.o. (21) | 1.1 (0.82-1.4) | 2.8 (1.4-3,036) | >0.14 |
| s.c. (13) | 0.1 (0.06-0.14) | 0.2 (0.15-0.45) | <0.0001 |
| Linezolide | |||
| CA-MRSA | |||
| p.o. (18) | 5.1 (3-8) | 16.6 (11.9-31.5) | <0.0087 |
| s.c. (18) | 3.7 (2-6) | 11.6 (8.3-22.8) | <0.0004 |
| Vancomycine | |||
| CA-MRSA | |||
| s.c. (32) | 12 (10-14) | 40.8 (29.1-69.4) | <0.0001 |
n, number of animals per group.
ED50s calculated using SAS version 5.
ED90s calculated using SAS version 9.1. NL, no limits obtained.
P values represent measurements of the significance of comparisons of dose-response profiles of survival incidence data for JNJ-Q2 and comparators. NA, not applicable.
Dosed BID, 1 and 3 h postinfection.
JNJ-Q2 was also compared to the anti-MRSA comparators linezolid and vancomycin with respect to activity against a CA-MRSA (OC 8525) strain in the murine septicemia model (Table 2). The activity (ED50) of JNJ-Q2 administered orally was less than that of linezolid (P < 0.0087) in this model; however, JNJ-Q2 was 2- and 8-fold more active than linezolid (P < 0.0004) and vancomycin (P < 0.0001), respectively, when the compounds were administered subcutaneously.
Lower respiratory tract infection model.
The efficacies (ED50s and ED90s) of JNJ-Q2, moxifloxacin, and gemifloxacin in the mouse S. pneumoniae lower respiratory tract infection model are detailed in Table 3. JNJ-Q2 was 2- and 10-fold more active than moxifloxacin by the oral (P < 0.0086) and subcutaneous (P < 0.0001) routes of administration, respectively. JNJ-Q2 displayed activity similar to that of gemifloxacin when administered either subcutaneously or orally in this model.
Table 3.
In vivo efficacy of single doses of JNJ-Q2 and comparators in a murine lower respiratory tract infection model with S. pneumoniae ATCC 6301
| Compound and dosing route (na) | ED50 in mg/kg/dayb (95% fiducial limits) | ED90 in mg/kg/dayc (95% fiducial limits) | P valued |
|---|---|---|---|
| JNJ-Q2 | |||
| p.o. (16) | 7.4 (5.3-10.1) | 19.7 (13.8-38.0) | NA |
| s.c. (16) | 1.9 (0.7-3.3) | 18.3 (9.5-80.3) | NA |
| Moxifloxacin | |||
| p.o. (16) | 14 (8-23) | 41.5 (28.3-84.2) | <0.0086 |
| s.c. (16) | 23 (NL) | NATC | <0.0001 |
| Gemifloxacin | |||
| p.o. (16) | 3.9 (2.2-5.8) | 25.7 (16.4-53.2) | <0.0066 |
| s.c. (16) | 2.0 (1.2-2.9) | 15.2 (8.9-40.6) | <0.0104 |
n, number of animals per group.
ED50s calculated using SAS version 5. NL, no limits obtained.
ED90s calculated using SAS version 9.1. NATC, program not able to calculate.
P values represent measurements of the significance of comparisons of dose-response profiles of survival incidence data for JNJ-Q2 and comparators. NA, not applicable.
Acute skin infection model.
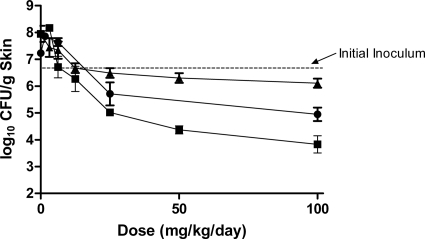
The effects of JNJ-Q2, linezolid, and vancomycin on acute skin infections mediated by a CA-MRSA strain (OC 8525) are shown in Fig. 1. In untreated control animals, the starting inoculum of approximately 6.8 log10 CFU increased to 7.5 log10 CFU (8 log10 CFU/g skin tissue) during the 48-h testing period. At every dose tested (1.6 to 100 mg/kg/day), JNJ-Q2 displayed greater reductions in bacterial burden in the skin of mice than linezolid (P < 0.0001) or vancomycin (P < 0.0045). At the highest dose tested (100 mg/kg/day), JNJ-Q2 reduced the bacterial burden in the skin by 2.5 and 1.3 log10 CFU/g more than linezolid and vancomycin, respectively, and reduced the bacterial titer by nearly 3 log10 CFU/g from the titer seen with the starting inoculum.
Fig. 1.
The effect of JNJ-Q2 (p.o., squares), linezolid (p.o., triangles), and vancomycin (s.c., circles) on acute CA-MRSA (OC 8525) skin infections. Each symbol represents the mean ± standard error for 8 to 20 mice. The starting inoculum was 6.8 log10 CFU/mouse, and untreated control values (48 h) were 8.0 log10 CFU/g skin tissue. The P values were generated by comparing the slopes of the dose-response curves for JNJ-Q2 versus linezolid (P value, <0.0001) and for JNJ-Q2 versus vancomycin (P value, <0.0045).
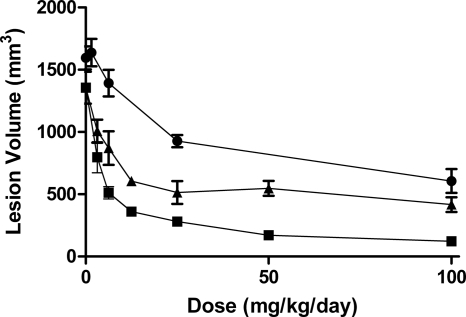
The skin lesion volumes resulting from infection with the CA-MRSA strain are shown in Fig. 2. The reductions in lesion volume were concordant with the reductions in CFU (Fig. 1). Animals treated with JNJ-Q2 had the smallest lesion volumes at every dose compared to those resulting from treatment with linezolid (P < 0.0001) and vancomycin (P < 0.0001).
Fig. 2.
The effect of JNJ-Q2 (p.o., squares), linezolid (p.o., triangles), and vancomycin (s.c., circles) on lesion volume in mice infected with CA-MRSA (OC 8525) in the acute skin infection model. Each symbol represents the mean ± standard error for 8 to 20 mice. The starting inoculum was 6.8 log10 CFU/mouse, and untreated control values (48 h) were 8.0 log10 CFU/g skin tissue. The P values were generated by comparing the slopes of the dose-response curves for JNJ-Q2 versus linezolid (P value, <0.0001) and for JNJ-Q2 versus vancomycin (P value, <0.0001) (note that the modeling contains a term for quadratic dose determinations).
Established skin infection model.
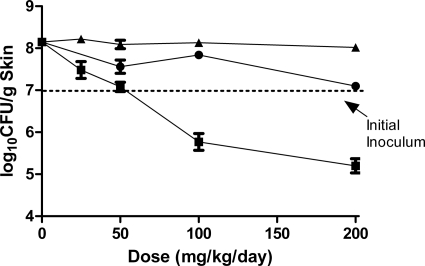
The efficacies of JNJ-Q2, ciprofloxacin, and moxifloxacin in mice with established (3-day) skin infections due to MRSA ATCC 43300 are shown in Fig. 3. Bacterial burdens in untreated control mice increased 1.4 log10 CFU to 7.7 log10 CFU (8.2 log10 CFU/g skin tissue) over the 6-day testing period. Oral treatment with JNJ-Q2 at 25, 50, 100, and 200 mg/kg/day resulted in dose-dependent reductions of 0.7, 1.1, 2.4, and 3.0 log10 CFU/g skin tissue. In contrast, treatment with either ciprofloxacin (P < 0.0007) or moxifloxacin (P < 0.0379) did not result in reductions in CFU below the initial infecting inocula (6.8 log10 CFU). At 200 mg/kg/day, the highest dose tested, JNJ-Q2 treatment resulted in 2.8- and 1.9-fold greater reductions of MRSA numbers in the skin of mice than ciprofloxacin and moxifloxacin, respectively.
Fig. 3.
The effect of JNJ-Q2 (p.o., squares), ciprofloxacin (i.p., triangles), and moxifloxacin (p.o., circles) on established MRSA ATCC 43300 skin infections. Each symbol represents the mean ± standard error for 14 to 16 mice. The starting inoculum was 7.0 log10 CFU/mouse, and untreated control values (3 days) were 8.2 log10 CFU/g skin tissue. The P values were generated by comparing the slopes of the dose-response curves for JNJ-Q2 versus ciprofloxacin (P value, <0.0007) and for JNJ-Q2 versus moxifloxacin (P value, <0.0379) (note that the modeling contains a term for dose * treatment interaction determinations).
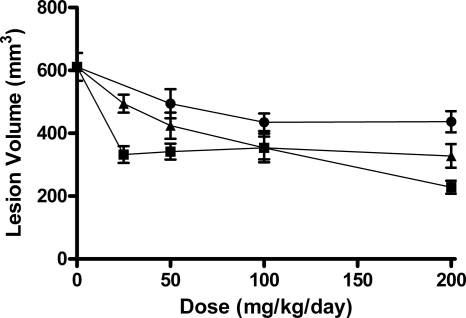
Skin lesion volumes for mice infected with MRSA ATCC 43300 and treated with JNJ-Q2, ciprofloxacin, or moxifloxacin are shown in Fig. 4. Control mice had a mean lesion volume of 611 mm3. Treatment with JNJ-Q2 resulted in lesion volumes that were 46 to 63% smaller than those in control mice, in comparison with reductions in lesion volume of 19 to 46% and 19 to 28% for ciprofloxacin (P < 0.0092) and moxifloxacin (P < 0.0001), respectively.
Fig. 4.
The effect of JNJ-Q2 (p.o., squares), ciprofloxacin (i.p., triangles), and moxifloxacin (p.o., circles) on lesion volume in mice with established MRSA ATCC 43300 skin infections. Each symbol represents the mean ± standard error for 14 to 16 mice. The starting inoculum was 7.0 log10 CFU/mouse, and untreated control values (3 days) were 8.2 log10 CFU/g skin tissue. The P values were generated by comparing the slopes of the dose-response curves for JNJ-Q2 versus ciprofloxacin (P value, <0.0092) and for JNJ-Q2 versus moxifloxacin (P value, <0.0001) (note that the modeling contains a term for quadratic dose determinations).
The propensities for JNJ-Q2, ciprofloxacin, and moxifloxacin to select for ciprofloxacin resistance in the established mouse skin infection model are summarized in Table 4. No resistant colonies were detected in any of the samples after treatment with JNJ-Q2 at 50 to 200 mg/kg/day. Samples from animals treated with JNJ-Q2 at 25 mg/kg/day contained low levels of resistant colonies, which grew in the presence of 2 μg/ml ciprofloxacin in 3/16 skin samples; however, counts were below the limit of reliable detection. In contrast, resistant colonies were recovered from each dose group of animals receiving ciprofloxacin. The density of resistant bacterial cells averaged approximately 3 log10 CFU/g skin tissue in each ciprofloxacin dose group. Ciprofloxacin-resistant colonies were also cultured from samples taken from moxifloxacin-treated animals (50 to 200 mg/kg/day), with resistant bacterial densities ranging from 2.6 to 3.2 log10 CFU/g skin tissue. The selection of resistance in infected animals treated with ciprofloxacin or moxifloxacin was statistically significant (P < 0.001) compared to the incidence of resistance following treatment of animals with JNJ-Q2.
Table 4.
Incidence of ciprofloxacin-resistant bacteria arising within ATCC 43300 (MRSA) mouse skin abscesses following treatment with JNJ-Q2 or comparators
| Dose (mg/kg/day) | No. of resistant colonies/total no. of coloniesa |
||
|---|---|---|---|
| JNJ-Q2b | Ciprofloxacin | Moxifloxacin | |
| 25 | 3/16 | 16/16 | NTc |
| 50 | 0/16 | 16/16 | 5/16 |
| 100 | 0/14 | 11/16 | 7/16 |
| 200 | 0/14 | 11/16 | 2/16 |
Values represent the total number of samples that had any MRSA growth on a TSA plate containing ciprofloxacin (2 μg/ml) versus the total number of samples tested.
P < 0.001 versus ciprofloxacin and moxifloxacin by logistic regression analysis.
NT, not tested.
DISCUSSION
The activity of the new fluoroquinolone JNJ-Q2 compared to the activity of other fluoroquinolones and anti-MRSA comparators in murine models of systemic, respiratory, and localized skin infections was assessed. The activities assessed included the efficacy of JNJ-Q2 in treating murine systemic and skin infections caused by MRSA and an established skin infection model with a virulent CA-MRSA isolate.
The fluoroquinolone moxifloxacin has demonstrated increased utility in treating respiratory tract infections, as it is associated with high rates of microbiological success in the clinic (3). In vitro, moxifloxacin displays activity against S. pneumoniae, including some ciprofloxacin-resistant isolates carrying quinolone resistance-determining region (QRDR) mutations; however, the MICs of JNJ-Q2 were 32-fold lower than those of moxifloxacin for these S. pneumoniae isolates (24). The greater in vitro potency of JNJ-Q2 was reflected in the relative activities of these agents in the murine lower respiratory tract infection model, in which JNJ-Q2 displayed lower ED50 and ED90 values than moxifloxacin.
The increased in vitro activity of JNJ-Q2, in comparison with other fluoroquinolone agents, against S. aureus, including MRSA and ciprofloxacin-resistant MRSA (24), was likewise reflected in the murine septicemia model with S. aureus MSSA and MRSA strains. Against MSSA, peroral ED50 values for JNJ-Q2 were comparable to moxifloxacin values, although JNJ-Q2 was more active by the subcutaneous route of administration. The moxifloxacin ED50 values for S. aureus Smith in our study closely matched those published previously for a systemic infection model (27). In the septicemia model with MRSA, JNJ-Q2 was more active than either of the anti-MRSA agents linezolid or vancomycin.
JNJ-Q2 exhibited dose-dependent reductions in bacterial load in an established MRSA mouse skin infection model, with limited evidence of resistance selection. In the MRSA established skin infection model, no resistant colonies were selected by JNJ-Q2 at doses of 50 to 200 mg/kg/day. This was in contrast to the comparator fluoroquinolone agents ciprofloxacin and moxifloxacin, which both selected for ciprofloxacin-resistant colonies within infected skin at the same doses. Poor efficacy in the established skin infection model observed in ciprofloxacin-treated animals was possibly due to the selection of resistant bacteria during treatment. Resistance selection in ciprofloxacin-treated animals was extensive, with 70 to 100% of treated mice yielding resistant colonies from infected skin samples. Given the clinical experience of ciprofloxacin, against which resistance in MRSA was observed to emerge rapidly (6), the reduced potential for resistance selection is a key attribute for a new fluoroquinolone developed to treat MRSA infections. Weight loss was noted in the high-ciprofloxacin-dose group (data not shown), which precluded increasing the dose to further evaluate efficacy. Concordant with our efficacy results, Cagni and colleagues (8) reported only minimal activity with ciprofloxacin (administered BID for 7 days) in a 21-day established rat tissue cage infection model; in contrast to our study, however, they did not isolate any resistant colonies following therapy. The difference in resistance selection observed here in the skin infection model reflects differences in the in vitro resistance rates observed for JNJ-Q2 and ciprofloxacin with MRSA, including isolates carrying QRDR mutations and displaying elevated ciprofloxacin MICs (24). Minimal efficacy was noted with moxifloxacin, even when administered at 200 mg/kg/day. As seen with ciprofloxacin treatment, resistant isolates were recovered at every dose level tested, but to a lesser extent, which may have negatively impacted the efficacy of moxifloxacin in this setting. This model of an established skin infection was fairly robust in that the infection involved a bacterial population of 8 log10 CFU/g tissue (7.7 log10 CFU total), permitting the differentiation of agents with disparate propensities for resistance selection.
In both the acute and established skin infection models, the observed reductions in lesion volume were generally dose dependent; however, lesions that were smaller overall were observed in animals in the established skin infection model. This may be reflective of staphylococcal infections being self-limiting in these murine models and could in part be due to the reported enhanced ability of S. aureus to bind hemoglobin derived from humans compared to that derived from other mammals, including mice (28).
The increased efficacy of JNJ-Q2 in comparison to ciprofloxacin and moxifloxacin in several mouse models of infection may have resulted from the lower MIC values of JNJ-Q2 for the infecting strains, and the pharmacokinetic exposures of JNJ-Q2 in the mouse underscore the potency of this compound. JNJ-Q2 is 67% bound to mouse plasma proteins and was 8% orally bioavailable in the mouse (S. Steller and A. Streeter, unpublished data). This compares to mouse oral bioavailability of 38% and 78% for ciprofloxacin and moxifloxacin, respectively (22, 30). JNJ-Q2 was 65% orally bioavailable in dogs, monkeys (G. Eichenbaum and S. Stellar, unpublished data), and humans (J. M. Davenport, P. Covington, L. Liverman, G. McIntyre, and J. Almenoff, presented at the Annual Meeting of the American College of Clinical Pharmacology, Chicago, IL, 2011). In the mouse, an oral dose of JNJ-Q2 administered at 10 mg/kg yielded an area under the curve (AUC) of 0.13 μg·h/ml (S. Steller and A. Streeter, unpublished data), a value that is 11-fold and 6-fold lower than the values seen with oral ciprofloxacin and moxifloxacin mouse exposures, respectively (23, 27). In the established skin infection model, studies with ciprofloxacin and moxifloxacin included doses of 150 and 200 mg/kg, respectively, achieving exposures in the mouse comparable to human doses of 750 and 400 mg, respectively (23, 26, 27). In the septicemia model, the lower ED50 value for the subcutaneous administration of JNJ-Q2 in comparison to that for moxifloxacin reflects the lower MIC values for JNJ-Q2, but the lower ED50 values for moxifloxacin following peroral administration likely resulted from its greater oral bioavailability in the mouse. In contrast, in the lower respiratory tract infection model, JNJ-Q2 displayed lower ED50 values than moxifloxacin by both oral and subcutaneous routes of administration, possibly resulting from increased in vitro potency and the potential for increased lung exposure for JNJ-Q2.
In conclusion, JNJ-Q2 displayed promising levels of efficacy in a variety of local and systemic mouse infection models and warrants further study.
ACKNOWLEDGMENTS
We are grateful to Karen Bush for her early leadership of this project. We thank Karen Amsler and Barbara Foleno for their assistance with the in vitro characterization of JNJ-Q2.
Footnotes
Published ahead of print on 12 September 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Adam H. J., Hoban D. J., Gin A. S., Zhanel G. G. 2009. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997-2006. Int. J. Antimicrob. Agents 34: 82–85 [DOI] [PubMed] [Google Scholar]
- 2. Almer L. S., Hoffrage J. B., Keller E. L., Flamm R. K., Shortridge V. D. 2004. In vitro and bactericidal activities of ABT-492, a novel fluoroquinolone, against gram-positive and gram-negative organisms. Antimicrob. Agents Chemother. 48: 2771–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. An M. M., et al. 2010. Moxifloxacin monotherapy versus beta-lactam-based standard therapy for community-acquired pneumonia: a meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents 36: 58–65 [DOI] [PubMed] [Google Scholar]
- 4. Ardanuy C., et al. 2009. Emergence of a multidrug-resistant clone (ST320) among invasive serotype 19A pneumococci in Spain. J. Antimicrob. Chemother. 64: 507–510 [DOI] [PubMed] [Google Scholar]
- 5. Bhagwat S. S., McGhee P., Kosowska-Shick K., Patel M. V., Appelbaum P. C. 2009. In vitro activity of the quinolone WCK 771 against recent U.S. hospital and community-acquired Staphylococcus aureus pathogens with various resistance types. Antimicrob. Agents Chemother. 53: 811–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blumberg H. M., Rimland D., Carroll D. J., Terry P., Wachsmuth I. K. 1991. Rapid development of ciprofloxacin resistance in methicillin-susceptible and -resistant Staphylococcus aureus. J. Infect. Dis. 163: 1279–1285 [DOI] [PubMed] [Google Scholar]
- 7. Bunce C., Wheeler L., Reed G., Musser J., Barg N. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60: 2636–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cagni A., Chuard C., Vaudaux P. E., Schrenzel J., Lew D. P. 1995. Comparison of sparfloxacin, temafloxacin, and ciprofloxacin for prophylaxis and treatment of experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 39: 1655–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CLSI 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—7th ed., vol. 29. CLSI document M07-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Dagan R., Givon-Lavi N., Leibovitz E., Greenberg D., Porat N. 2009. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J. Infect. Dis. 199: 776–785 [DOI] [PubMed] [Google Scholar]
- 11. Davies T. A., et al. 2008. Effects of the 7-valent pneumococcal conjugate vaccine on U.S. levofloxacin-resistant Streptococcus pneumoniae. Microb. Drug Resist. 14: 187–196 [DOI] [PubMed] [Google Scholar]
- 12. Davies T. A., Yee Y. C., Goldschmidt R., Sahm D. F., Evangelista A. T. 2008. Decline in the prevalence of pandemic clones Spain23F-1 and Spain9V-3 among US fluoroquinolone-resistant Streptococcus pneumoniae TRUST surveillance isolates since 2001. Postgrad. Med. 120: 39–45 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez J., et al. 2010. In vivo activity of ceftobiprole in murine skin infections due to Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54: 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandez J., et al. 2010. Efficacy of a new fluoroquinolone (FQ) JNJ-Q2 in murine models of Staphylococcus aureus and Streptococcus pneumoniae infection, abstr. F1-2093. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fritsche T. R., Sader H. S., Jones R. N. 2007. Potency and spectrum of garenoxacin tested against an international collection of skin and soft tissue infection pathogens: report from the SENTRY antimicrobial surveillance program (1999-2004). Diagn. Microbiol. Infect. Dis. 58: 19–26 [DOI] [PubMed] [Google Scholar]
- 16. Hilliard J. J., et al. 2009. In vivo activity of the pyrrolopyrazolyl-substituted oxazolidinone RWJ-416457. Antimicrob. Agents Chemother. 53: 2028–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoshino K., et al. 2008. In vitro and in vivo antibacterial activities of DC-159a, a new fluoroquinolone. Antimicrob. Agents Chemother. 52: 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones R. N., Mendes R. E., Sader H. S. 2010. Ceftaroline activity against pathogens associated with complicated skin and skin structure infections: results from an international surveillance study. J. Antimicrob. Chemother. 65: 17–31 [DOI] [PubMed] [Google Scholar]
- 19. Klevens R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763–1771 [DOI] [PubMed] [Google Scholar]
- 20. Li M., et al. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106: 5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Little R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., Schabenbgerger O. 2006. SAS for mixed models. SAS Institute, Inc., Cary, NC [Google Scholar]
- 22. Liu X. G., Li R. C. 2001. Effects of repeated rifabutin administration on the pharmacokinetics of intravenous and oral ciprofloxacin in mice. J. Chemother. 13: 563–568 [DOI] [PubMed] [Google Scholar]
- 23. Miyazaki E., Miyazaki M., Chen J. M., Chaisson R. E., Bishai W. R. 1999. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 43: 86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morrow B. J., et al. 2010. In vitro antibacterial activities of JNJ-Q2, a new broad-spectrum fluoroquinolone. Antimicrob. Agents Chemother. 54: 1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morrow B. J., et al. 2011. Antistaphylococcal activities of the new fluoroquinolone JNJ-Q2. Antimicrob. Agents Chemother. 55: 5512–5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owens R. C., Jr., Ambrose P. G. 2002. Pharmacodynamics of quinolones, p. 155–176 In Nightingale C. H., Murakawa T., Ambrose P. G. (ed.), Antimicrobial pharmacodynamics in theory and clinical practice. Marcel Dekker Inc., New York, NY [Google Scholar]
- 27. Patel M. V., et al. 2004. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob. Agents Chemother. 48: 4754–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pishchany G., et al. 2010. Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell Host Microbe 8: 544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. SAS Institute 2008. The logistic procedure; SAS/STAT 9.2 user's guide. SAS Institute, Inc., Cary, NC [Google Scholar]
- 30. Siefert H. M., et al. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43: 69–76 [DOI] [PubMed] [Google Scholar]
- 31. Trong H. N. G., Prunier A.-L., Leclercq R. 2005. Hypermutable and fluoroquinolone-resistant clinical isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 49: 2098–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe S., Ito T., Hiramatsu K. 2007. Susceptibilities of healthcare- and community-associated methicillin-resistant staphylococci to the novel des-F(6)-quinolone DX-619. J. Antimicrob. Chemother. 60: 1384–1387 [DOI] [PubMed] [Google Scholar]
- 33. Westfall P. H. T., Rom R. D. D., Wolfinger R. D., Hochberg Y. 1999. Multiple comparisons and multiple tests using the SASTM system. SAS Institute Inc., Cary, NC [Google Scholar]