Abstract
Dihydroartemisinin-piperaquine is a fixed-dose artemisinin-based combination treatment. Some antimalarials have altered pharmacokinetics in pregnancy. Pregnant women in the 2nd or 3rd trimester and matched nonpregnant women with uncomplicated falciparum malaria were treated with a total of 6.4 mg/kg of body weight dihydroartemisinin and 51.2 mg/kg piperaquine once daily for 3 days. Venous blood samples were drawn at prespecified time points over 9 weeks. Plasma dihydroartemisinin and piperaquine concentrations were analyzed by liquid chromatography-mass spectrometry. Piperaquine and dihydroartemisinin pharmacokinetics were well described. There were no significant differences in total piperaquine exposure (P = 0.80) or drug exposure during the terminal elimination phase (72 h to infinity) (P = 0.64) between the two groups. The apparent volume of distribution of piperaquine was significantly smaller (602 liters/kg versus 877 liters/kg) in pregnant women than in nonpregnant women (P = 0.0057), and the terminal elimination half-life was significantly shorter (17.8 days versus 25.6 days; P = 0.0023). Dihydroartemisinin exposure after the first dose was significantly lower (844 h × ng/ml versus 1,220 h × ng/ml, P = 0.0021) in pregnant women, but there were no significant differences in total dihydroartemisinin exposure or maximum concentrations between the two groups. There were no significant differences in any pharmacokinetic parameters between the second and third trimester. These results obtained through noncompartmental analysis suggest that in the treatment of falciparum malaria, there are no clinically important differences in the pharmacokinetics of dihydroartemisinin or piperaquine between pregnant and nonpregnant women. However, a more detailed analysis using population pharmacokinetic modeling is needed to fully investigate the differences found for some of the pharmacokinetic parameters, such as the terminal half-life.
INTRODUCTION
In the eastern and western border areas of Thailand, Plasmodium falciparum has developed resistance to almost every antimalarial drug (5). This poses particular problems for the treatment of pregnant women, a group especially vulnerable to P. falciparum infections. The World Health Organization (WHO) recommends the use of artemisinin combination therapy (ACT) (short-course, 3-day treatments) in the second and third trimester of pregnancy (37). However, pregnant women often have lower antimalarial blood concentrations than nonpregnant women and are therefore at risk of undertreatment (13–16, 34, 36). Dihydroartemisinin-piperaquine (DHA-PPQ) is one of the most promising ACTs. It is a coformulation of dihydroartemisinin and piperaquine. Randomized clinical trials in Asia, Africa, and South America indicate excellent tolerability and high cure rates in nonpregnant populations (2–4, 8, 25, 31, 35, 38). Recently, DHA-PPQ was found to be well tolerated and effective in the treatment of multiple recrudescent P. falciparum infections in 50 pregnant women in Thailand (27) and in 104 pregnant women with uncomplicated P. falciparum and Plasmodium vivax infections in West Papua, Indonesia (24). Pharmacokinetic studies, including population pharmacokinetics of PPQ, have been reported in adults and children (10, 20, 21, 25, 29, 32, 33) but not in pregnant women. Plasma concentrations of the artemisinin derivatives artesunate or artemether and their common active metabolite DHA are reported to be low in pregnancy (14, 15, 17, 23), but this has not been studied for DHA-PPQ. In this study, we compared the pharmacokinetic parameters of DHA and PPQ in the treatment of uncomplicated P. falciparum malaria in pregnant and matched nonpregnant women living on the western border of Thailand.
MATERIALS AND METHODS
Antenatal clinics.
The study was carried out in the WangPha clinic of the Shoklo Malaria Research Unit (SMRU). This clinic is based on the western border of Thailand where malaria transmission is low and seasonal; P. falciparum and P. vivax are the predominant species. All pregnant women routinely have a dating ultrasound scan at their first antenatal clinic (ANC) attendance (26) and are invited to attend the frequent (weekly) screening program to detect and treat all malaria episodes (22) and prevent maternal mortality (28). Women receive ferrous sulfate and folic acid supplements from the first ANC consultation until delivery. If they become anemic, treatment doses are provided. Women were encouraged to deliver under the supervision of trained Karen midwives, certified in Advanced Life Support Obstetrics (ALSO) procedures and supervised by doctors. Birth weights were measured on a Seca digital scale with a precision of ±10 g, and infant length and head and arm circumference were measured with a Seca measuring tape (28). Nonpregnant women consulted the outpatient department in the same clinic.
Ethics.
Approval of the study was obtained from the ethics committee of the Faculty of Tropical Medicine, Bangkok (MUTM 2007-111), and the Oxford Tropical Research Ethics Committee (OxTREC 017-07).
Matching.
Pregnant women with a viable singleton pregnancy in the second or third trimester with P. falciparum monoinfection or mixed (P. falciparum and P. vivax) infection, a field-sample hematocrit of more than 25%, and no signs of severe malaria were eligible for inclusion. Every pregnant woman was matched prospectively to a nonpregnant woman (confirmed by urine test) presenting with falciparum malaria by age, parasitemia, smoking, raised measured temperature (≥37.5°C) on admission, and history of fever in the last 2 days. Before enrolment, the purpose of the study was explained in the patient's own language and written consent was obtained (by thumb print if she was unable to read or write). A full medical history and physical examination were carried out, and baseline blood samples were taken.
DHA-PPQ dosing regimen.
The patients were treated with a total dose of 6.4 mg/kg of body weight DHA and 51.2 mg/kg PPQ (one DHA-PPQ tablet contained 40/320 mg of DHA/PPQ; Holleypharm, People's Republic of China). The combination was given with water every 24 h, i.e., at 0, 24, and 48 h, under direct observation. The amount of tablets was calculated from the actual weight of the (pregnant) woman, and the tablets were divided to the nearest quarter.
Sampling regimen.
Blood samples (2 ml) were obtained from a catheter inserted in a vein and were taken into 1-ml lithium heparin tubes (for PPQ) and 1-ml prechilled sodium fluoride-potassium oxalate tubes (for DHA) at baseline (before the first dose) and at hours 0.5, 1.5, 4, 8 (before the 2nd dose), 24.5, 25.5, 28, and 32 (before the third dose), and 48.25, 48.5, 49, 50, 51, 52, 54, 56, 60, and 72. The catheter was removed, and additional samples were taken for PPQ by venous puncture at days 5, 7, 14, 21, 28, 35, 42, 49, 56, 63, 77, and 84. Whole-blood samples for PPQ drug concentrations were centrifuged at room temperature at 1,500 to 2,000 × g for 10 min to obtain plasma. Immediately after centrifugation, the plasma was transferred into a screw-cap cryovial and frozen at −20°C in a laboratory freezer. Within 2 months the frozen plasma samples were transferred to a −80°C freezer. Blood samples for DHA analysis were placed on wet ice and processed immediately after collection. Whole blood was centrifuged at 4°C at 2,000 × g for 7 min to obtain plasma, transferred into a screw-cap cryovial, and frozen in liquid nitrogen. The sampling and storage times were recorded, and a note made in the case of visible hemolysis. All samples were analyzed at the Clinical Pharmacology Laboratory, MORU, Bangkok, Thailand. Plasma DHA and PPQ concentrations were analyzed by liquid chromatography-mass spectrometry (LC-MS).
Pharmacokinetic analysis.
Individual concentration-time data were evaluated using a noncompartmental analysis approach in WinNonlin version 5.3 (Pharsight Corporation, CA). Total exposure up to the last measured concentration (AUC0-last) was calculated using the linear trapezoidal method for ascending concentrations and the logarithmic trapezoidal method for descending concentrations. Drug exposure was extrapolated from the last observed concentration to time infinity by Clast/λZ for each individual subject to compute total drug exposure (AUC0-∞). The terminal elimination half-life (t1/2) was estimated by log-linear regression of at least three observed concentrations in the terminal elimination phase. Maximum concentration (Cmax) and time to maximum concentration (Tmax) were taken directly from the observed data. Apparent volume of distribution (VZ/F) and oral clearance (CL/F) were computed individually according to standard procedures.
Observed DHA and PPQ exposure, Tmax, and Cmax were computed after every dose. Residual PPQ exposure from doses one and two could not be accurately subtracted from the PPQ exposure of the last dose because of its multicompartment pharmacokinetics and a long terminal elimination half-life. Therefore, the total dose of PPQ base (i.e., the sum of the three doses) was used as the input dose together with all observed concentration-time data in the noncompartmental analysis of PPQ to compute pharmacokinetic parameters. DHA has a short terminal elimination half-life, and dense samples suitable for noncompartmental analysis were collected after the last dose. Therefore, observed concentration-time data from the last dose was used in the noncompartmental analysis of DHA to compute pharmacokinetic parameters.
Statistical analysis.
Data were described using the statistical program SPSS for Windows version 18 (Predictive Analytics SoftWare, United States) and EpiInfo (Centers for Disease Control and Prevention). Continuous data were compared by Student's t test or the Mann-Whitney U test. Treatment efficacy was compared by Kaplan-Meier survival analysis.
Efficacy and safety assessment.
At enrolment, a full medical history and physical examination were carried out by a physician and a midwife. The gestational age of the pregnancy and fetal viability were confirmed by ultrasound. Complete blood count (CBC), parasite count, and biochemistry were measured on admission and at day 14. On each of the first 5 days of the study, patients had a clinical and parasitological evaluation. Daily malaria smears were made for assessment of parasite clearance. Adverse effects were evaluated daily until day 5 and weekly thereafter until 6 weeks. Parasitological follow up was continued for 9 weeks (63 days) in total or, in pregnant women, until delivery, whichever occurred later. Reappearance of P. vivax parasites was treated with chloroquine (25 mg base/kg total dose; Government Pharmaceutical Organization, Thailand). In the case of reappearance of P. falciparum parasites, a PCR blood spot sample was obtained, and pregnant women were treated with artesunate (2 mg/kg/day for 7 days; Guilin, People's Republic of China) and clindamycin (300 mg three times daily for 7 days; Siam Pharmaceutical Company Ltd., Bangkok, Thailand) and nonpregnant women with artesunate (4 mg/kg/day for 3 days) and mefloquine (25 mg base/kg in two divided doses; Mephaquine, Atlantic Laboratories Corp. Ltd., Bangkok, Thailand). Each newborn was examined by a trained physician for the presence of congenital abnormalities. Mothers and their babies were invited for follow-up visits to check growth and for malaria infection and to provide care if needed.
RESULTS
From June to December 2008, 25 pregnant women and 24 matched nonpregnant women with acute P. falciparum (with or without P. vivax) malaria consented to participate in the study. One pregnant woman vomited the drug within 1 h of the first dose and was excluded from further analysis and replaced by another pregnant woman (Table 1). There were 13 women in the second and 11 women in the third trimester. Twenty-seven women (11 pregnant and 16 nonpregnant) reported taking paracetamol before enrolment; two of them also reported self-treatment with antimalarials (chloroquine and primaquine), whereas two others took unknown medicines in a small packet (yaa-chud) (19). Pregnant women received a higher dose of DHA-PPQ than nonpregnant women (P = 0.034); the median (range) total doses of DHA were 6.7 (6.1 to 7.3) versus 6.3 (6.0 to 7.3) mg/kg and of PPQ were 53.3 (49.0 to 58.5) versus 50.3 (48.0 to 58.5) mg/kg for pregnant women and nonpregnant women, respectively. The median (range) time between the last meal and the treatment was 150 (6 to 591) minutes and did not differ between the groups.
Table 1.
Demographic characteristics on admission of pregnant Karen women and matched nonpregnant women with falciparum malariaa
| Characteristic | Pregnant women (n = 24) | Nonpregnant women (n = 24) | P value |
|---|---|---|---|
| Age (yr) | 28.1 ± 8.0 | 30.5 ± 9.0 | 0.29 |
| Gravidity | 3 (1–9) | NA | NA |
| Parity | 2 (0–8) | NA | NA |
| Primigravida | 16.6 (4) | NA | NA |
| Smoker | 20.8 (5) | 20.8 (5) | 1.0 |
| Weight (kg) | 49 ± 6 | 47 ± 8 | 0.06 |
| Height (cm) | 153 ± 4 | 154 ± 6 | 0.49 |
| Temperature (°C) | 37.7 ± 0.9 | 37.7 ± 0.7 | 0.88 |
| Days of fever | 2 (0–4) | 1 (0–4) | 0.19 |
| Gestational age (wk) | 24.6 ± 5.9 | NA | NA |
| 3rd trimester | 45.8 (11) | NA | NA |
| Hematocrit on admission (%) | 31.0 ± 4.5 | 34.8 ± 4.6 | 0.008 |
| Anemic | 41.7 (10) | 16.7 (4) | 0.059 |
| Geometric mean parasitemia/μl (range) | 7,349.06 (96–176,594) | 5,968.72 (32–135,645) | 0.628 |
| Mixed (P. falciparum and P. vivax) infection | 16.7 (4) | 8.3 (2) | 0.67 |
| Hepatomegaly | 20.8 (5) | 16.7 (4) | 0.72 |
| Splenomegaly | 8.3 (2) | 4.2 (1) | 0.56 |
Data are presented as mean ± standard deviation, median (range), or number (%). NA, not applicable.
Pharmacokinetics.
In this study, PPQ and DHA pharmacokinetics were well described in pregnant and nonpregnant women with malaria (Tables 2 and 3; Fig. 1 and 2).
Table 2.
Noncompartmental analysis of piperaquine
| Parametera | Median (range) |
P valuec | |
|---|---|---|---|
| Pregnant women (n = 24) | Nonpregnant women (n = 23b) | ||
| Body wt (kg) | 51.0 (36.0–58.0) | 48.0 (37.0–78.0) | 0.0619 |
| Total dose (mg/kg) | 28.5 (23.8–37.6) | 26.2 (19.7–42.8) | 0.1863 |
| Cmax 1 (ng/ml) | 138 (39.3–328) | 71.6 (10.1–239) | 0.0269 |
| Cmax 2 (ng/ml) | 201 (58.2–455) | 136 (13.6–393) | 0.0342 |
| Cmax 3 (ng/ml) | 309 (138–575) | 245 (53.4–798) | 0.1478 |
| CL/F (liter/hr) | 51.6 (32.7–111) | 47.4 (23.3–466) | 0.8315 |
| CL/F (liter/hr/kg) | 1.00 (0.680–2.26) | 1.10 (0.324–11.1) | 0.7984 |
| V/F (liter) | 30,400 (15,100–57,900) | 39,700 (14,900–80,500) | 0.0136 |
| V/F (liter/kg) | 602 (407–1,180) | 877 (300–1,840) | 0.0057 |
| t1/2 (days) | 17.8 (8.88–24.9) | 25.6 (4.78–39.9) | 0.0023 |
| AUC0–24 (h × ng/ml) | 1,480 (506–3,270) | 869 (157–2,940) | 0.0083 |
| AUC24–48 (h × ng/ml) | 2,400 (734–4,400) | 1,710 (167–4,740) | 0.0351 |
| AUC48–72 (h × ng/ml) | 3,660 (1,160–5,010) | 2,750 (500–8,280) | 0.0269 |
| AUC0–last (h × ng/ml) | 28,100 (10,800–40,200) | 21,300 (2,370–49,400) | 0.2418 |
| AUC0–∞ (h × ng/ml) | 29,200 (11,600–41,800) | 24,100 (2,750–60,900) | 0.5232 |
| AUC0–∞/dose [h × ng/ml/(mg/kg)] | 1,000 (442–1,470) | 907 (90.1–3,080) | 0.7984 |
| Day 7 concn (ng/ml) | 31.8 (13.3–80.2) | 25.9 (6.80–56.6) | 0.0410 |
| Day 14 concn (ng/ml) | 19.5 (7.76–49.3) | 16.7 (2.24–59.2) | 0.2873 |
| Day 28 concn (ng/ml) | 10.7 (3.70–31.4) | 9.17 (5.14–47.6) | 0.6356 |
Cmax, maximum observed plasma concentration after dose 1, 2, and 3; CL, elimination clearance; V apparent volume of distribution; t1/2, terminal elimination half-life; AUC0-24, observed area under the plasma concentration-time curve from zero time to 24 h (i.e., 1st dose); AUC24-48, observed area under the plasma concentration-time curve from 24 h to 48 h (i.e., 2nd dose); AUC48-72, observed area under the plasma concentration-time curve from 48 h to 72 h (i.e., 3rd dose); AUC0-last, observed area under the plasma concentration-time curve from zero time to last observed concentration; AUC0-∞, predicted area under the plasma concentration time curve after the last dose from zero time to infinity; F, oral bioavailability.
One subject in the nonpregnant group was lost after 7 days during follow-up.
P values using the two-sample Wilcoxon rank-sum (Mann-Whitney) test are given. Values in boldface are significant.
Table 3.
Noncompartmental analysis of dihydroartemisinin
| Parametera | Median (range) |
P valueb | |
|---|---|---|---|
| Pregnant women (n = 24) | Nonpregnant women (n = 24) | ||
| Body wt (kg) | 51.0 (36.0–58.0) | 47.5 (37.0–78.0) | 0.0737 |
| Total dose (mg/kg) | 6.66 (6.12–7.32) | 6.33 (6.00–7.32) | 0.0135 |
| Cmax 1 (ng/ml) | 366 (113–1,020) | 432 (91.4–1,240) | 0.0696 |
| Cmax 2 (ng/ml) | 595 (73.0–1,160) | 472 (82.4–1,140) | 0.9753 |
| Cmax 3 (ng/ml) | 553 (201–881) | 538 (141–1,280) | 0.6952 |
| CL/F (liter/hr) | 106 (49.0–461) | 81.6 (38.4–236) | 0.0696 |
| CL/F (liter/hr/kg) | 2.14 (1.14–9.04) | 1.74 (0.799–5.50) | 0.1171 |
| V/F (liter) | 170 (60.4–800) | 149 (67.0–457) | 0.3429 |
| V/F (liter/kg) | 3.31 (1.4–15.7) | 3.42 (1.34–9.91) | 0.7105 |
| t1/2 (h) | 1.07 (0.46–2.5) | 1.40 (0.837–2.07) | 0.3753 |
| AUC0–24 (h × ng/ml) | 844 (260–2,170) | 1,220 (396–2,780) | 0.0021 |
| AUC24–48 (h × ng/ml) | 1,100 (195–2,060) | 1,270 (348–3,750) | 0.0909 |
| AUC48–72 (h × ng/ml) | 1,040 (254–2,030) | 1,300 (414–2,580) | 0.1489 |
| AUC48–∞ (h × ng/ml) | 1,050 (260–2,040) | 1,310 (423–2,610) | 0.1489 |
| AUC48–∞/dose [h × ng/ml/(mg/kg)] | 468 (111–878) | 576 (182–1,250) | 0.1171 |
Cmax, maximum observed plasma concentration after dose 1, 2, and 3; CL, elimination clearance; V apparent volume of distribution; t1/2, terminal elimination half-life; AUC0-24, observed area under the plasma concentration-time curve from zero time to 24 h (i.e., 1st dose); AUC24-48, observed area under the plasma concentration-time curve from 24 h to 48 h (i.e., 2nd dose); AUC48-72, observed area under the plasma concentration-time curve from 48 h to 72 h (i.e., 3rd dose); AUC0-∞, predicted area under the plasma concentration time curve after the last dose from zero time to infinity; F, oral bioavailability.
P values are given using the Two-sample Wilcoxon rank-sum (Mann-Whitney) test. Values in boldface are significant.
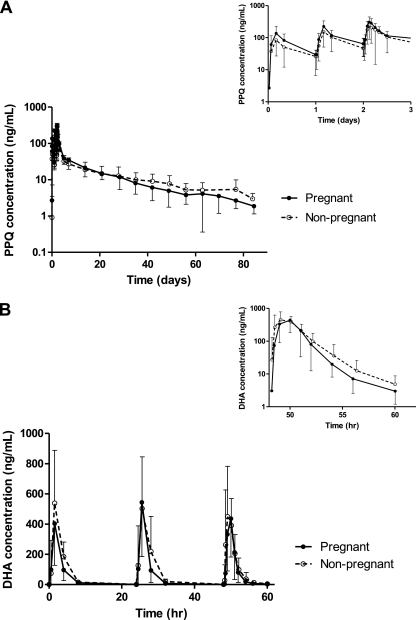
Fig. 1.
Mean (standard deviation) plasma concentration-time profiles of piperaquine (A) and dihydroartemisinin (B) in pregnant (solid line) and nonpregnant (dashed line) women with P. falciparum malaria. Insets show detailed plasma piperaquine profiles over the first 3 days (top right) and plasma DHA concentrations after the final dose (bottom right).
Fig. 2.
Individual plasma concentration-time profiles of piperaquine (A and B) and dihydroartemisinin (C and D) in pregnant (A and C) and nonpregnant (B and D) women with P. falciparum malaria. The solid black lines represent the women with recrudescent P. falciparum infections.
Piperaquine pharmacokinetics.
There were no significant differences in total PPQ exposure (P = 0.80) (Table 2) or drug exposure during the terminal elimination phase (72 h to infinity) between pregnant and nonpregnant women; the median (range) AUC72-∞ was 19,900 (9,180 to 33,900) h × ng/ml versus 19,400 (1,930 to 50,500) h × ng/ml (P = 0.64), respectively. There were no significant differences (P > 0.05) in any pharmacokinetic parameters between the second and third trimester. There were higher PPQ concentrations at day 7 (P = 0.041) in pregnant women than in nonpregnant women, but this difference disappeared 1 week later, resulting in no significant differences in PPQ concentrations at day 14 or day 28 (P = 0.29 and P = 0.64, respectively). However, when the PPQ concentration was normalized for the actual dose/weight received, there was no difference (P > 0.15) in median day 7 (or day 14 or 28) concentrations. The PPQ concentration at day 7 and total PPQ exposure were well correlated (data not shown). The apparent volumes of distribution of PPQ were significantly smaller in pregnant women than in nonpregnant women (P = 0.0057), resulting in significantly higher maximum concentrations in pregnant women than in nonpregnant women after the first dose (P = 0.027) and second dose (P = 0.034) but not after the third dose (P = 0.15) (Table 2). This decrease in apparent volume of distribution was associated with a significantly shorter terminal elimination half-life of PPQ in pregnant women than in nonpregnant women (P = 0.0023). Pregnant women had increased 24-h PPQ exposures after the first dose (P = 0.0083), the second dose (P = 0.035), and the third dose (P = 0.027) compared to nonpregnant women. Despite receiving a normal dose (30.6 mg/kg), one nonpregnant patient had a very high elimination clearance (CL/F of 11.1 liters/hr/kg) and apparent volume of distribution (VZ/F of 1,840 liters/kg) of PPQ with a substantially shorter terminal elimination half-life (4.8 days) than the rest of the women in this study (Fig. 2). No altered pharmacokinetic properties of DHA could be seen for this patient. It is likely that this woman had impaired absorption of the lipophilic PPQ. This woman presented with a recrudescence at day 21, presumably as a direct result of the very low PPQ exposure after DHA had cleared the majority of malaria parasites.
Dihydroartemisinin pharmacokinetics.
There were no significant differences in total DHA exposure or Cmax (P > 0.05) between the two groups. DHA exposure after the first dose was significantly lower (844 h × ng/ml versus 1,220 h × ng/ml, P = 0.0021) in pregnant women than in nonpregnant women (Table 3). No other significant differences in the pharmacokinetic properties (i.e., clearance or apparent volume of distribution) of dihydroartemisinin could be seen in pregnant women than in nonpregnant women. There were no significant differences (P > 0.05) in any pharmacokinetic parameters between the second and third trimester. There was a trend toward higher elimination clearance in pregnant women than in nonpregnant women, but this difference did not reach statistical significance (P = 0.12). The high interindividual variability for this drug might mask differences when using noncompartmental analysis. No indication of DHA autoinduction (7) could be seen in these data with comparable DHA exposure and maximum concentrations after all three doses. However, the sampling was not identical after all doses and this might mask a small difference between doses.
Efficacy and adverse effects.
Using Kaplan-Meier survival analysis, the nonadjusted cure rate (95% confidence interval [CI]) by day 63 for any recurrence (P. falciparum or P. vivax) was 45.8% (25.8 to 65.8) for pregnant women and 55.4% (34.8 to 76.0) for nonpregnant women, P = 0.48. Of the recurrent infections in pregnant women, 77% were P. vivax (10/13), with a median (range) time to the next P. falciparum or P. vivax infection of 70 (31 to 180) days or 56 (28 to 77) days, respectively. The P. falciparum PCR-adjusted cure rate (95% CI) by day 63 was 95.7% (87.3 −100) for both pregnant and nonpregnant women; there was one recrudescent infection at day 31 and one at day 21, respectively. The individual plasma concentrations of DHA and of PPQ for these two women are shown in Fig. 2.
There were no suspected drug-related serious adverse events. All adverse events were mild; the most common reported events by day 42 were abdominal pain (32%), palpitations (37%), dizziness (38%), sleep problems (38%), and headache (48%), and these were not significantly different between the two groups. No woman developed severe malaria. The parasite and fever clearance times were rapid (median [range], 2 [1 to 4] days and 1 [1 to 2] day, respectively), with no significant difference between the groups. There were no clinically significant abnormalities in biochemistry and hematology results.
Pregnancy outcome.
Two women left the study area before delivery. The remaining 22 women delivered live, normal, singleton babies. All placentas were malaria smear negative. Two mothers did not come back after delivery for baby follow-up after delivery. Of the remaining 20 babies, all completed 1 month of follow-up and there were no neonatal deaths.
DISCUSSION
DHA-PPQ is an important ACT and a promising candidate drug for interventions to reduce malaria in pregnancy. In this study, the pharmacokinetic properties were evaluated using a noncompartmental analysis approach, and the results suggest no risk of lower total drug exposure of PPQ or DHA in pregnant women treated for falciparum malaria. Unlike lumefantrine, proguanil, and atovaquone, where dose adjustments in pregnancy are recommended, the overall PPQ pharmacokinetic parameters seen in this study were comparable with previously published results in nonpregnant adults and children (6, 10, 11, 21, 29, 30, 32). However, pregnant women tended to have a smaller PPQ volume of distribution, a shorter terminal half-life, a higher Cmax after the first two doses, and a higher AUC during the first 72 h than nonpregnant women. As shown previously, PPQ exhibits considerable interindividual pharmacokinetic variability (32). For PPQ, the day 7 plasma concentration may provide a useful indicator of drug exposure and a predictor of efficacy. Malaria patients with a day 7 PPQ plasma concentration below 30 ng/ml were more likely to have a recurrence in Papua, Indonesia (25). In this study, both women (i.e., one pregnant and one nonpregnant woman) with recrudescent infections had low day 7 PPQ concentrations (24 and 6.8 ng/ml, respectively). Previous reports indicate that the absorption of PPQ is dependent on concomitant intake of fat in healthy volunteers. However, studies in malaria patients indicate no difference in total drug exposure but higher variability when given in the fasting state (1, 30). The half-life of only 18 days in pregnant women and the low day 28 concentrations of PPQ suggest that at least monthly doses would be required when DHA-PPQ is considered for intermittent preventive treatment in pregnancy or chemoprophylaxis.
Pharmacokinetic parameter estimates (clearance and apparent volume of distribution) for DHA were slightly higher than those previously reported in nonpregnant adult patients but lower than those reported in healthy volunteers (6, 9, 12, 18, 20). DHA exposure after the first dose was significantly lower in pregnant women; however, the differences were relatively small and may be explained by the high interindividual variability.
DHA-PPQ was safe in this small study, and there were no serious adverse events. All women cleared their parasites and fever quickly. There were no hematological or biochemical adverse events, and no baby had any congenital abnormalities. The day 63 PCR-adjusted cure rate for P. falciparum was high and similar to the rates in previous studies in nonpregnant women (38), and it was slightly higher than observed when DHA-PPQ was used as rescue treatment in pregnancy in the same population (27). Inferior cure rates in treating recrudescent infections in pregnant women have been reported before (16).
In conclusion, these results obtained through noncompartmental analysis suggest that in the treatment of falciparum malaria, there are no clinically important differences in the pharmacokinetics of dihydroartemisinin or piperaquine between pregnant and nonpregnant women and dose adjustment may not be warranted. However, a more detailed analysis using population pharmacokinetic modeling is needed to fully investigate the differences found for some of the pharmacokinetic parameters, such as the terminal half-life.
ACKNOWLEDGMENTS
We thank the patients and staff of SMRU and MORU for their contributions. Members of the Data Safety Monitoring Board were Ishag Adam, Karen Barnes, Philippe Brasseur, and Patrice Piolat. We thank Zhejiang Holley Nanhu Pharmaceutical Co., People's Republic of China, for supplying the tablets.
The scientific input, field work, and publication costs were supported by the Wellcome-Trust Mahidol University Oxford Tropical Medicine Research Program, which is supported by the Wellcome Trust of Great Britain. The assays were supported by a grant from the MiP Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine.
We declare that none of the authors had a conflict of interest.
Footnotes
Published ahead of print on 26 September 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Annerberg A., et al. 2011. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob. Agents Chemother. 55: 3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arinaitwe E., et al. 2009. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin. Infect. Dis. 49: 1629–1637 [DOI] [PubMed] [Google Scholar]
- 3. Ashley E. A., et al. 2005. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41: 425–432 [DOI] [PubMed] [Google Scholar]
- 4. Bassat Q., et al. 2009. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS One 4: e7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brockman A., et al. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94: 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinh N. T., et al. 2009. Pharmacokinetics and bioequivalence evaluation of two fixed-dose tablet formulations of dihydroartemisinin and piperaquine in Vietnamese subjects. Antimicrob. Agents Chemother. 53: 828–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordi T., et al. 2005. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br. J. Clin. Pharmacol. 59: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grande T., et al. 2007. A randomised controlled trial to assess the efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Peru. PLoS One 2: e1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hong X., et al. 2008. Pharmacokinetics of dihydroartemisinin in Artekin tablets for single and repeated dosing in Chinese healthy volunteers. Biopharm. Drug Dispos. 29: 237–244 [DOI] [PubMed] [Google Scholar]
- 10. Hung T. Y., et al. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br. J. Clin. Pharmacol. 57: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karunajeewa H. A., et al. 2008. Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria. Antimicrob. Agents Chemother. 52: 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Thi D. T., Le N. H., Nguyen C. H., Phan Thi D., Na-Bangchang K. 2008. Pharmacokinetics of a five-day oral dihydroartemisinin monotherapy regimen in patients with uncomplicated falciparum malaria. Drug Metab. Pharmacokinet. 23: 158–164 [DOI] [PubMed] [Google Scholar]
- 13. McGready R., et al. 2003. The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur. J. Clin. Pharmacol. 59: 545–552 [DOI] [PubMed] [Google Scholar]
- 14. McGready R., et al. 2006. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 62: 1021–1031 [DOI] [PubMed] [Google Scholar]
- 15. McGready R., et al. 2006. Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 62: 367–371 [DOI] [PubMed] [Google Scholar]
- 16. McGready R., et al. 2008. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated plasmodium falciparum treatment in pregnancy. PLoS Med. 5: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris C. A., et al. 2011. Population pharmacokinetics of artesunate and dihydroartemisinin in pregnant and non-pregnant women with malaria. Malar J. 10: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Na-Bangchang K., Thanavibul A., Tippawangkosol P., Karbwang J. 2005. Pharmacokinetics of the four combination regimens of dihydroartemisinin/mefloquine in acute uncomplicated falciparum malaria. Southeast Asian J. Trop. Med. Public Health 36: 23–33 [PubMed] [Google Scholar]
- 19. Newton P. N., et al. 2008. Characterization of “Yaa Chud” Medicine on the Thailand-Myanmar border: selecting for drug-resistant malaria and threatening public health. Am. J. Trop. Med. Hyg. 79: 662–669 [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen D. V., et al. 2009. Pharmacokinetics and ex vivo pharmacodynamic antimalarial activity of dihydroartemisinin-piperaquine in patients with uncomplicated falciparum malaria in Vietnam. Antimicrob. Agents Chemother. 53: 3534–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen T. C., et al. 2008. Pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 79: 620–623 [PubMed] [Google Scholar]
- 22. Nosten F., ter Kuile F., Maelankirri L., Decludt B., White N. J. 1991. Malaria during pregnancy in an area of unstable endemicity. Trans. R. Soc. Trop. Med. Hyg. 85: 424–429 [DOI] [PubMed] [Google Scholar]
- 23. Onyamboko M. A., et al. 2011. Pharmacokinetics and pharmacodynamics of artesunate and dihydroartemisinin following oral treatment in pregnant women with asymptomatic Plasmodium falciparum infections in Kinshasa DRC. Malar. J. 10: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poespoprodjo J. R., et al. 2008. Adverse pregnancy outcomes in an area where multidrug-resistant plasmodium vivax and Plasmodium falciparum infections are endemic. Clin. Infect. Dis. 46: 1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price R. N., et al. 2007. Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria. Antimicrob. Agents Chemother. 51: 4090–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rijken M. J., et al. 2009. Obstetric ultrasound scanning by local health workers in a refugee camp on the Thai-Burmese border. Ultrasound Obstet. Gynecol. 34: 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rijken M. J., et al. 2008. Dihydroartemisinin-piperaquine rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Am. J. Trop. Med. Hyg. 78: 543–545 [PubMed] [Google Scholar]
- 28. Rijken M. J., et al. 2011. Malaria in pregnancy: the difficulties in measuring birthweight. BJOG 118: 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roshammar D., Hai T. N., Friberg Hietala S., Van Huong N., Ashton M. 2006. Pharmacokinetics of piperaquine after repeated oral administration of the antimalarial combination CV8 in 12 healthy male subjects. Eur. J. Clin. Pharmacol. 62: 335–341 [DOI] [PubMed] [Google Scholar]
- 30. Sim I. K., Davis T. M., Ilett K. F. 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob. Agents Chemother. 49: 2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smithuis F., et al. 2006. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet 367: 2075–2085 [DOI] [PubMed] [Google Scholar]
- 32. Tarning J., et al. 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob. Agents Chemother. 52: 1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tarning J., et al. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob. Agents Chemother. 49: 5127–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tarning J., et al. 2009. Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 53: 3837–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tran T. H., et al. 2004. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet 363: 18–22 [DOI] [PubMed] [Google Scholar]
- 36. White N. J., McGready R. M., Nosten F. H. 2008. New medicines for tropical diseases in pregnancy: catch-22. PLoS Med. 5: e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. WHO 2010. Guidelines for the treatment of malaria, 2nd ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 38. Zwang J., et al. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4: e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]