Abstract
Topical microbicides are potentially an alternative method to vaccines for reducing the spread of herpes simplex virus (HSV). We have previously shown (S. Liu et al., Biochim. Biophys. Acta 1723:270–281, 2005) that the catechin (−)-epigallocatechin gallate (EGCG) inactivates HSV at neutral pH; however, to function in the female genital tract EGCG must also be effective at acidic pH. EGCG inactivated HSV-1 and HSV-2 at pH 8.0 by 3 log10 to 4 log10 but was ineffective at pH 5.7. The EGCG digallate dimers theasinensin A, P2, and theaflavin-3,3′-digallate (TF-3) inactivated both viruses by 3 log10 to 4 log10 at pH 5.7 and as much as 5 log10 at pH 8.0. TF-3 inactivated HSV-1 and HSV-2 by 4 to 5 log10 in the pH range of 4.0 to 5.7. Dimers with one gallate moiety had antiviral activity intermediate between the activities of EGCG and digallate dimers. Confocal and electron microscopy showed that theasinensin A did not damage Vero cells. All EGCG dimers inactivated enveloped viruses with class I, class II, and class III (HSV-1, HSV-2) fusion proteins more effectively than did monomeric EGCG. EGCG had no activity against the nonenveloped viruses tested, but TF-3 reduced the titer of 4 of 5 nonenveloped viruses by ≅2 to 3.5 log10. Results also showed that HSV-1 glycoprotein B (gB) was aggregated more rapidly by theasinensin A than EGCG, which, when taken together with the nonenveloped virus data, suggests that dimers may inhibit the function of viral proteins required for infectivity. Digallate dimers of EGCG appear to have excellent potential as microbicidal agents against HSV at acidic and neutral pHs.
INTRODUCTION
Herpes simplex virus (HSV) is the leading cause of genital ulcers in the developed world (16). In the United States alone 40 to 60 million people are infected with HSV-2 (23). In some cities in North America and Europe, HSV-1, which is usually found orally, causes more than half of the new cases of genital herpes, particularly in women (16).
In the absence of a fully effective HSV vaccine (1), topical microbicides represent an important potential strategy for preventing the sexual and mother-to-child spread of HSV. It has been shown for both HSV (33) and human immunodeficiency virus (HIV) (20) that the viral load in genital secretions is the chief predictor of viral transmission, suggesting that if a microbicide reduces the concentration of excreted HSV, the incidence of viral transmission will be reduced.
(−)-Epigallocatechin gallate (EGCG), the primary catechin in green tea (5), has been previously shown in our laboratory to inactivate multiple clinical isolates of HSV-1 and HSV-2 (12). These studies were carried out at neutral pH. While efficacy at neutral pH would be fine for an anti-HSV compound utilized in the eye (14), a topical microbicide utilized vaginally (27) or on the skin (30) to reduce HSV transmission must function across a pH range that is more acidic than neutral. Vaginal pH will vary over a range of 3.9 to 5.7 (21) and then become more neutral in the presence of semen, rising to pH 6 to 7 (27). The pH of the outermost layer of skin (stratum corneum) ranges from 4.5 to 5.5 (30).
EGCG is the largest of the catechins found in green tea but is still a small molecule, with a molecular weight (MW) of 458.4 (10). When EGCG is oxidized in green tea, it forms two digallate dimers, theasinensin A (MW 914) and P2 (MW 884), and when oxidized in black tea, it forms the digallate dimer TF-3 (MW 868.7) (10, 24) (Fig. 1). The results in the present study indicate that the EGCG dimers theasinensin A, P2, and TF-3 inactivate HSV-1 and HSV-2 more effectively between pHs 4.0 and 6.6 than the EGCG monomer and have anti-HSV activity that is equivalent to or greater than that of the monomer at pH 7.4 to 8.0. Consequently, EGCG dimers can potentially reduce HSV transmission over most of the pH range found in the vagina.
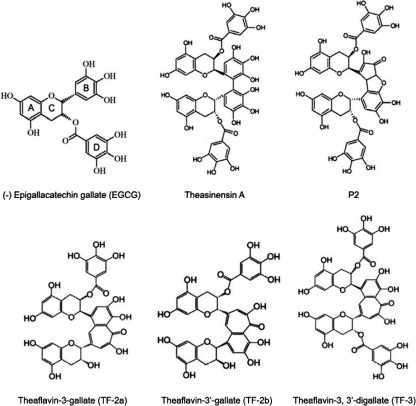
Fig. 1.
Structures of tea flavonoids with one gallate moiety (ring D), EGCG, TF-2a, and TF-2b, and two gallate moieties, theasinensin A, P2, and TF-3.
MATERIALS AND METHODS
Cells, viruses, catechins, and HSV proteins.
HSV-1 (strain F1) was obtained from R. Rubenstein (New York State Institute for Basic Research), and HSV-2 (strain 333) was obtained from Mary K. Howett (Penn State University). Vesicular stomatitis virus (VSV) strain Indiana, Semliki Forest virus (SFV), respiratory syncytial virus (RSV), measles virus, coxsackie B4 virus, coxsackie A9 virus, poliovirus 1, poliovirus 3, echovirus 6, and cells were obtained from ATCC (Manassas, VA). HSV-2 was grown in CV-1 cells, and all other viruses were grown in Vero cells. Cells were grown in RPMI 1640 medium (Cellgro) (30) with 10% fetal calf serum (FCS). The maintenance medium for Vero and CV-1 cells was as described above but with 1% FCS. Theasinensin A, P2, theaflavin-3,3′-gallate, and the mixture of theaflavin-3-gallate and 3′-gallate were purchased from Chromadex (Irvine, CA). EGCG was obtained from Sigma (St. Louis, MO). Purified HSV-1 glycoproteins gB (isolate 730) (New C#8) and gD-1 (isolate 306) (C#226) were provided by Gary H. Cohen and Roselyn J. Eisenberg (University of Pennsylvania).
Virus inactivation and titration.
Catechins were dissolved in dimethyl sulfoxide (DMSO) and diluted in phosphate-buffered saline or 1% FCS medium just before using. The final DMSO concentration in both control and catechin samples was 0.5%. Viruses were incubated with the appropriate catechin at the indicated concentration for 1 h at 37° in phosphate-buffered saline (PBS) at the indicated pH. Addition of catechins to the medium did not change the pH. All viruses were titrated by inoculation of 10-fold dilutions into Vero cell cultures in 96-well microtiter tissue culture plates (Falcon). A virus dilution (0.1 ml) in maintenance medium was inoculated into each well, with three wells per dilution (28). Each experiment was performed in triplicate, and each data point presented is the mean ± the standard deviation of three separate experiments. The plates were incubated for 5 days at 37°C and examined daily for a cytopathic effect. Virus titers were calculated by the method of Reed and Muench (22).
Treatment of cells with theasinensin A and EGCG.
Vero cell monolayers were treated with either theasinensin A or EGCG in 1% FCS medium for 1 h at 37°C. Control or treated monolayers were then exposed to propidium iodide and examined by phase-contrast microscopy (×20 magnification).
Virus purification.
Vero cells were grown in RPMI 1640 supplemented with 1% FCS (12). The monolayer of Vero cells was infected with HSV-1 at a multiplicity of infection of 10. After 1 h of adsorption, the cells were washed and then incubated for 24 h at 37°C in 5% CO2. The cells were frozen at −80°C and thawed at 4°C. The suspensions were clarified by centrifugation at 1,000 rpm for 25 min and then by centrifugation at 3,000 rpm for 10 min. The virus was pelleted from the supernatant by centrifugation at 12,500 rpm for 2 h in a Beckman SS34 rotor. The pellet was suspended in TNE buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA) and laid onto a three-step discontinuous sucrose gradient consisting of 2 ml each of 50, 40, and 30% sucrose in TNE buffer. After a 2-h centrifugation at 22,000 rpm in a Beckman SW41 rotor, virus at the interphase between the 40 and 50% sucrose layers was aspirated, diluted 1:4 with PBS, and pelleted in a Beckman SW50.2 rotor for 1 h at 35,000 rpm. The virus pellet was suspended in PBS, left untreated or treated with TF-3 (100 μM) for 2 h at 37°C, and then spun in a Beckman TLA 100.4 rotor for 2 h at 20,000 rpm. The virus pellet was fixed in a 4% glutaraldehyde-0.1 M PBS (pH 7.4) buffer in preparation for electron microscopy (EM).
EM of purified virus.
TF-3-treated and control HSV-1 virions were fixed with 1% glutaraldehyde and 1% paraformaldehyde in 0.1 M PBS (pH 7.4) for 60 min and washed with 0.1 M PBS (pH 7.4). The samples were dehydrated through 70% ethanol and then embedded into LR White embedding medium directly from 70% ethanol. Osmium tetroxide was not used, in order to preserve antigenicity. Sections were cut with either a Sorvall MT-2 or MT-5000 ultramicrotome and collected on nickel grids. Grids were immersed in 1% bovine serum albumin (BSA)–0.1 M PBS-NaN3 for 30 min and then placed into a blocking solution of 20% normal goat serum. Photographs were taken of purified control and catechin-treated virions.
EM of Vero cells.
Medium with 1% FCS was added to control cells, medium with 1% FCS and 100 μM EGCG was added to treated cells, and control and EGCG-treated cells were incubated at 37°C for 24 h. They were then placed in microcentrifuge vials and spun at 1,000 rpm in an Eppendorf microcentrifuge for 5 min to obtain cell pellets. The pellets were first rinsed in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) for 30 min and then fixed in a 4% solution of glutaraldehyde in 0.1 M PBS (pH 7.4) for 60 min as previously described (12). Briefly, cells were rinsed again in 0.1 M PBS (pH 7.4 for 10 min), postfixed with 1% osmium tetroxide for 90 min, rinsed with PBS for 10 min, dehydrated with graded (50, 70, 85, 95, and twice 100%) ethanol for 10 min in each step, infiltrated with propylene oxide and plastic Spurr medium resin (26), and finally embedded in BEEM capsules and polymerized in a 70°C oven overnight. Ultrathin sections were obtained by using a Sorvall MT-2 or MT-5000 ultramicrotome with a diamond knife. The ultrathin sections obtained were placed on uncoated 200-mesh copper grids, stained with saturated uranyl acetate in 50% ethanol for 5 min, rinsed with 0.22-μm-pore-size Millipore-filtered distilled water for at least 50 s to obtain a clean section without any residue of uranyl acetate, and then stained with Reynold's lead citrate for 5 min and rinsed again with Millipore-filtered distilled water for 50 s. They were then air dried, examined with a Hitachi 7500 transmission electron microscope operated at 80 kV, and photographed with an Advanced Microscopy Techniques digital camera system.
SDS-PAGE of EGCG-treated proteins.
A mixture of flavonoid (550 μM) and gB or gD (500 to 700 μg/ml) in a 0.1 M K2HPO4-KH2PO4 buffer (pH 7.0) was incubated at 35°C in a water bath for 1 or 24 h, and the products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12). Ten microliters of each product was mixed with an equal volume of sample buffer and denatured at 50°C for 15 min. The samples were separated on 6.5% polyacrylamide gel and visualized with Coomassie brilliant blue R-250.
RESULTS
Digallate EGCG dimers inactivate HSV and VSV at acidic, neutral, and alkaline pHs.
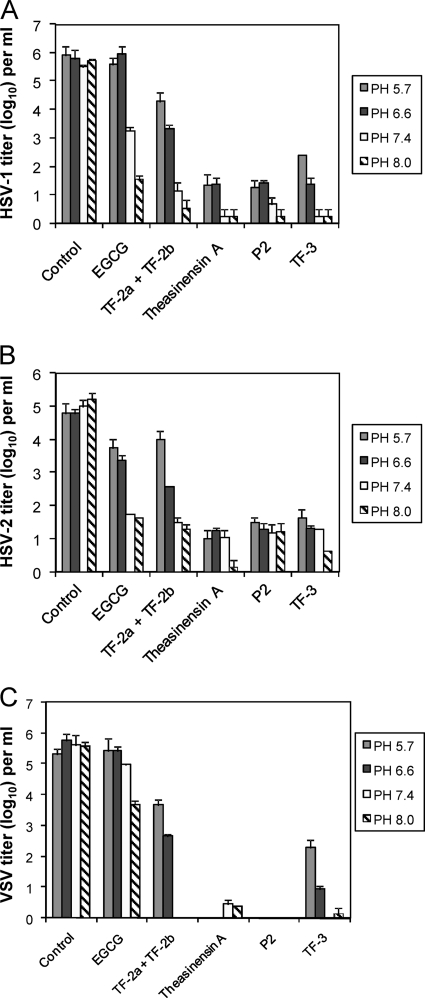
EGCG and other catechins can be oxidatively coupled to form dimers (Fig. 1) some of which have one gallate moiety (ring D of EGCG) (TF-2a, TF-2b) and others of which have two gallates (theasinensin A, P2, TF-3). The results in Fig. 2 show that the presence of two gallates substantially increases antiviral activity at pH 5.7 and pH 6.6. EGCG had almost no activity against HSV-1 (Fig. 2A) below pH 7.4. The mixture of TF-2a and TF-2b, which are dimers with one gallate, had some anti-HSV-1 activity at acidic pH, but the digallate dimers reduced HSV-1 titers by 2 to 3 log10 more than TF2a and TF-2b and 3.5 to 4.5 log10 more than EGCG. While EGCG inactivated HSV-1 by >2 log10 at pH 7.4 and >4 log10 at pH 8.0, the digallate dimers had 2 to 3 log10 greater activity at pH 7.4 and at least 10-fold greater activity at pH 8.0 than EGCG. The monogallate dimers were more active than EGCG at pHs 7.4 and 8.0 and almost as active as the digallate dimers.
Fig. 2.
Effect of pH on the antiviral activity of monogallate and digallate tea flavonoids. Each compound was used at a concentration of 100 μM and incubated with virus from a clarified cell supernatant for 1 h at 37°C prior to titration with Vero cells. (A) HSV-1; (B) HSV-2; (C) VSV. Titers are the means ± standard deviations of results of three separate experiments.
HSV-2 (Fig. 2B) inactivation in the pH 5.7 to 8.0 range showed a pattern similar to that with HSV-1. While EGCG inactivated HSV-2 by 1 log10 at pHs 5.7 and 6.6, the digallate dimers had 100-fold greater anti-HSV-2 activity in the same pH range. The monogallate dimer mixture had a pH-dependent viral inactivation profile similar to that of EGCG. HSV-2 appears to be equally as sensitive to inactivation to all compounds at pHs 7.4 and 8.0. The results in Fig. 2A and B show that the presence of two gallate groups provides increased HSV inactivation at acidic pH compared to that with monomeric EGCG or dimeric EGCG with only one gallate group.
Since the VSV G protein is structurally homologous to HSV-1 gB (11, 23) and both glycoproteins are essential for viral infectivity, we also examined the effects of the tea polyphenols (Fig. 1) on VSV infectivity (Fig. 2C). As with HSV-1 and HSV-2, EGCG had little or no activity against VSV at acidic pH. In contrast to HSV-1 and HSV-2, EGCG had little activity against VSV at pH 7.4, and while EGCG did inactivate VSV at pH 8.0 by almost 2 log10, this was significantly less antiviral activity than was seen with HSV-1 and HSV-2 at pH 8.0. This result suggests that VSV is inherently less sensitive to EGCG monomer inactivation than HSV. The digallate dimers inactivated VSV across the entire pH range tested. Theasinensin A and P2 inactivated VSV by >5 log10 at all pHs. TF-3 was somewhat less active than the other digallate dimers at pH 5.7 but comparably inactivated VSV at pHs 6.6, 7.4, and 8.0. The monogallate dimer mixture was less active than all three digallate compounds at acidic pH but was equally as active at pHs 7.4 and 8.0. VSV inactivation at pHs 5.7 and 6.6 appears to be dependent on the presence of gallate moieties, with two gallates providing greater VSV inactivation than one. The presence of one gallate moiety in a dimer at pHs 7.4 and 8.0 provided VSV inactivation comparable to that with the digallate dimers, just as with HSV-1 and HSV-2.
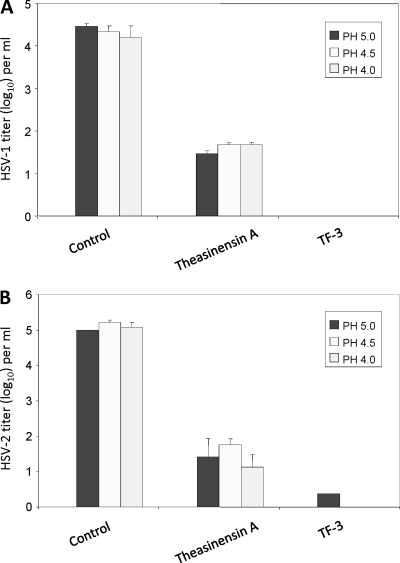
Because vaginal pH can be as low as ≅4.0, we examined the efficacy of TF-3 and theasinensin A against HSV-1 (Fig. 3A) and HSV-2 (Fig. 3B) at pHs 4.0, 4.5, and 5.0. TF-3 and theasinensin A reduced the titer of HSV-1 by >4 log10 and ≅3 log10, respectively, at each acidic pH. HSV-2 titers were reduced by ≅5 log10 using TF-3 and by >3 log10 with theasinensin A between pHs 4.0 and 5.0. The results in Fig. 2 and 3 indicate that the presence of two gallates in EGCG dimers provided antiviral activity over a broader pH range than EGCG. In most instances digallate dimers were as strongly antiviral at pH 4.0 as at pH 8.0.
Fig. 3.
Efficacy of digallate dimers in the pH range found vaginally. (A) HSV-1; (B) HSV-2. Each compound was used at a concentration of 100 μM. Isolates were prepared and titers were determined as described for Fig. 2.
Effect of pH on the antiviral concentration ranges of EGCG, theasinensin A, and TF-3.
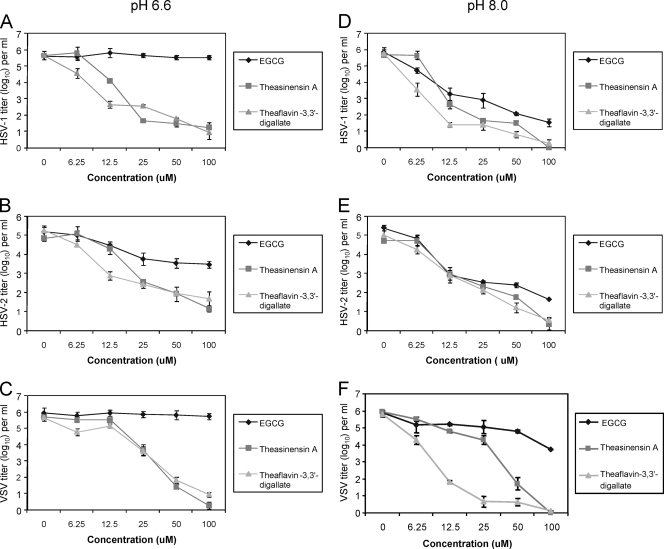
The results in Fig. 4A to C show that at pH 6.6 TF-3 reduced the titers of HSV-1 and HSV-2 by 2 to 3 log10 at a concentration of 12.5 μM. When the concentration was raised to 25 μM the digallate dimers theasinensin A and TF-3 reduced the titers of HSV-2 and VSV by at least 2 log10 and the titer of HSV-1 by 3 log10 (TF-3) to 4 log10 (theasinensin A). EGCG did not reduce the titers of HSV-1 and VSV at any concentration, and HSV-2 titers were reduced by only ≅1 log10 between 25 and 100 μM.
Fig. 4.
Effect of pH on the antiviral concentration range of monogallate and digallate tea flavonoids against HSV-1, pH 6.6 (A), HSV-2, pH 6.6 (B), VSV, pH 6.6 (C), HSV-1, pH 8.0 (D), HSV-2, pH 8.0 (E), and VSV, pH 8.0 (F). Isolates were prepared and titers were determined as described for Fig. 2.
When EGCG, theasinensin A, and TF-3 were tested against HSV-1, HSV-2, and VSV at pH 8.0 (Fig. 4D to F), HSV-1 and HSV-2 titers were reduced at a concentration of 12.5 μM equally by EGCG and both dimers while VSV showed greater sensitivity to TF-3. When the concentration of each polyphenol was raised to 100 μM, the titers of HSV-1 and HSV-2 were comparably reduced but the VSV titer was reduced by 3 log10 less by EGCG than the dimers. These VSV results showed that even under conditions which provide for maximum EGCG antiviral activity the dimers were more effective against a broader range of viruses.
Effect of EGCG, theasinensin A, and TF-3 on Vero cells.
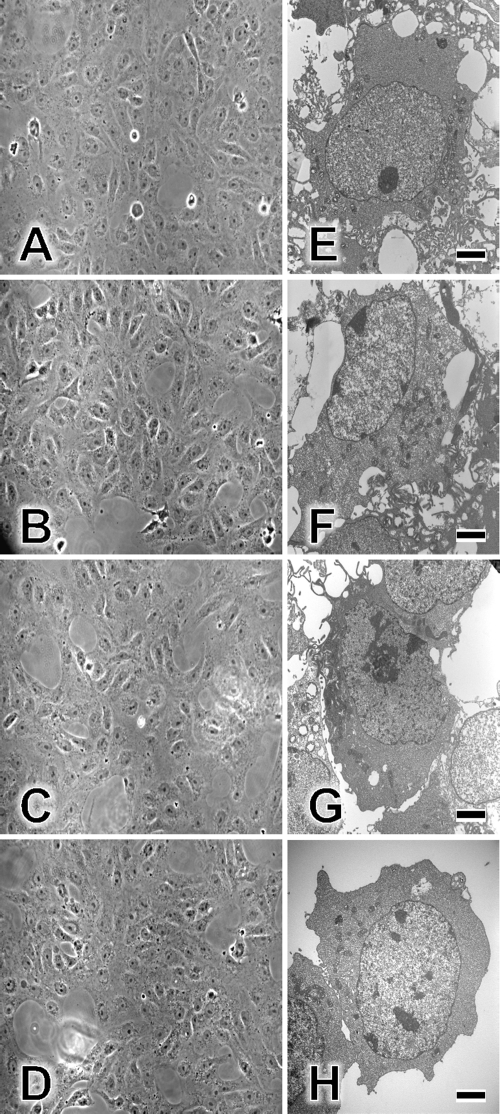
Uninfected Vero cell monolayers were treated with 100 μM EGCG, theasinensin A, or TF-3 in tissue culture medium (pH 7.4) and examined by phase-contrast microscopy (Fig. 5). When untreated cell monolayers (Fig. 5A) were compared with EGCG (Fig. 5B)-, theasinensin A (Fig. 5C)-, and TF-3 (Fig. 5D)-treated monolayers, no differences were seen. Vero cell monolayers were also examined using the cell titer 96 AQueous nonradioactive cell proliferation assay (Promega, Madison, WI) and separately with propidium iodide, which penetrates only the membranes of dead cells (data not shown). The cell proliferation assay did not show any differences following exposure of cells for 1 or 24 h to EGCG, theasinensin A, or TF-3, and propidium iodide-dependent red fluorescence was not seen in any of the treated cell cultures. When individual cells that were either untreated (Fig. 5E), EGCG treated (Fig. 5F), theasinensin A treated (Fig. 5G), or TF-3 treated (Fig. 5H) were examined by EM, no damage was seen to the treated cells. There were no indications of apoptosis observed following exposure of Vero cells to EGCG or EGCG dimers. Also, we have previously shown (12) that treatment of Vero cells for periods ranging from 1 to 48 h prior to HSV-1 or HSV-2 exposure did not reduce the production of infectious virus. Exposure of Vero cells to EGCG dimers also had no effect on virus replication (results not shown).
Fig. 5.
Effects of TF-3, theasinensin A, and EGCG on Vero cells. Vero cell monolayers were incubated for 1 h at 37°C in tissue culture medium with 1% FCS (A), medium with 100 μM EGCG (B), medium with 100 μM theasinensin A (C), or medium with 100 μM TF-3 (D). Monolayers were then examined by phase-contrast microscopy and photographed at ×20 magnification. Individual Vero cells were examined by EM as described in Materials and Methods. EGCG and dimers were utilized at 100 μM. (E) Control cells; (F) EGCG-treated cells; (G) theasinensin A-treated cells; (H) TF-3-treated cells. Scale bars are 2 μm (panels E to H).
Comparison of the effect of TF-3 on HSV-1 particle number and infectivity.
Following incubation of TF-3 with HSV-1 virions for 1 h (Table 1), the number of viral particles decreased by 28% while infectivity declined by >5 log10. This suggests that many virions which appear undamaged visually are inactivated by TF-3.
Table 1.
Quantitation of HSV-1 particles in the presence and absence of TF-3
| Cell typea | Result of assay |
|
|---|---|---|
| No. of virionsb | Infectivity (TCID50)c | |
| Control | 30.15 ± 9.83 | 105.71±0.07 |
| TF-3 | 21.73 ± 12.98 | 100.25±0.22 |
| TF-3/control (%) | 72.0 | 0.00035 |
| P value | <0.02 | <0.0001 |
Control samples with HSV-1 in PBS and treated samples with HSV-1, PBS, and TF-3 (100 μM) were incubated at pH 8.0 for 1 h.
At ×60,000 magnification, 26 separate fields (7.6 μm2) were counted for both control and TF-3 samples, and means ± standard deviations were calculated.
50% tissue culture infective dose (TCID50) values are the means ± standard deviations of results of three separate experiments.
Inactivation of enveloped and nonenveloped viruses by EGCG and dimers.
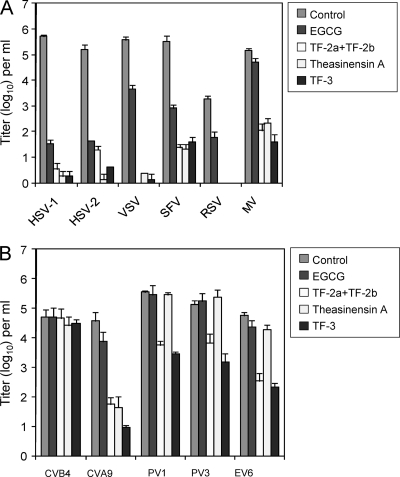
HSV-1 and HSV-2 are DNA alpha herpesviruses, while VSV is a negative-strand RNA rhabdovirus; however, all three are enveloped viruses utilizing class III fusion proteins (32). We, therefore, also examined the sensitivity of other enveloped viruses which have class I and class II fusion proteins (Fig. 6A). Respiratory syncytial virus (RSV) and measles virus (MV) are both paramyxoviruses with class I fusion proteins, and Semliki Forest virus (SFV) is an alphavirus with a class II fusion protein (32). The results in Fig. 6A show that HSV virions with class III fusion proteins were reduced in titer by ≅4 log10 by EGCG (pH 8.0) while VSV was reduced by 2 log10. Class II SFV is reduced by 2.5 log10, and RSV and MV, which have class I fusion proteins, are the least sensitive to EGCG inactivation, with titers reduced by 1.5 log10 and 0.5 log10, respectively. The EGCG dimers reduced the titers of viruses with class I, II, and III fusion proteins more effectively than EGCG itself, and MV, which was minimally sensitive to EGCG, was inactivated by ≥3 log10 by theasinensin A, TF-3, and TF-2a plus TF-2b. The variability in sensitivity to inactivation by EGCG suggests that inactivation of enveloped viruses is not solely due to membrane disruption but may also involve protein binding following interaction with the viral envelope.
Fig. 6.
Inactivation of enveloped and nonenveloped viruses by EGCG and EGCG dimers. Each compound was used at a concentration of 100 μM and incubated for 1 h at pH 8.0. (A) Enveloped viruses: herpes simplex virus (HSV), vesicular stomatitis virus (VSV), Semliki Forest virus (SFV), respiratory syncytial virus (RSV), and measles virus (MV). (B) Nonenveloped viruses: coxsackie B4 virus (CVB4), coxsackie A9 virus (CVA9), poliovirus 1 (PV1), poliovirus 3 (PV3), and echovirus 6 (EV6). Viral titers are the means ± standard deviations of results of three separate experiments.
We examined the effect of EGCG and EGCG dimers on a group of enteroviruses which are nonenveloped (Fig. 6B). EGCG did not reduce the titers of any of the viruses. Theasinensin A reduced the titer of coxsackie A9 virus by ≅3 log10 but was ineffective against polioviruses 1 and 3, coxsackie B4 virus, and echovirus 6. The theaflavin dimer, TF-3, had a broader range of activity than theasinensin A, inactivating echovirus 6, poliovirus 1, and poliovirus 3 by ≥2 log10 and coxsackie A9 virus by ≥3.5 log10. TF-2a plus TF-2b had an inactivation profile similar to that of TF-3. None of the compounds reduced the titer of coxsackie B4 virus. These results indicate that an EGCG dimer can inactivate susceptible nonenveloped viruses as well as enveloped viruses.
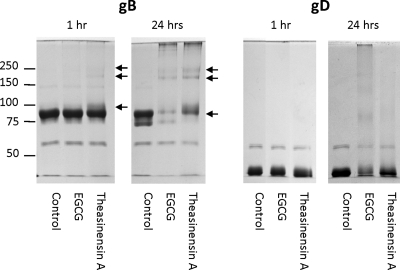
The inactivation of enveloped viruses by EGCG dimers could be solely due to localization into viral envelopes (25) or binding to envelope glycoproteins following membrane binding (Fig. 6). However, the results with nonenveloped enteroviruses (Fig. 6B) suggested that dimers can inactivate virions by interacting with proteins required for infectivity. Two HSV-1 glycoproteins involved in viral entry are gB and gD, and we had previously shown that macromolecular complexes were produced when EGCG was incubated with gB and gD for 24 h (12). The results in Fig. 7 showed that following a 1-h incubation, theasinensin A produced shifts in the banding pattern of gB (see arrows). Following a 24-h incubation of gB and theasinensin A, the molecular weight shifts were more pronounced than at 1 h (see arrows). When EGCG was incubated with gB for 1 h, the protein pattern was identical to that of the control lane, but at 24 h, macromolecular complexes were produced. Theasinensin A affected gB protein aggregation more rapidly than EGCG in 1 h. By 24 h, both compounds produced major though not identical alterations in the gB banding pattern. When theasinensin A and EGCG were incubated with gD for 1 h, no difference was seen between the banding patterns of the treated sample and that of the control. At 24 h, the EGCG-treated sample showed a greater change in banding pattern than the theasinensin A sample. The change in gB banding pattern following a 1-h incubation with theasinensin A, but not EGCG, at pH 7.4 (Fig. 7) is in line with the 1-h HSV-1 inactivation results (Fig. 2A, pH 7.4), where theasinensin A and EGCG reduced the titer of HSV-1 by ≅5 log10 and 2 log10, respectively.
Fig. 7.
Effects of EGCG and theasinensin A on the formation of macromolecular complexes by HSV-1 envelope glycoproteins gB and gD. Theasinensin A or EGCG at a concentration of 550 μM was incubated with recombinant purified gB or gD for 1 or 24 h and then analyzed by SDS-PAGE, followed by Coomassie blue staining. The arrows indicate shifts in the banding pattern of gB following incubation with EGCG or theasinensin A.
DISCUSSION
The present study indicates that when EGCG is oxidized to produce dimers with two gallate groups, the effective antiviral pH range is expanded from the neutral into the acidic range. EGCG inactivates HSV-1 and HSV-2 as effectively as digallate dimers at pH 8.0 but has minimal or no anti-HSV activity at pH 5.7 or below, while the dimers inactivate HSV from pH 4.0 to 8.0. EGCG dimers are also more effective against other enveloped viruses with class I, II, and III fusion proteins at neutral to slightly alkaline pH. Both digallate and monogallate dimers reduced the titer of VSV, which, along with HSV has a class III fusion protein, by 3 log10 more than EGCG at pH 8.0. Also at neutral pH, EGCG dimers reduced the titers of viruses with class I and class II fusion proteins more effectively than EGCG. MV (class I) was not inactivated by EGCG at neutral pH but had its titer reduced by the dimer by ≥3 log10 at the same pH. At pH 5.7, the monogallate dimers and EGCG had similarly low antiviral activities against HSV, indicating that it is the presence of two gallates in addition to the dimerization that is needed for antiviral activity as the pH becomes more acidic. The importance of gallate groups for antimicrobial activity has also been shown when tea catechins and theaflavins were used as bactericidal agents (7, 9) or against human cancer cells (8). As gallate ester groups were added to the theaflavin molecule, antibacterial and antitumor activities increased. Our results suggest that the digallate dimers of EGCG (theasinensin A, P2, and TF-3), with their strong inactivation of HSV between pHs 4.0 and 8.0, would potentially function well as active ingredients in a topical microbicide. This is especially important in order for a microbicide to reduce vaginal transmission of HSV from male to female and female to male, because the vaginal pH can range from pH 3.6 to 4.5 in most women, from pH 5.0 to 6.5 in women with bacterial vaginosis, and from pH 6.0 to 7.0 when semen temporarily neutralizes vaginal pH (2, 21, 27).
TF-3 and theasinensin A were not toxic to Vero cells in the present study. In other studies utilizing TF-3, the growth of all human cancer cell lines and normal human liver cells was inhibited but the growth of normal human lung cells was unaffected (8). In a clinical study, tea polyphenols appeared to lack toxicity following human consumption (29). These in vitro and in vivo toxicity studies, when considered with the fact that cells in the female genital tract are protected by a mucosal barrier, suggest that antiviral EGCG dimers have the potential for use in a microbicide.
The results in this study suggest that inactivation of viruses by theasinensin A and TF-3 can involve interaction with viral proteins. The decrease in titers of the nonenveloped coxsackie A9 virus by EGCG dimers can only be due to interaction with the viral capsid. The variability in sensitivity to dimer inactivation between the five enteroviruses tested suggests that TF-3 and the other dimers do not bind equally as well to even closely related viruses. The ability of EGCG dimers to inactivate enveloped viruses from all three fusion classes suggests that the dimers may bind directly to all fusion proteins or, more likely, that following hydrogen binding to viral envelopes (25), the dimers may be able to move to essential glycoproteins and prevent them from functioning. It is also possible that the hydrogen binding itself between these catechins and envelope lipid bilayers may alter the function of fusion-required glycoproteins without direct interaction between the catechin and the glycoprotein. It has been shown that TF-3 inhibits HIV-1 entry into target cells by preventing envelope glycoprotein-mediated membrane fusion (15). This TF-3-dependent anti-HIV activity was correlated with the ability to block formation of the gp41 six-helix bundle. The use of TF-3 and other digallate dimers could provide direct inactivation of HSV and HIV, thereby reducing the titer of both pathogens involved in the synergistic relationship between HSV and HIV (6, 31). It is essential to prevent initial HSV infection, since once the virus is established, HIV-1 receptor-positive cells can persist at the sites of herpes virus infection for months after healing, even with acyclovir therapy (35). This may explain the failure of acyclovir treatment to reduce HIV-1 acquisition (3). Our results clearly indicate that digallate dimers of EGCG have the capability to reduce the titer of HSV-1 and HSV-2 virions by 3 to 5 log10, thereby reducing HSV transmission and consequently HIV transmission.
Approximately half of the drugs currently in clinical use are of natural product origin (19). Many of the most promising natural lead compounds are available only in extremely small amounts; however, this is not the situation with theaflavin and catechin derivatives, which are the major polyphenols in black tea and green tea, respectively. Catechins account for 6 to 16% of the dry weight of green tea leaves and theaflavins for about 2% of the dried water extract of black tea (10). It has been suggested (13) that natural products such as TF-3 and EGCG possess superior specificity and potency compared to artificially designed molecules due to evolutionary selection. As importantly, the structures of the biological targets of natural products, e.g., protein binding sites, are often well conserved among proteins of markedly different genetic sequences (18). This is evidenced by the structural homology found between the class III fusion proteins gB from HSV-1 and glycoprotein G from VSV (11, 23). Both gB and G are viral envelope proteins that are essential for viral fusion with cell membranes, and their X-ray structures indicate that they are similar in domain organization despite having different primary sequences (11).
A number of studies have shown that compounds with anti-HSV activity function by binding to gB from either HSV-1 or HSV-2. Among 22 human cytokines examined, MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5 had anti-HSV-1 activity and were shown to strongly bind to HSV-1 virions via envelope gB (17). The θ defensin retrocyclin 2, which protects cells from infection by inhibiting viral adhesion and entry, was shown by surface plasmon resonance studies to strongly bind to gB from HSV-2 (34). Also, a number of sulfated or sulfonated polysaccharides which inhibit the cell-to-cell spread of HSV bind to gB (4). It is, therefore, possible that the interaction of EGCG dimers with gB has a similar antiviral effect on HSV infectivity and that the inactivation of all the susceptible enveloped and nonenveloped viruses examined in this study is the result of an alteration of the structure of essential fusion proteins by EGCG dimers. EGCG dimers are lower-molecular-weight and inexpensive alternatives to polyanions, chemokines, and defensins and can potentially be utilized to inactivate HSV and HIV in a topical microbicide.
ACKNOWLEDGMENTS
We thank Gary H. Cohen and Roselyn J. Eisenberg for providing purified HSV-1 gB and gD, as well as Richard A. Kascsak for helpful suggestions during the preparation of the manuscript and Anna Parese and Mary Ellen Cafaro for helping to prepare the manuscript.
This work was supported by Public Health Service grant AI 51611-05 from the National Institute of Allergy and Infectious Diseases and by the New York State Office of Mental Retardation and Developmental Disabilities.
Footnotes
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Aurelian L. 2004. Herpes simplex virus type 2 vaccines: new ground for optimism? Clin. Diagn. Lab. Immunol. 11:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boskey E. R., Telsch K. M., Whaley K. J., Moench T. R., Cone R. A. 1999. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67:5170–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Celum C., et al. 2008. Effect of acyclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomized, double-blind, placebo-controlled trial. Lancet 371:2109–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheshenko N., et al. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 48:2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu D.-C., Juneja L. R. 1997. General chemical composition of green tea and its infusion, p. 13–22.In Yamamoto T., Juneja L. R., Chu D.-C., Kim M. (ed.), Chemistry and applications of green tea. CRC Press, New York, NY. [Google Scholar]
- 6. Corey L., Wald A., Celum C. L., Quinn T. C. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35(5):435–445 [DOI] [PubMed] [Google Scholar]
- 7. Friedman M. 2007. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 51:116–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman M., et al. 2007. Structure-activity relationships of tea compounds against human cancer cells. J. Agric. Food Chem. 55:243–253 [DOI] [PubMed] [Google Scholar]
- 9. Friedman M., Henika P. R., Levin C. E., Mandrell R. E., Kozukue N. 2006. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J. Food Prot. 69:354–361 [DOI] [PubMed] [Google Scholar]
- 10. Hamilton-Miller J. M. T. 1995. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 39:2375–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heldwein E. E., et al. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220 [DOI] [PubMed] [Google Scholar]
- 12. Isaacs C. E., et al. 2008. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 52:962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koehn F. E., Carter G. T. 2005. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4:206–220 [DOI] [PubMed] [Google Scholar]
- 14. Lin M. C., Graham A. D., Polse K. A., McNamara N. A., Tieu T. G. 2000. The effects of one-hour wear of high-Dk soft contact lenses on corneal pH and epithelial permeability. CLAO J. 26:130–133 [PubMed] [Google Scholar]
- 15. Liu S., et al. 2005. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta 1723:270–281 [DOI] [PubMed] [Google Scholar]
- 16. Mindel A. 1998. Genital herpes—how much of a public-health problem? Lancet 351:16–18 [DOI] [PubMed] [Google Scholar]
- 17. Nakayama T., et al. 2006. Novel antiviral activity of chemokines. Virology 350:484–492 [DOI] [PubMed] [Google Scholar]
- 18. Overington J. P., Al-Lazikani B., Hopkins A. L. 2006. How many drug targets are there? Nature 5:993–996 [DOI] [PubMed] [Google Scholar]
- 19. Paterson I., Anderson E. A. 2005. The renaissance of natural products as drug candidates. Science 310:451–453 [DOI] [PubMed] [Google Scholar]
- 20. Quinn T. C., et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921–929 [DOI] [PubMed] [Google Scholar]
- 21. Ravel J., et al. 2011. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108:4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reed L. J., Muench M. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493–497 [Google Scholar]
- 23. Roche S., Bressanelli S., Rey F. A., Gaudin Y. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187–191 [DOI] [PubMed] [Google Scholar]
- 24. Sang S., Lee M.-H., Ho C.-T., Yang C. S. 2005. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 53:9478–9484 [DOI] [PubMed] [Google Scholar]
- 25. Sirk T. W., Friedman M., Brown E. F. 2011. Molecular binding of black tea theaflavins to biological membranes: relationship to bioactivities. J. Agric. Food Chem. 59:3780–3787 [DOI] [PubMed] [Google Scholar]
- 26. Spur A. R. 1969. A low viscosity epoxy resin and embedding medium for electron microscopy. J. Ultrustruct. Res. 26:31–43 [DOI] [PubMed] [Google Scholar]
- 27. Tevi-Bénissan C., et al. 1997. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 4:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thormar H., Isaacs C. E., Brown H. R., Barshatzky M. R., Pessolano T. 1987. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 31:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ullmann U., et al. 2003. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 31:88–101 [DOI] [PubMed] [Google Scholar]
- 30. Wagner H., Kostka K.-H., Lehr C. M., Schaefer U. F. 2003. pH profiles in human skin: influence of two in vitro test systems for drug delivery testing. Eur. J. Pharm. Biopharm. 55:57–65 [DOI] [PubMed] [Google Scholar]
- 31. Wald A. 2004. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes 11:3:70–76 [PubMed] [Google Scholar]
- 32. White J. M., Delos S. E., Brecher M., Schornberg K. 2008. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 43:189–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whitley R. J. 1994. Herpes simplex virus infections of women and their offspring: implications for a developed society. Proc. Natl. Acad. Sci. U. S. A. 91:2441–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yasin B., et al. 2004. θ defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu J., et al. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature 15:886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]