Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is an increasingly common cause of health care-associated urinary tract infections. Antimicrobials with in vitro activity against CRKP are typically limited to polymyxins, tigecycline, and often, aminoglycosides. We conducted a retrospective cohort study of cases of CRKP bacteriuria at New York-Presbyterian Hospital from January 2005 through June 2010 to compare microbiologic clearance rates based on the use of polymyxin B, tigecycline, or an aminoglycoside. We constructed three active antimicrobial cohorts based on the active agent used and an untreated cohort of cases that did not receive antimicrobial therapy with Gram-negative activity. Microbiologic clearance was defined as having a follow-up urine culture that did not yield CRKP. Cases without an appropriate follow-up culture or that received multiple active agents or less than 3 days of the active agent were excluded. Eighty-seven cases were included in the active antimicrobial cohorts, and 69 were included in the untreated cohort. The microbiologic clearance rate was 88% in the aminoglycoside cohort (n = 41), compared to 64% in the polymyxin B (P = 0.02; n = 25), 43% in the tigecycline (P < 0.001; n = 21), and 36% in the untreated (P < 0.001; n = 69) cohorts. Using multivariate analysis, the odds of clearance were lower for the polymyxin B (odds ratio [OR], 0.10; P = 0.003), tigecycline (OR, 0.08; P = 0.001), and untreated (OR, 0.14; P = 0.003) cohorts than for the aminoglycoside cohort. Treatment with an aminoglycoside, when active in vitro, was associated with a significantly higher rate of microbiologic clearance of CRKP bacteriuria than treatment with either polymyxin B or tigecycline.
INTRODUCTION
Klebsiella pneumoniae is among the leading causes of health care-associated infections (12). Until recently, K. pneumoniae isolates were reliably susceptible to carbapenems, even when resistant to other antimicrobial classes (23). Unfortunately, carbapenem-resistant K. pneumoniae (CRKP) has emerged and is now endemic in many New York City hospitals (3, 4). Of the K. pneumoniae isolates from New York hospitals reported to the Centers for Disease Control and Prevention's National Healthcare Safety Network, 21% were resistant to carbapenems (12). Furthermore, this emerging pathogen is no longer limited to the New York area, as CRKP has now been identified in at least 33 U.S. states and many countries (13).
In the United States, carbapenem resistance among K. pneumoniae is almost exclusively due to production of Klebsiella pneumoniae carbapenemase (KPC) (4, 6, 19), an enzyme that hydrolyzes all currently available beta-lactam antimicrobials. KPC is typically encoded by a blaKPC gene carried by a plasmid containing additional genes that confer resistance to other antimicrobial classes (5, 6). Antimicrobial agents that most often demonstrate in vitro activity against CRKP include polymyxins and tigecycline, which are active against most isolates, and the aminoglycosides gentamicin and amikacin, which are active against approximately one-half of isolates (5, 6). In our institution, CRKP is most frequently isolated from urinary tract specimens. Unfortunately, minimal clinical data exist to guide selection of an appropriate antimicrobial treatment regimen for CRKP urinary tract infection (UTI).
Neither the polymyxins nor tigecycline is approved by the U.S. Food and Drug Administration (FDA) for the treatment of UTI. Evaluations of polymyxin B (20) and tigecycline (2, 8, 10, 14, 21) for the treatment of Gram-negative bacterial UTI are limited to case reports. In addition to limited clinical data, pharmacokinetic data raise concerns over the use of polymyxin B and tigecycline for UTI. Although urinary recovery of colistin has varied (11, 16), the only modern pharmacokinetic study of polymyxin B demonstrated that less than 1% of an intravenous dose administered to critically ill patients was recovered in the urine (27). Renal clearance of tigecycline accounts for only 15% of total systemic clearance, and only 10 to 22% of a tigecycline dose is excreted unaltered in the urine (9, 22). Unlike polymyxin B and tigecycline, aminoglycosides are almost exclusively renally excreted (26), achieve high urinary levels (15, 26), are efficacious for UTI caused by other susceptible bacteria (25), and are FDA approved for the treatment of UTI. The objective of this study was to compare the microbiologic clearance rates associated with the administration of at least 3 days of polymyxin B, tigecycline, and aminoglycosides (gentamicin or amikacin) for the treatment of CRKP bacteriuria.
MATERIALS AND METHODS
Study setting, design, and definitions.
This retrospective cohort study was performed at the Weill Cornell Medical College (WCMC) and Columbia University Medical Center (CUMC) campuses of New York-Presbyterian Hospital, two large academic medical centers in Manhattan, NY. At both centers, polymyxin B was the only polymyxin available, and use of polymyxin B, tigecycline, or amikacin required approval from an infectious diseases physician or pharmacist. The study was approved by the Institutional Review Board at both centers.
Cases of CRKP bacteriuria between January 2005 and June 2010 that met specific criteria (see “Case identification” below) were included in one of three active antimicrobial cohorts (polymyxin B, tigecycline, or aminoglycoside) corresponding to the agent administered or an untreated cohort if an antimicrobial with intrinsic Gram-negative activity was not administered before the follow-up urine culture. Cases that received only inactive Gram-negative antimicrobials, defined as an agent with intrinsic Gram-negative activity but to which the index CRKP was resistant in vitro, were excluded. The primary outcome was microbiologic clearance, defined as having a follow-up urine culture (the first urine culture obtained 3 to 21 days after initiation of the active antimicrobial) from which CRKP was not isolated. Outcomes applied to the untreated cohort used the date of index bacteriuria as the reference date rather than the date of active antimicrobial initiation. Additional analyses were performed to compare clearance rates in the following four subgroups: bacteriuria with pyuria, catheter-associated bacteriuria, monotherapy (defined as cases that received ≤2 days of an accompanying inactive Gram-negative antimicrobial), and cases in which the CRKP isolate was susceptible to an aminoglycoside.
Secondary outcomes included development of CRKP bacteremia, isolation of a subsequent CRKP isolate that was not susceptible to the active agent used, nephrotoxicity, overall mortality, and CRKP-associated mortality within 30 days of active antimicrobial initiation. Nephrotoxicity was defined by the Acute Kidney Injury Network (AKIN) criteria (17). CRKP-associated mortality was defined as having clinical evidence of CRKP infection within 3 days of death.
Microbiological testing.
The clinical microbiology laboratories primarily used Vitek 2 (bioMérieux Inc., Durham, NC) to determine carbapenem susceptibilities among K. pneumoniae isolates. At CUMC, isolates that tested resistant to ertapenem were considered to be resistant to imipenem and meropenem. At WCMC, isolates that tested resistant to ertapenem but not to imipenem or meropenem underwent modified Hodge testing (MHT) (1) to assess for carbapenemase production. In this study, carbapenem resistance was defined as being ertapenem resistant by Vitek2, an imipenem or meropenem MIC of ≥4 μg/ml by Vitek2 or Etest, or detection of carbapenemase by MHT. Polymyxin B and tigecycline susceptibilities were determined by Etest until the final study year, when susceptibility testing for tigecycline was performed using Vitek 2. The Clinical and Laboratory Standards Institute (CLSI) breakpoint for non-Enterobacteriaceae of ≤2 μg/ml was used for determining susceptibility to polymyxin B, and the FDA breakpoint of ≤2 μg/ml was used for tigecycline. Aminoglycoside susceptibilities were determined by Vitek 2 using CLSI breakpoints. Organisms were not routinely identified to the species level in either laboratory if they were recovered from urine cultures yielding <104 CFU/ml or if more than two different organisms were present.
Case identification.
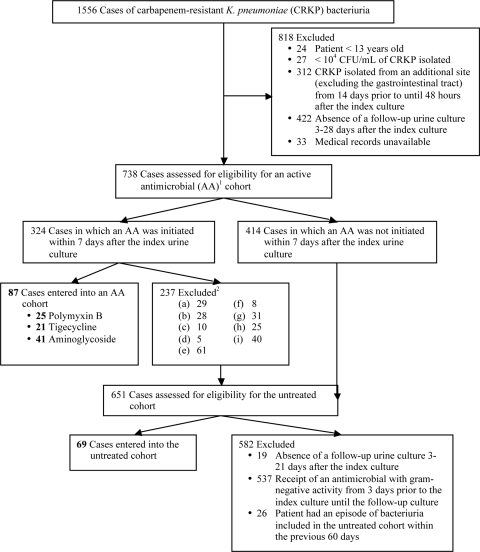
Microbiology records were reviewed to identify all urine cultures that yielded ≥104 CFU/ml of CRKP between January 2005 and June 2010. Cases were excluded if the isolate was obtained from a patient under 13 years of age or whose medical record was not accessible, an appropriate follow-up urine culture was not obtained, or CRKP was isolated from an additional anatomic site (Fig. 1). Of the remaining cases, those in which treatment with polymyxin B, tigecycline, gentamicin, or amikacin was initiated within 7 days after the index bacteriuria were assessed for eligibility for the corresponding active antimicrobial cohort. Cases were excluded from an active antimicrobial cohort if an appropriate follow-up urine culture was not obtained, the antimicrobial was not active in vitro, the patient received two different active antimicrobials, intravesicular therapy, or less than 3 days of the active antimicrobial, or the last urine culture before active antimicrobial initiation yielded <104 CFU/ml of CRKP (Fig. 1).
Fig. 1.
Flowchart of identification of cases for cohort entry. Active antimicrobials (AAs) include polymyxin B, tigecycline, gentamicin, and amikacin. Exclusion criteria for an AA cohort are the following: (a) no follow-up urine culture 3 to 21 days after AA initiation; (b) index CRKP isolate, or any subsequent urine CRKP isolate before AA initiation, was not susceptible to the AA; (c) receipt of any antimicrobial to which the index CRKP was not resistant during the 3 days prior to the index bacteriuria; (d) last urine culture before AA initiation yielded <104 CFU/ml of CRKP; receipt of any of the following from 3 days prior to the index bacteriuria until the follow-up culture: (e) 2 antimicrobials to which the index CRKP was not resistant, (f) 2 of the antimicrobial agents under investigation, (g) <3 days of the AA, (h) intravesicular antimicrobial therapy, (i) if multiple occurrences of CRKP bacteriuria were identified from a patient before AA initiation, the culture closest to the date of AA initiation was entered into the cohort and the earlier cultures were excluded.
All cases that met initial inclusion criteria but were not entered into an active antimicrobial cohort were assessed for eligibility for the untreated cohort. Cases were excluded from this cohort if there was not an appropriate follow-up urine culture, an antimicrobial with Gram-negative activity was initiated from 3 days prior to the index bacteriuria until the follow-up culture, or the patient had an episode of bacteriuria included in the untreated cohort within the previous 60 days (Fig. 1).
Data collection.
Medical records of all included cases were reviewed to obtain data on demographics and baseline characteristics (Charlson comorbidity index score [7], serum creatinine level and white blood cell [WBC] count closest to the date of the index bacteriuria, receipt of renal replacement therapy, urinary tract abnormality, mortality prediction model III [MPM3] score [24], CRKP colony count, identification of other organisms in the index culture, degree of pyuria, urinary tract catheterization, fever, patient location, hospital service, length of hospitalization prior to collection of the index culture, and intensive care unit [ICU] stay within 30 days of the index culture). We also obtained data on bacteriuria management (antimicrobial therapy, urinary catheter management, and receipt of input from a physician with specialty training in infectious diseases) and outcomes.
Data management and statistics.
Differences in baseline characteristics, treatments, and outcomes between cohorts were assessed using Pearson's χ2 or Fisher's exact test for dichotomous or categorical variables and Kruskal-Wallis test for continuous variables. Pairwise comparisons of microbiologic clearance rates were then performed between the aminoglycoside cohort and all other cohorts. A backward, stepwise, multivariate logistic regression model was constructed to determine the odds of microbiologic clearance with use of polymyxin B, tigecycline, or no Gram-negative antimicrobial therapy compared to with use of an aminoglycoside, after adjusting for other covariates. All covariates were initially entered into the model, but only those with P values of ≤0.10 in multivariate stepwise regression plus three other predetermined important potential confounding variables (presence of a urinary tract abnormality, urinary catheter retention, and receipt of an active antimicrobial on the day of the follow-up culture) were included in the final model. P values were two tailed, and values of <0.05 were considered significant. STATA version 10.0 (StataCorp, College Station, TX) was used for statistical analysis.
RESULTS
A total of 1,556 cases of CRKP bacteriuria were identified, of which 1,400 were excluded (Fig. 1). Eighty-seven cases, occurring in 85 different patients, met criteria for entry into one of the following active antimicrobial cohorts: polymyxin B (n = 25), tigecycline (n = 21), and aminoglycoside (n = 41). Sixty-nine cases, occurring in 58 different patients, met criteria for entry into the untreated cohort.
Baseline and treatment characteristics.
Of the 156 cases, the median age was 69 years, 39% were male, 49% were white, and 59% received care at CUMC. The following baseline characteristics differed significantly between cohorts (Table 1): the proportion of cases occurring as an outpatient (active antimicrobial cohorts, 6%; untreated cohort, 17%), the proportion of inpatient cases on a medical (polymyxin B, 29%; other cohorts, 75%) or surgical (polymyxin B, 46%; other cohorts, 17%) service, and the degree of pyuria (64% of cases in the untreated cohort had >5 WBC per high-powered field [HPF] on microscopic urinalysis compared to 92% of cases in the active antimicrobial cohorts).
Table 1.
Baseline and treatment-associated characteristics of cases, stratified by cohort
| Characteristic | Cohort valuea |
P valueb | |||
|---|---|---|---|---|---|
| Aminoglycoside (n = 41) | Polymyxin B (n = 25) | Tigecycline (n = 21) | Untreated (n = 69) | ||
| Age (yr) | 71 (18–102) | 70 (26–89) | 63 (44–91) | 69 (27–94) | 0.445 |
| Male | 11 (27) | 12 (48) | 9 (43) | 29 (42) | 0.284 |
| Charlson comorbidity index score | 3 (1–5) | 3 (2–4) | 3 (2–5) | 3 (2–5) | 0.449 |
| Baseline serum creatinine (mg/dl) | 0.9 (0.4–5.7) | 1.2 (0.4–4.4) | 1.2 (0.3–5.7) | 1.4 (0.3–9.7) | 0.056 |
| On renal replacement therapy | 2 (5) | 3 (12) | 1 (5) | 7 (10) | 0.665 |
| Urinary tract abnormalityc | 5 (12) | 7 (28) | 7 (33) | 10 (14) | 0.096 |
| MPM3 score (% mortality)d | 10 (1–65) | 12 (1–27) | 10 (2–56) | 9 (0–49) | 0.935 |
| Index culture colony count of >105 CFU/ml | 30 (73) | 22 (88) | 13 (62) | 45 (66) | 0.139 |
| Polymicrobial index culturee | 3 (7) | 3 (12) | 0 | 13 (19) | 0.083 |
| Degree of pyuriaf | <0.001 | ||||
| >50 WBC/HPF | 29 (73) | 12 (52) | 12 (57) | 21 (32) | |
| 6–50 WBC/HPF | 7 (17) | 9 (39) | 8 (38) | 21 (32) | |
| ≤5 WBC/HPF | 4 (10) | 2 (9) | 1 (5) | 24 (36) | |
| Catheter associatedg | 20 (49) | 13 (52) | 11 (52) | 30 (43) | 0.831 |
| Fever (temp, ≥38.0°C) | 11 (27) | 9 (36) | 4 (19) | 10 (14) | 0.117 |
| WBC count (blood) (×103/μl) | 10.8 (1.3–28.8) | 8.4 (0.2–18.2) | 11.4 (1.8–24.5) | 9.3 (0.3–32.9) | 0.381 |
| Patient location | |||||
| Outpatient | 1 (2) | 1 (4) | 3 (14) | 12 (17) | 0.046 |
| Inpatienth | 40 (98) | 24 (96) | 21 (86) | 57 (83) | |
| ICU | 5 (12) | 5 (21) | 3 (17) | 6 (11) | 0.579 |
| Non-ICU | 35 (88) | 19 (79) | 15 (83) | 51 (89) | |
| Medical service | 28 (70) | 7 (29) | 13 (72) | 45 (79) | <0.001 |
| Surgical service | 6 (15) | 11 (46) | 5 (28) | 8 (14) | 0.012 |
| Other service | 6 (15) | 6 (25) | 0 | 4 (7) | 0.042 |
| Hospital day | 4 (0–71) | 13 (0–160) | 6 (0–57) | 13 (0–169) | 0.143 |
| ICU stay within past 30 days | 16 (39) | 11 (44) | 9 (43) | 24 (35) | 0.827 |
| Catheter managementi | 0.359 | ||||
| Catheter removed | 4 (22) | 5 (50) | 2 (23) | 6 (23) | |
| Catheter exchanged | 10 (56) | 4 (40) | 3 (33) | 9 (35) | |
| Catheter retained | 4 (22) | 1 (10) | 4 (44) | 11 (42) | |
| Input from infectious diseasesj | 23 (56) | 18 (72) | 11 (53) | 23 (33) | 0.005 |
| Days from index culture until active antimicrobial initiation | 2 (0–7) | 2 (0–6) | 2 (0–5) | N/A | 0.287 |
| Days of active antimicrobial until follow-up culture | 6 (3–13) | 4 (3–11) | 6 (3–17) | N/A | 0.147 |
| Receipt of active antimicrobial on the day of follow-up culture | 21 (51) | 17 (68) | 13 (62) | N/A | 0.382 |
| ≥3 days of an inactive Gram-negative antimicrobial before the follow-up culture | 22 (54) | 18 (72) | 10 (47) | N/A | 0.198 |
| ≥3 days of an inactive beta-lactam | 16 (39) | 16 (64) | 8 (38) | N/A | 0.118 |
| Days between index and follow-up cultures | 8 (3–25) | 7 (3–14) | 7 (5–23) | 4 (3–21) | <0.001 |
Data are median values(ranges) for continuous variables and number (percentage) of cases for dichotomous variables, unless noted. N/A, not applicable.
P values comparing all cohorts are based on the χ2 or Fisher's exact test for dichotomous or categorical variables and the Kruskal-Wallis test for continuous variables.
Ileal conduit, indwelling urinary hardware other than a bladder catheter, obstructive or symptomatic nephrolithiasis, or chronic urinary retention.
Calculated based on data from the date of the index bacteriuria.
Count of ≥104 CFU/ml for an organism other than CRKP.
Only cases with microscopic urinalysis were analyzed (aminoglycoside, n = 40; polymyxin B, n = 23; tigecycline, n = 21; untreated, n = 66).
Indwelling urinary catheter at the time of or within 48 h prior to the index culture.
Only inpatient cases were analyzed for all inpatient subgroups.
Only cases with an indwelling urinary catheter at the time of the index culture were analyzed (aminoglycoside, n = 18; polymyxin B, n = 10; tigecycline, n = 9; untreated, n = 26).
Input from a physician with specialty training in infectious diseases other than an antimicrobial request approval.
Active antimicrobials were initiated a median of 2 days after the index urine culture and continued for a median of 5 days until the follow-up culture. Among cases with normal baseline renal function (creatinine clearance, ≥60 ml/min), the median daily doses of polymyxin B, gentamicin, and amikacin were 2.25 mg/kg of body weight/day (range, 1.1 to 3.3 mg/kg/day), 4.7 mg/kg/day (range, 0.5 to 7.6 mg/kg/day), and 15.4 mg/kg/day (range, 7.8 to 16.1 mg/kg/day), respectively, based on the ideal body weight for nonobese patients and adjusted body weight for obese patients. All cases in the tigecycline cohort received an initial dose of 100 mg, followed by 50 mg every 12 h. The only treatment-associated variable that differed significantly between cohorts was the proportion of cases that received input from infectious diseases (polymyxin B, 72%; aminoglycoside, 56%; tigecycline, 53%; untreated, 33%). No statistically significant differences were identified between cohorts for any other baseline or treatment-associated variables, although cases in the untreated cohort had less days between the index and follow-up urine cultures (median, 4 days) than cases in the active antimicrobial cohorts (median, 7 to 8 days) (Table 1).
Microbiologic clearance.
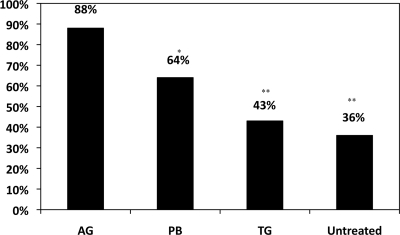
The microbiologic clearance rate for the aminoglycoside cohort, 88%, was greater than the clearance rates for the polymyxin B cohort (64%; P = 0.02), tigecycline cohort (43%; P < 0.001), and untreated cohort (36%; P < 0.001) (Fig. 2 and Table 2). In all subgroups, clearance rates in the aminoglycoside cohort were significantly greater than those in the tigecycline and untreated cohorts. In the stepwise, multivariate logistic regression model (Table 3), the following four variables remained in the model based on a P value of ≤0.10: fever, MPM3 score, coadministration of an inactive Gram-negative antimicrobial, and antimicrobial cohort. Compared to cases in the aminoglycoside cohort, cases in the polymyxin B (OR, 0.10; P = 0.003), tigecycline (OR, 0.08; P = 0.001), and untreated (OR, 0.14; P = 0.003) cohorts had lower odds of microbiologic clearance in the multivariate model.
Fig. 2.
Microbiologic clearance rates by the antimicrobial treatment cohort. AG, aminoglycoside; PB, polymyxin B; TG, tigecycline; *, P = 0.02; **, P < 0.001. P values calculated using the χ2 test to compare the clearance rate of the cohort to that of the AG cohort.
Table 2.
Microbiologic clearance of carbapenem-resistant K. pneumoniae from urine, stratified by treatment cohort
| Characteristic | Cohort valuea |
P valueb | |||
|---|---|---|---|---|---|
| Aminoglycoside (n = 41) | Polymyxin B (n = 25) | Tigecycline (n = 21) | Untreated (n = 69) | ||
| Overall study population | 36 (88)c | 16 (64) | 9 (43) | 25 (36) | <0.001 |
| Associated pyuriad | 32/36 (89) | 13/21 (62) | 8/20 (40) | 11/42 (26) | <0.001 |
| Catheter associatede | 16/20 (80) | 8/13 (62) | 4/11 (36) | 9/30 (30) | 0.003 |
| Monotherapyf | 17/19 (89) | 3/7 (43) | 2/11 (18) | N/A | <0.001 |
| Susceptible to an aminoglycoside | 36/41 (88) | 8/10 (80) | 6/13 (46) | 15/41 (37) | <0.001 |
Data are the number(percentage) of cases for the overall study population and the number of cases/number of cases eligible (percentage) for subgroups. N/A, not applicable.
P values comparing all cohorts are based on the χ2 or Fisher's exact test.
The microbiologic clearance rate was 86% (25/29) for gentamicin and 92% (11/12) for amikacin.
Counts of >5 white blood cells/high-powered field on microscopic urinalysis.
Indwelling urinary catheter at the time of or within 48 h prior to the index culture.
Received ≤2 days of an accompanying inactive Gram-negative antimicrobial.
Table 3.
Univariate and multivariate analyses of factors associated with microbiologic clearance of carbapenem-resistant K. pneumoniae from urinea
| Variable | Univariate |
Multivariateb |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (yr) | 0.98 (0.96-1.00) | 0.087 | ||
| Male | 0.84 (0.44-1.60) | 0.591 | ||
| Charlson comorbidity index (weighted comorbidity) | 0.95 (0.81-1.10) | 0.487 | ||
| Baseline serum creatinine (mg/dl) | 0.80 (0.62-1.03) | 0.088 | ||
| Urinary tract abnormalityc | 0.71 (0.32-1.60) | 0.412 | 0.47 (0.16-1.36) | 0.163 |
| MPM3 score (% mortality) | 0.97 (0.94-1.00) | 0.046 | 0.95 (0.91-0.98) | 0.005 |
| Index culture colony count of >105 CFU/ml | 0.81 (0.41-1.63) | 0.563 | ||
| Polymicrobial index culturec | 1.14 (0.43-3.00) | 0.796 | ||
| Pyuria (>5 WBC/HPF) | 0.76 (0.35-1.66) | 0.486 | ||
| Catheter associatedc | 0.67 (0.36-1.27) | 0.222 | ||
| Fever (temp of ≥38.0°C) | 3.36 (1.40-8.00) | 0.006 | 3.75 (1.27-11.02) | 0.016 |
| WBC count (blood) (×103/μl) | 1.00 (0.95-1.06) | 0.974 | ||
| Patient location | ||||
| Inpatient, not ICU | 1.00 | Ref | ||
| Inpatient, ICU | 1.36 (0.50-3.68) | 0.550 | ||
| Outpatient | 0.55 (0.20-1.55) | 0.261 | ||
| Inpatient service | ||||
| Medical | 1.00 | Ref | ||
| Surgical | 0.86 (0.38-1.97) | 0.726 | ||
| Other inpatient service | 1.26 (0.42-3.75) | 0.681 | ||
| N/A (Outpatient) | 0.53 (0.18-1.51) | 0.233 | ||
| ICU stay within past 30 days | 0.89 (0.46-1.70) | 0.722 | ||
| Urinary catheter retentiond | 0.50 (0.19-1.29) | 0.151 | 0.62 (0.19-2.09) | 0.445 |
| Input from infectious diseasesc | 1.63 (0.86-3.07) | 0.135 | ||
| Duration of active antimicrobial until follow-up culture (days) | 1.20 (1.08-1.33) | 0.001 | ||
| Receipt of active antimicrobial on the day of the follow-up culture | 3.47 (1.66-7.26) | 0.001 | 2.47 (0.75-8.07) | 0.136 |
| ≥3 days of an inactive Gram-negative antimicrobial before follow-up culture | 4.45 (2.06-9.62) | <0.001 | 2.70 (0.83-8.75) | 0.097 |
| Duration between index and follow-up cultures (days) | 1.02 (0.95-1.10) | 0.542 | ||
| Antimicrobial cohort | ||||
| Aminoglycoside | 1.00 | Ref | 1.00 | Ref |
| Polymyxin B | 0.25 (0.07-0.85) | 0.027 | 0.10 (0.02-0.47) | 0.003 |
| Tigecycline | 0.10 (0.03-0.37) | <0.001 | 0.08 (0.02-0.35) | 0.001 |
| Untreated | 0.08 (0.03-0.23) | <0.001 | 0.14 (0.04-0.51) | 0.003 |
CI, confidence interval; OR, odds ratio; Ref, reference group.
All variables were initially entered into the multivariate model, but only those with P values of <0.10 in the multivariate model plus urinary tract abnormality, urinary catheter retention, and receipt of active antimicrobial on the day of the follow-up culture were included in the final model.
Definitions can be found in the footnotes to Table 1.
Indwelling urinary catheter at the time of index culture, with the catheter not removed or exchanged before the follow-up culture.
Secondary outcomes.
Of the 136 cases for which 30-day follow-up data were available, 8 (6%) developed CRKP bacteremia within 30 days (6 in the untreated cohort and 2 in the polymyxin B cohort), and 22 (16%) died, of which 9 (7%) were CRKP associated. Bacteremia and mortality rates did not differ significantly between cohorts. Of the 81 cases in an active antimicrobial cohort not receiving renal replacement therapy, 13 (16%) developed acute kidney injury, as follows: 5 in the aminoglycoside cohort (5/39; 13%), 8 in the polymyxin B cohort (8/22; 37%), and none in the tigecycline cohort (P = 0.004). Only three of these cases were AKIN stage 2 or 3. Rates of subsequent isolation of a nonsusceptible CRKP isolate did not significantly differ between active antimicrobial cohorts, although the highest rate was found in the tigecycline cohort (tigecycline, 43%; polymyxin B, 24%; aminoglycoside, 20%; P = 0.14). Microbiologic clearance rates did not correlate with antimicrobial MICs for the initial isolates.
DISCUSSION
In this study, treatment with at least 3 days of an aminoglycoside achieved statistically significantly higher rates of microbiologic clearance of CRKP bacteriuria than did treatment with at least 3 days of polymyxin B or tigecycline. These differences persisted in all subgroups, and aminoglycoside use was independently associated with microbiologic clearance in multivariate analysis. We controlled for important potential confounding variables, including urinary catheter use and management, urinary tract abnormalities, measures of acute and chronic illness, bacteriuria colony counts, duration of antimicrobial use, timing of the follow-up culture in relation to antimicrobial use, and coadministration of inactive Gram-negative antimicrobials. We also included a cohort of cases that did not receive Gram-negative antimicrobial therapy, allowing for comparisons of the active antimicrobial cohorts to untreated cases. Of note, the microbiologic clearance rate associated with tigecycline use (43%) was similar to that of the untreated cases (36%).
Given the domestic and international spread of CRKP (19) and the significant epidemiologic role of Klebsiella pneumoniae as a cause of health care-associated UTI, CRKP UTI will become increasingly common. Thus, it is important to have an understanding of the comparative effectiveness of available antimicrobials for the treatment of such infections. This study is the first to compare the effectiveness of antimicrobial agents with in vitro activity against CRKP in eradicating the organism from the urinary tract. We suspect that differences in microbiologic clearance rates associated with aminoglycosides, polymyxin B, and tigecycline are related to differences in urinary tract concentrations achieved by these agents (9, 15, 22, 26, 27).
As a retrospective analysis, this study has limitations. The choice of antimicrobial was not randomized, and thus, unmeasured or unadjusted confounding may have biased associations between antimicrobial use and microbiologic clearance. However, given the magnitude of differences in odds of clearance and statistical significance in the multivariate model, it is unlikely that residual confounding would completely account for the large differences in clearance rates between the aminoglycoside cohort and other cohorts. Because the study was retrospective, certain important variables were not standardized, including antimicrobial duration and dosing and timing of follow-up cultures. It was not possible to accurately assess recurrence after treatment given that the timing of and rationale for obtaining additional urine cultures were not standardized. We were also unable to accurately assess clinical signs and symptoms and thus could not determine whether cases represented UTI or asymptomatic bacteriuria or assess clinical response.
The majority of CRKP bacteriuria episodes were excluded, potentially limiting the generalizability of the study. However, we believe strict inclusion criteria were essential to establish mutually exclusive cohorts and permit a fair comparison of the agents under investigation. The requirement of a follow-up urine culture may have led to a sampling bias, as cases without a follow-up culture may have been more likely to respond clinically. However, cases without a follow-up culture also may have been more likely to represent colonization rather than infection. Furthermore, this requirement was applied to all cohorts and thus was unlikely to generate a selection bias that differed among cohorts. In order to maintain a more homogeneous study population and limit confounding by indication, the study excluded cases in which CRKP was also isolated from a nonurine specimen, including blood. Thus, the findings are not applicable to bacteremic UTIs.
Although cases that received two active agents were excluded, the study allowed for coadministration of inactive Gram-negative antimicrobials. Concomitant therapy with an inactive Gram-negative agent was associated with increased odds of microbiologic clearance (Table 3), suggesting that these agents either had partial clinical activity or synergistic activity with the active agents. This finding merits further investigation to elucidate the role of inactive Gram-negative agents in the treatment of CRKP UTI, particularly agents such as beta-lactams that are highly concentrated in the urinary tract. In order to minimize confounding bias related to concomitant use of inactive Gram-negative agents, we incorporated their use in the multivariate model and analyzed a subgroup of cases in which the active agent was given as monotherapy.
Although the mechanism of carbapenem resistance among isolates was not determined, previous studies have demonstrated that carbapenem resistance among K. pneumoniae in New York City has been almost exclusively due to KPC production (3, 4, 6). Since most isolates were likely KPC producers, it is unknown if these results can be extrapolated to cases caused by K. pneumoniae strains in which carbapenem resistance is due to another mechanism. The pharmacokinetics of colistin differ from polymyxin B, as studies have shown higher percentages of urinary tract excretion of colistin (11, 16). Thus, results related to polymyxin B in this study might not be applicable to colistin. We also did not assess the role of intravesicular or combination antimicrobial therapy.
This study indicates that when active in vitro, short courses of aminoglycosides (a median of 6 days) are highly effective in clearing CRKP from urine, particularly compared to polymyxin B or tigecycline. Of note, tobramycin, which rarely displays in vitro activity against CRKP (5), was not evaluated. These results, combined with clinical data demonstrating their efficacy for the treatment of UTI caused by other bacteria (25), support the use of gentamicin or amikacin for CRKP UTIs, provided that the benefits of treatment are not outweighed by the risks of aminoglycoside administration. The low microbiologic clearance rates associated with tigecycline use, combined with consistent pharmacokinetic data indicating minimal urinary excretion of the drug (9, 22) and the high rate of subsequent isolation of a tigecycline-nonsusceptible isolate, lead us to discourage the use of tigecycline for CRKP UTI. Although polymyxin B use was associated with a higher clearance rate than tigecycline, this rate was significantly lower than that associated with aminoglycoside use. This finding, combined with polymyxin B's low urinary concentrations (27) and potential for nephrotoxicity (18), suggests that it may be suboptimal for the treatment of CRKP UTI. Further research in the form of a prospective cohort study or randomized trial would be ideal to confirm the findings of this study, assess clinical response, and evaluate the role of alternate treatment options for CRKP UTI, particularly for infections caused by aminoglycoside-resistant isolates. However, until then, we conclude that aminoglycosides are likely more effective than polymyxin B and tigecycline for clearance of urinary tract infections due to aminoglycoside-susceptible CRKP.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant T32 AI007613) and by the Clinical and Translational Science Center at Weill Cornell Medical College (grant UL1RR024996). We have no potential conflicts of interest to disclose.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Footnotes
Published ahead of print on 3 October 2011.
REFERENCES
- 1. Anderson K. F., et al. 2007. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J. Clin. Microbiol. 45:2723–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anthony K. B., et al. 2008. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin. Infect. Dis. 46:567–570 [DOI] [PubMed] [Google Scholar]
- 3. Bratu S., et al. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430–1435 [DOI] [PubMed] [Google Scholar]
- 4. Bratu S., et al. 2005. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob. Agents Chemother. 49:3018–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bratu S., et al. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128–132 [DOI] [PubMed] [Google Scholar]
- 6. Castanheira M., Sader H. S., Deshpande L. M., Fritsche T. R., Jones R. N. 2008. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 52:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383 [DOI] [PubMed] [Google Scholar]
- 8. Cunha B. A., McDermott B., Nausheen S. 2007. Single daily high-dose tigecycline therapy of a multidrug-resistant (MDR) Klebsiella pneumoniae and Enterobacter aerogenes nosocomial urinary tract infection. J. Chemother. 19:753–754 [DOI] [PubMed] [Google Scholar]
- 9. Curcio D. 2008. Treatment of recurrent urosepsis with tigecycline: a pharmacological perspective. J. Clin. Microbiol. 46:1892–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geerlings S. E., van Donselaar-van der Pant K. A., Keur I. 2010. Successful treatment with tigecycline of two patients with complicated urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. J. Antimicrob. Chemother. 65:2048–2049 [DOI] [PubMed] [Google Scholar]
- 11. Gobin P., Lemaitre F., Marchand S., Couet W., Olivier J. C. 2010. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 54:1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hidron A. I., et al. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 13. Kitchel B., et al. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53:3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krueger W. A., et al. 2008. Treatment with tigecycline of recurrent urosepsis caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J. Clin. Microbiol. 46:817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanao J. M., Pedraz J. L., Navarro A. S., Dominguez-Gil A. 1984. Influence of dose in the urinary excretion of amikacin. Int. J. Clin. Pharmacol. Ther. Toxicol. 22:538–542 [PubMed] [Google Scholar]
- 16. Li J., et al. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J. Antimicrob. Chemother. 53:837–840 [DOI] [PubMed] [Google Scholar]
- 17. Mehta R. L., et al. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendes C. A., Cordeiro J. A., Burdmann E. A. 2009. Prevalence and risk factors for acute kidney injury associated with parenteral polymyxin B use. Ann. Pharmacother. 43:1948–1955 [DOI] [PubMed] [Google Scholar]
- 19. Nordmann P., Cuzon G., Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 20. Pastewski A. A., et al. 2008. Parenteral polymyxin B use in patients with multidrug-resistant gram-negative bacteremia and urinary tract infections: a retrospective case series. Ann. Pharmacother. 42:1177–1187 [DOI] [PubMed] [Google Scholar]
- 21. Reid G. E., Grim S. A., Aldeza C. A., Janda W. M., Clark N. M. 2007. Rapid development of Acinetobacter baumannii resistance to tigecycline. Pharmacotherapy 27:1198–1201 [DOI] [PubMed] [Google Scholar]
- 22. Rello J. 2005. Pharmacokinetics, pharmacodynamics, safety and tolerability of tigecycline. J. Chemother. 17(Suppl. 1):12–22 [DOI] [PubMed] [Google Scholar]
- 23. Streit J. M., Jones R. N., Sader H. S., Fritsche T. R. 2004. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001). Int. J. Antimicrob. Agents 24:111–118 [DOI] [PubMed] [Google Scholar]
- 24. Vasilevskis E. E., et al. 2009. Mortality probability model III and simplified acute physiology score II: assessing their value in predicting length of stay and comparison to APACHE IV. Chest 136:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vidal L., et al. 2007. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 60:247–257 [DOI] [PubMed] [Google Scholar]
- 26. Wilson T. W., Mahon W. A., Inaba T., Johnson G. E., Kadar D. 1973. Elimination of tritiated gentamicin in normal human subjects and in patients with severely impaired renal function. Clin. Pharmacol. Ther. 14:815–822 [DOI] [PubMed] [Google Scholar]
- 27. Zavascki A. P., et al. 2008. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin. Infect. Dis. 47:1298–1304 [DOI] [PubMed] [Google Scholar]