Abstract
The need to identify improved therapy against cystic echinococcosis (CE) has motivated pharmacology-based research. The comparative pharmacological performances of the benzimidazole compounds flubendazole (FLBZ) and albendazole (ABZ) were addressed here. The goals of the work were as follows: (i) to evaluate the ex vivo activities of FLBZ, ABZ, and their respective metabolites against Echinococcus granulosus protoscoleces, (ii) to compare the plasma and cyst disposition kinetics for the two drugs in infected mice, and (iii) to compare the clinical efficacies of FLBZ and ABZ against CE in mice. For the ex vivo study, E. granulosus protoscoleces were incubated with FLBZ, reduced FLBZ (R-FLBZ), ABZ, and ABZ-sulfoxide (ABZSO) (10 nmol/ml). Protoscolex viability was monitored by the methylene blue exclusion test and scanning electron microscopy (SEM). For the pharmacokinetic study, BALB/c mice with CE were allocated to two different groups and orally treated with either FLBZ or ABZ (5 mg/kg of body weight), both formulated as a cyclodextrin-based solution. Blood and cyst samples were taken up to 12 h posttreatment and analyzed by high-performance liquid chromatography (HPLC). For the efficacy study, CE-infected BALB/c mice were divided into three groups: the unmedicated control group and the FLBZ- and ABZ-treated groups. Oral treatments were performed twice a day during 25 days. After treatment, all animals were killed and the weight of the cysts was recorded. Loss of protoscolex viability was observed after drug incubation. FLBZ was detected in plasma (area under the concentration-versus-time curve [AUC] = 1.8 μg·h/ml) and cysts (AUC = 0.3 μg·h/g) collected from treated infected animals. Conversely, ABZSO was the only active molecule measured in plasma (AUC = 4.4 μg·h/ml) and cysts (AUC = 1.5 μg·h/g) after ABZ treatment. FLBZ induced a 90% reduction in cyst weight in comparison to those collected from untreated control mice (P < 0.05). However, no differences in cyst weight were observed between the ABZ-treated (8.2 g) and unmedicated control (10.5 g) groups. Due to these results, we consider flubendazole to have great potential to become a drug of choice in the treatment of cystic echinococcosis.
INTRODUCTION
Cystic echinococcosis (CE) disease in humans occurs as a result of infection by the larval stages of taeniid cestodes of the genus Echinococcus, named Echinococcus granulosus. The life cycle of E. granulosus involves dogs and other canids as definitive hosts for the intestinal tapeworm, as well as domestic and wild ungulates as intermediate hosts for the tissue-invading metacestode (larval) stage. The metacestode (hydatid cyst) is a fluid-filled, spherical, unilocular cyst that consists of an inner germinal layer of cells supported by a laminated membrane (34). Each cyst is surrounded by a host-produced layer of granulomatous adventitial reaction. Small vesicles, called brood capsules, bud internally from the germinal layer and produce multiple protoscoleces (PSC) by asexual division. In humans, the slowly growing hydatid cysts can attain a volume of several liters and contain many thousands of PSC. The signs and symptoms depend on the location and size of hydatid cysts (33).
Currently, treatment of the disease involves surgical removal of either the entire cyst or its contents by puncture and aspiration (PAIR) (14), as well as chemotherapy based on the use of benzimidazole (BZ) methylcarbamate compounds (30). As a chemical class, the BZ methylcarbamates have only limited water solubility, which allows their preparation only as tablets for oral administration in humans. Small differences in drug solubility may have a major influence on their absorption and resultant pharmacokinetic behavior (21). It has been previously reported that the use of complexing agents, such as hydroxypropyl-β-cyclodextrins (CDs), increases both the water solubility of the BZ methylcarbamates flubendazole (FLBZ) and albendazole (ABZ) (6) and their systemic drug exposure in different species (8, 13). ABZ formulated as either a suspension or capsules is the drug most frequently used to treat CE in humans. Although beneficial results (a positive therapeutic response in ∼75% of cases) have been reported in different studies (10, 32), more often the success rate is somewhat lower than expected. Furthermore, the prolonged treatment period required for CE therapy is frequently associated with side effects (17). FLBZ, an alternative BZ methylcarbamate compound, failed to affect E. granulosus cysts in humans after its oral administration as a suspension or tablets (9, 29). However, the improved kinetic behavior of FLBZ administered as a CD-based solution resulted in enhanced FLBZ clinical efficacy against CE developed in mice (8), showing that the increased FLBZ bioavailability induced by CDs would impact the amount of drug reaching the hydatid cysts and its overall antiparasitic effect.
Anthelmintic efficacy after a drug treatment may depend not only on the activity of the parent drug but also on the activity of the active metabolite/s. In the case of ABZ, two metabolites are found in the systemic circulation after its administration to different animal species, ABZ-sulfoxide (ABZSO) and ABZ-sulfone (ABZSO2) (4, 23, 28, 31). While ABZSO is an active metabolite, the sulfone derivative does not exert anthelmintic action. The main metabolic pathways for FLBZ include reduction to form reduced FLBZ (R-FLBZ) and hydrolysis of the methylcarbamate group to form the hydrolyzed-FLBZ metabolite (H-FLBZ). R-FLBZ was the main analyte recovered from the bloodstream of sheep (25) and mice (8) treated with FLBZ, in which only trace amounts of H-FLBZ were detected. Likewise, while H-FLBZ is an inactive metabolite, some biological activity has been described for R-FLBZ (1). In order to gain further insight into the activities of drugs used against CE, the potential contribution of the parent compound and its active metabolites to the final anthelmintic effect need to be considered.
The ability of drugs to reach high and sustained concentrations at the site of parasites in the body depends on their pharmacokinetic and metabolic patterns in the host. Thus, the characterization of the pharmacokinetic and/or pharmacodynamic behavior of drugs/metabolites has been relevant in achieving optimized drug treatments for different parasitic infections (24). In the search for improved therapy for CE, the goals of the current work were as follows: (i) to compare the ex vivo activities of FLBZ, ABZ, and their respective metabolites against E. granulosus PSC, (ii) to characterize the plasma drug exposure and cyst concentration profiles for FLBZ and ABZ in mice infected with E. granulosus, and (iii) to compare the clinical efficacies of FLBZ and ABZ orally administered as a CD solution to mice infected with E. granulosus.
MATERIALS AND METHODS
Chemicals.
Janssen Animal Health (Beerse, Belgium) kindly provided pure analytical standards of FLBZ and its metabolites, R-FLBZ and H-FLBZ. Reference standards of ABZ, ABZSO, and oxibendazole (OBZ) (used as an internal standard) were purchased from Sigma-Aldrich (Dorset, United Kingdom). The solvents used for the chemical extraction and chromatographic analysis were high-performance liquid chromatography (HPLC) grade (Baker, Inc., Phillipsburg, NJ). The CD was kindly supplied by Cargill Inc. (Hammond, IN).
FLBZ and ABZ solutions and formulations.
The FLBZ, R-FLBZ, ABZ, and ABZSO solutions used for ex vivo studies were prepared by dissolution of 10 mg of pure standard of each drug in 100 ml of methanol. The FLBZ and ABZ formulations used in the efficacy and pharmacokinetic studies were prepared by dissolution of 50 mg of pure FLBZ or ABZ and 10 g of CD in 100 ml of deionized water. The pH was adjusted to 1.2 using hydrochloric acid (25 mM). The formulations were shaken during a 48-h period (40°C) and then were filtrated through a 0.2-μm filter (Whatman, Clifton, NJ). The final FLBZ/ABZ concentration was confirmed by HPLC.
Protoscolex collection.
PSC of E. granulosus were collected aseptically from hydatid cysts obtained from livers of infected cattle slaughtered in an abattoir located in the southeast of Buenos Aires province, Argentina. Vitality was assessed by muscular movements (evaluated under a light microscope), motility of flame cells, and methylene blue exclusion test (5).
Experimental design. (i) Ex vivo study.
The culture protocols were carried out as previously described (11) using 199 medium (Gibco, Invitrogen, Buenos Aires, Argentina) supplemented with 100 IU penicillin, 100 μg/ml streptomycin, and 4 mg/ml glucose. Cultures were performed (in triplicate) in 10 ml of incubation medium at 37°C without changes of medium (12). Viable and free PSC (1,500 per Leighton tube) were incubated with 10 nmol/ml of FLBZ, R-FLBZ, ABZ, or ABZSO. PSC incubated with culture medium alone or culture medium supplemented with methanol were used as controls. Culture tubes were followed microscopically every day. Samples of PSC (approximately 70 to 90 PSC in 180 μl of incubation medium) from both control and treated groups were taken every 6 days for up to 54 days. Vitality of the PSC was monitored using the methylene blue exclusion technique. Additionally, ultrastructure studies with scanning electron microscopy (SEM) were performed. Samples were fixed with 2.5% glutaraldehyde in sodium cacodylate buffer for 48 h at 4°C. Then, several washes in cacodylate buffer were made. The specimens were dehydrated by sequential incubations in increasing concentrations of ethanol and were finally immersed in hexamethyl-disilazane for 5 min, 1 h, and then overnight. They were then sputter coated with gold (100 Å thick) and inspected on a Jeol JSM-6460 LV (Peabody, MA) scanning electron microscope operating at 15 kV.
(ii) In vivo studies.
BALB/c mice (4 months old at the start of the experiments) were used. The animals were housed in a temperature-controlled (21 ± 2°C), light-cycled (12-h light/dark cycle) room. Food and water were provided ad libitum. Animal procedures and management protocols were approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02) of the Faculty of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Tandil, Argentina (http://www.vet.unicen.edu.ar). The animals were used for the following studies.
(a) Pharmacokinetic study.
BALB/c mice (n = 88) were infected by intraperitoneal (i.p.) inoculation of 1,500 PSC/animal, suspended in 0.5 ml of medium 199. Six months postinfection, the animals were allocated to two experimental groups (44 animals/group): the FLBZ and ABZ groups received a single dose of either FLBZ- or ABZ-CD solution, respectively. Both formulations were given orally at the same dose rate (5 mg/kg of body weight), using an intragastric tube. Blood and cyst samples were obtained from sacrificed animals (n = 4 per collection point) at the following times posttreatment: 5, 15, and 30 min and 1, 2, 3, 4, 6, 8, 10, and 12 h. Blood samples were centrifuged at 2,000 × g for 15 min, and the recovered plasma was stored at −20°C until analysis by HPLC. Cyst samples were washed several times with physiologic solution and stored at −20°C until analysis by HPLC.
HPLC and pharmacokinetic analysis of the concentration data were as follows. Plasma (100-μl) and cyst (0.5-g) samples were spiked with OBZ as an internal standard (1 μg/ml). After 5 min, plasma and cyst samples were supplemented with 1.5 ml of acetonitrile, HPLC grade. Later samples were shaken for 15 min in a multitube vortexer (VWR Scientific Products, West Chester, PA) and centrifuged at 3,800 × g for 10 min. The supernatant was concentrated to dryness in a vacuum concentrator (Speed-Vac; Savant) and then reconstituted with 150 μl of mobile phase. Finally, 50 μl of this solution was injected into the chromatographic system.
Chromatography was performed on Shimadzu HPLC equipment (Shimadzu Corporation, Kyoto, Japan) with a UV visible spectrophotometric detector (SPD-10A) reading at 292 nm and a C18 reversed-phase column (5 μm, 250 mm by 4.6 mm; Kromasil, Sweden) used for analysis of FLBZ, ABZ, and its metabolites. The calibration curves for each analyte, constructed by least-squares linear regression analysis, showed good linearity, with correlation coefficients of ≥0.994. Mean absolute recovery percentages for concentrations ranging between 0.01 and 4 μg/ml (n = 5) were 90.2 (H-FLBZ), 92.1 (R-FLBZ), and 90.2% (FLBZ), with coefficients of variation (CV) of 10.7, 7.4, and 10.6%, respectively, and 81.1 (ABZSO), 76.4 (ABZSO2), and 92.4% (ABZ), with CV of 5.2, 4.3, and 6.4%, respectively. The limit of quantification (LOQ) was defined as the lowest measured concentration with a CV of ≤20%, accuracy of ±20%, and an absolute recovery of ≥70%. The LOQ for all assayed analytes were 0.01 μg/ml.
The peak concentration (Cmax) and time to peak concentration (Tmax) were read from the plotted concentration-time curves of each analyte. The plasma and cyst area under the concentration time-curve (AUC) were calculated by the trapezoidal rule (15), using the PK Solutions computer program (Summit Research Services, Ashland, OR).
(b) Efficacy study.
BALB/c mice (n = 30; body weight, 25 ± 5 g) were infected with PSC of E. granulosus as described above (see “Pharmacokinetic study”). At 9 months postinfection, the animals were allocated into the following experimental groups (10 animals/group): unmedicated control group, animals receiving CD (10%) as a placebo; FLBZ group, animals treated with the FLBZ-CD solution; and ABZ group, animals treated with the ABZ-CD solution. All treatments were performed by intragastric inoculation (0.3 ml/animal) every 12 h for 25 consecutive days. The FLBZ and ABZ dose rate was 5 mg/kg. At the end of the treatment period, the animals were sacrificed, and necropsy was carried out immediately thereafter. The cysts present were removed from the peritoneal cavity. The weight of the cysts collected from each animal was recorded.
Statistical analysis.
The results obtained in the ex vivo studies are presented as vitality (percent, arithmetic means ± standard deviations [SD]). The observed differences were compared by analysis of variance (ANOVA). Tukey's range test was used to indicate the order of significance when a significant F value was obtained. The pharmacokinetic parameters are presented as arithmetic means ± SD. A nonparametric test (Mann-Whitney) was used for the statistical comparison of the pharmacokinetic data obtained from the two experimental groups. For the clinical efficacy study, cyst weights (arithmetic mean ± SD) were compared by ANOVA. Tukey's range test was used to indicate the order of significance when a significant F value was obtained. In all cases, a P value of <0.05 was considered statistically significant. The statistical analysis was performed using the Instat 3.0 software program (GraphPad Software, San Diego, CA).
RESULTS
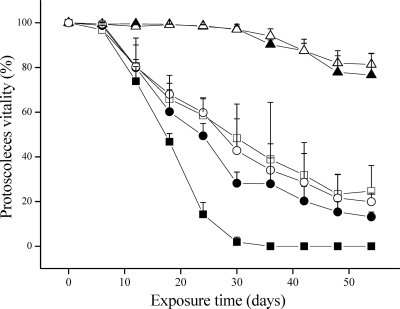
Figure 1 shows the survival of E. granulosus PSC exposed to FLBZ, R-FLBZ, ABZ, or ABZSO (10 nmol/ml). Control PSC incubated in the absence of drug were not altered and remained viable (94.1% ± 1.8%) after 36 days of incubation. In contrast, a loss of PSC viability in FLBZ-treated cultures was observed after 6 days, with a 35.4% ± 0.7% reduction in the number of viable parasites. The number of dead PSC increased with the drug exposure time, and the viability decreased to 54.8% after 18 days of culture. The maximal protoscolicidal effect of FLBZ was observed after 25 days of incubation, when the percentage of vital PSC was only 17.3%. From day 30 onward, viable parasites could no longer be observed in cultures treated with 10 nmol/ml of FLBZ. The treatment with either ABZ or ABZSO also showed a protoscolicidal effect, which was reached later than that observed for FLBZ, with 61% (ABZ) and 67.4% (ABZSO) of parasites remaining viable in culture after 18 days, and the percentage of vital PSC decreased to 29.1% (ABZ) and 52.2% (ABZSO) at 30 days of culture. Likewise, the PSC incubation with R-FLBZ was associated with a loss of viability similar to that observed for ABZSO. In this case, the viability diminished from 68.4% at 18 days of incubation to 65.9% (24 days) and 58.5% (30 days) (Fig. 1).
Fig. 1.
Survival (mean ± SD) of E. granulosus protoscoleces after exposure to either culture medium (alone or supplemented with methanol; control group), flubendazole (FLBZ), reduced flubendazole (R-FLBZ), albendazole (ABZ), or albendazole sulfoxide (ABZSO) at the same concentrations (10 nmol/ml) over 54 days. ▴, control medium; ▵, control medium + methanol; ▪, FLBZ; □, R-FLBZ; •, ABZ; ○, ABZSO.
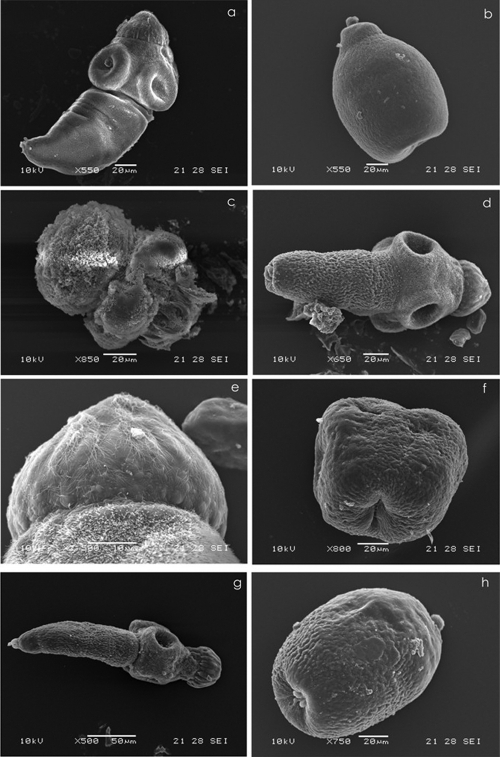
The primary site of drug damage was the parasite tegument, where the presence of numerous blebs could be observed using an inverted microscope (data not shown). SEM analysis demonstrated the drug-induced morphological and structural damage in FLBZ-, R-FLBZ-, ABZ-, and ABZSO-treated PSC (Fig. 2). Hence, the results of vitality tests coincide with the tissue damage observed at the ultrastructural level by SEM. However, the damage was faster and appeared to be broader after FLBZ treatment, where a complete loss of morphology was observed at 25 days postinfection (p.i.). At this time, all PSC showed rostellar disorganization and complete shedding of microtriches. The internal tissue was severely affected, resulting in the loss of its integrity and an increase in the number of lipid droplets (Fig. 2).
Fig. 2.
Representative images of the scanning electron microscopy (SEM) of protoscoleces cultured in vitro for 6 days in the presence of medium containing methanol (10 μl) (control group) or FLBZ, R-FLBZ, ABZ, or ABZSO. (a) Evaginated control protoscolex. (b) Invaginated control protoscolex. (c) Evaginated protoscolex cultured with FLBZ. Note the extensive drug-induced damage with contraction in the soma region, the tegument being markedly altered and also shedding microtriches, and disorder on the rostellum. (d, e, and f) Evaginated protoscolex cultured with R-FLBZ. The altered tegument of the soma region and loss of microtriches on the rostellum can be observed. Details of the rostellum from protoscolex are shown in panel d. and invaginated protoscolex with an extensive damage affecting the tegument. (g) Evaginated altered protoscolex in the presence of ABZ. The shedding of microtriches can be observed in the scolex region either on suckers or on the rostellum. (h) Invaginated protoscolex, clearly altered after culture in the presence of ABZSO.
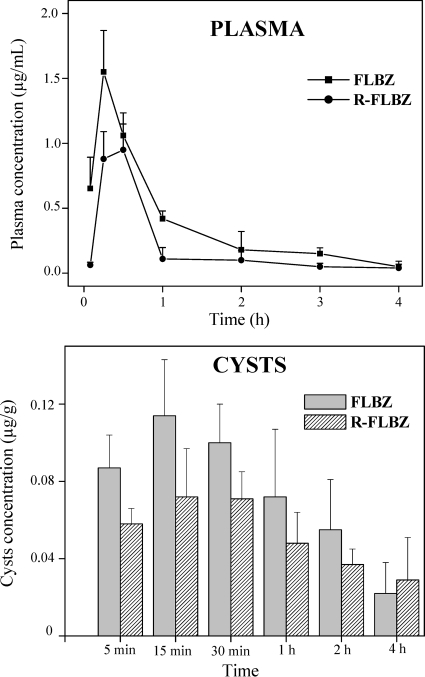
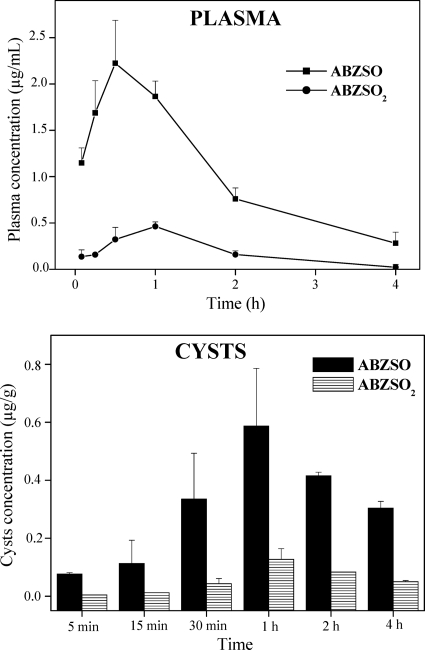
FLBZ and R-FLBZ were the main analytes detected in plasma and cysts obtained after the oral administration of FLBZ to infected mice. The concentration profiles (mean ± SD) of FLBZ and R-FLBZ either in plasma or within recovered cysts are shown in Fig. 3. FLBZ achieved the peak plasma concentration at 30 min after its oral administration, being detected up to 6 h posttreatment (Fig. 3, shown only up to 4 h). Cmax values of 1.56 ± 0.3 and 1.02 ± 0.1 μg/ml were observed for FLBZ and R-FLBZ, respectively. Table 1 summarizes the main pharmacokinetic parameters obtained for FLBZ and R-FLBZ after the oral administration of FLBZ to mice. There were no significant differences (P > 0.05) between the concentrations of FLBZ and R-FLBZ measured in cyst samples (Table 1). Both the cyst concentrations of FLBZ and R-FLBZ were 10-fold lower than those observed in plasma. The main pharmacokinetic parameters obtained for ABZ metabolites in plasma and cysts of infected mice are shown in Table 2. The parent drug was not detected at any time posttreatment, either in plasma or in cysts. ABZSO and ABZSO2 were the main metabolites recovered in plasma. Similarly, both metabolites were measured in cysts recovered from ABZ-treated mice. Higher peak plasma concentrations (Cmax) were observed for ABZSO (2.60 ± 0.5 μg/ml) than for ABZSO2 (0.40 ± 0.1 μg/ml). A similar pattern was observed in cysts, where ABZSO concentrations were 5-fold higher than those observed for ABZSO2. These results were reflected in a higher AUC value for ABZSO both in plasma (4.40 ± 0.4 μg·h/ml) and in cysts (1.50 ± 0.1 μg·h/g) compared to those obtained for ABZSO2 (0.70 ± 0.2 μg·h/ml and 0.30 ± 0.1 μg·h/g in plasma and cysts, respectively) (Table 2).
Fig. 3.
Plasma and cystic concentration profiles (mean ± SD) for FLBZ and R-FLBZ after FLBZ oral administration (5 mg/kg), as a hydroxypropyl-β-cyclodextrin solution, to infected mice.
Table 1.
Pharmacokinetic parameters for FLBZ and its reduced metabolite (R-FLBZ) obtained in plasma and cystsa
| Pharmacokinetic parameter | Value for drugd |
|||
|---|---|---|---|---|
| Plasma |
Cysts |
|||
| FLBZ | R-FLBZ | FLBZ | R-FLBZ | |
| AUC0–t (μg·h/ml[g]b) | 1.80 ± 0.3 A | 1.35 ± 0.3 A | 0.35 ± 0.1 B | 0.28 ± 0.1 B |
| Cmax (μg/ml[g]c) | 1.56 ± 0.3 A | 1.02 ± 0.1 B | 0.12 ± 0.1 C | 0.10 ± 0.0 C |
| Tmax (h) | 0.30 ± 0.0 A | 0.40 ± 0.1 A | 0.10 ± 0.8 A | 0.60 ± 0.3 A |
| Dp | 5 min–6 h | 5 min–6 h | 5 min–6 h | 5 min–6 h |
AUC0–t, area under the concentration-versus-time curve from time zero to t; Cmax, peak plasma concentration; Tmax, time to Cmax; Dp, detection period. Different letters next to values indicate statistical differences in the pharmacokinetic parameter (P < 0.05).
Plasma AUC is expressed as μg·h/ml and cyst AUC as μg·h/g.
Plasma Cmax is expressed as μg/ml and cyst Cmax as μg/g.
Values are means ± SD for drug parameters after oral administration of FLBZ to mice (5 mg/kg).
Table 2.
Pharmacokinetic parameters for ABZSO and ABZSO2 obtained in plasma and cystsa
| Pharmacokinetic parameter | Value for drug |
|||
|---|---|---|---|---|
| Plasma |
Cysts |
|||
| ABZSO | ABZSO2 | ABZSO | ABZSO2 | |
| AUC0–t (μg·h/ml[g]b) | 4.40 ± 0.4 A | 0.70 ± 0.2 A | 1.50 ± 0.1 B | 0.30 ± 0.1 C |
| Cmax (μg/ml[g]c) | 2.60 ± 0.5 A | 0.40 ± 0.1 B | 0.50 ± 0.1 B | 0.10 ± 0.0 C |
| Tmax (h) | 0.50 ± 0.1 A | 0.50 ± 0.1 A | 1.00 ± 0.0 A | 1.00 ± 0.0 A |
| Dp | 5 min–6 h | 5 min–4 h | 5 min–6 h | 5 min–4 h |
See footnote a of Table 1 for abbreviations. Different letters next to values indicate statistical differences in the pharmacokinetic parameter (P < 0.05). Values are means ± SD for drug parameters after oral administration of ABZ to mice (5 mg/kg).
Plasma AUC is expressed as μg·h/ml and cyst AUC as μg·h/g.
Plasma Cmax is expressed in μg/ml and cyst Cmax in μg/g.
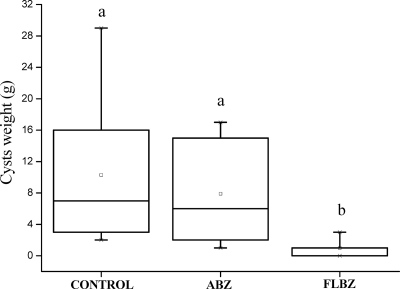
All infected animals involved in the efficacy study developed cysts in their abdominal cavity. Figure 4 shows the weight (mean ± SD) of the cysts recovered from unmedicated control and treated mice. There were no statistical differences (P > 0.05) between cyst weights recovered from the unmedicated group (10.5 ± 8.7 g) and those from the ABZ-treated group (8.23 ± 6.6 g). Conversely, after oral administration of FLBZ to mice, the cyst weights (0.98 ± 0.8 g) were significantly lower (P < 0.05) than those obtained in both the unmedicated and ABZ-treated groups.
Fig. 4.
Box plot representation showing the comparative distribution of the weight (g) of hydatid cysts recovered from either unmedicated or FLBZ- or ABZ-treated mice. Both treatments were given at 5 mg/kg every 12 h over 25 days. A significant cyst weight reduction (P < 0.05) was achieved in FLBZ-treated animals compared to results for the control group.
DISCUSSION
The pharmacological activity mediating BZ action is based on binding to parasite β-tubulin, which produces a subsequent disruption of the tubulin-microtubule dynamic equilibrium (18). While the sulfides (ABZ and fenbendazole) have a greater affinity for β-tubulin, their oxidized metabolites (ABZSO and oxfendazole, respectively) bind to a lesser extent. As a consequence, the parent compounds have a higher anthelmintic potency than the sulfoxide metabolites, as was previously demonstrated in different in vitro (19, 22) and ex vivo (27) studies. The anthelmintic potency of FLBZ appears to be similar to that described for ABZ or fenbendazole (20, 27). No data are available on the anthelmintic activity of R-FLBZ, which is the main FLBZ metabolite found in plasma of sheep (25) and mice (8), with the exception of an observed effect on Fasciola hepatica egg hatching for that metabolite (1). In the current experiment, all compounds involved in the ex vivo study (FLBZ, R-FLBZ, ABZ, and ABZSO) decreased PSC vitality. Nevertheless, the PSC cultured in the presence of FLBZ were killed more effectively and faster than those incubated with ABZ. While PSC vitality was reduced 70 or 50% after 30 days of incubation by ABZ or ABZSO, respectively, FLBZ killed 99% of PSC in the same time period. The faster activity of FLBZ has been previously demonstrated by Elissondo et al. (12), who obtained a similar outcome after culture of PSC with different concentrations of FLBZ. Furthermore, the current study describes for the first time the protoscolicidal effect of R-FLBZ, with results similar to those observed for ABZSO.
In agreement with kinetic data previously obtained with mice (8), a high FLBZ plasma concentration was observed after its administration as a CD solution. Furthermore, current observations show similar behavior after ABZ administration as a CD-based solution in mice compared to that obtained after the administration of ABZ as a conventional suspension (7). CDs increased FLBZ and ABZ water solubility (6), which accounted for enhanced absorption and bioavailability in mice compared with results for the conventional suspension formulation (8). After oral administration of FLBZ to mice, FLBZ and R-FLBZ were the main analytes recovered in plasma. The plasma AUC FLBZ/R-FLBZ ratio was 1.33 as a result of the predominance of FLBZ over R-FLBZ in mouse plasma after the administration of FLBZ. This plasma profile is different from that found in sheep, where R-FLBZ was the main metabolite found in the bloodstream (25). In the current work, as previously reported for other species (23, 28, 4, 31) following ABZ administration, ABZSO and ABZSO2 were the only analytes recovered in plasma, with ABZSO being the main metabolite measured up to 4 h (Fig. 5) and accounting for 86% of the total analytes measured in plasma.
Fig. 5.
Plasma and cystic concentration profiles (mean ± SD) for ABZSO and ABZSO2 after oral administration of ABZ (5 mg/kg) as a hydroxypropyl-β-cyclodextrin solution to infected mice.
The higher the concentration profiles achieved at the tissue/fluid of the parasite location, the greater the amount of drug reaching the target parasite (2). Consequently, the enhancement in plasma drug exposure induced by formulation in CD impacts on the amount of drug reaching the hydatid cysts and its overall antiparasitic effect. The FLBZ/R-FLBZ pattern found in cysts was similar to that observed in plasma, resulting in a cyst AUC ratio of 1.25. However, concentrations in cysts represented only 19 (FLBZ) and 21% (R-FLBZ) of those measured in plasma. ABZ metabolite accumulation in cysts appears to be higher, representing 34 (ABZSO) and 43% (ABZSO2) of the plasma concentrations.
Even when animals were treated at the same dose (5 mg/kg) of both FLBZ and ABZ, a higher concentration of ABZ metabolites was observed in cysts than that measured for FLBZ/R-FLBZ. The greater ABZSO concentrations in cysts could be explained by its higher concentrations in the bloodstream. Furthermore, since in the murine model hydatid cysts develop “free” in the whole abdominal cavity, the drug can reach the cyst only from the peritoneal fluid. The greater water solubility of ABZSO than that reported for FLBZ or R-FLBZ (26) could determine its high concentration in the peritoneal fluid and consequently the greater amount of drug reaching the target parasite. In fact, ABZSO has been detected in high concentrations in organic aqueous fluids as intestinal fluids (3) and urine (16).
After a 25-day treatment period, a clear reduction in cyst weight was observed following the administration of the FLBZ solution compared to results for the unmedicated control mice (Fig. 4). The mean cyst weight was reduced by 90% in the FLBZ-treated mice, which agrees with previously reported results (8). On the contrary, under our experimental conditions, ABZ treatment did not result in significant changes in cyst weight compared to that of the unmedicated mice (14% reduction; P > 0.05). Since ABZ has previously demonstrated excellent activity against hydatid cysts developed in the murine model (7, 35, 36), it is likely that the lack of a clinical effect observed in the current work may be related to differences in the infectious material (age of cysts, intermediated host) and in its management at the time of collection. Furthermore, the relatively short treatment period may limit ABZ action. The shorter period of time required by FLBZ to exert its action is consistent with the in vitro results, where FLBZ required 24 days of incubation to kill 90% of protoscoleces, while ABZSO killed 40% in the same time period. The only active molecule that reaches the hydatid cyst after ABZ treatment is the ABZSO active metabolite, and accumulation in cysts (AUC) was 4-fold higher than that observed for FLBZ. However, this difference decreases when the sum of FLBZ plus R-FLBZ cyst exposure is considered.
In conclusion, while ABZ efficacy is based on the capacity of its ABZSO metabolite to reach the cyst, after FLBZ administration the ability of both the parent drug and its reduced metabolite to accumulate in the hydatid cyst may account for its improved in vivo activity. The low FLBZ/R-FLBZ concentration in cysts may be compensated by the higher anthelmintic potency of the parent drug. The current results clearly show that if systemic availability of FLBZ is improved by different drug formulation strategies, FLBZ may be a highly useful alternative for CE treatment in humans. The outcome for the drug reported here contributes greatly with comparative data on the pharmacological performances of two BZ compounds for use in treatment of CE.
ACKNOWLEDGMENTS
This work was partially supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Técnica (ANPCyT) (both from Argentina).
We acknowledge Kathleen Vlaminck, Leo Van Leemput (Janssen Animal Health, Beerse, Belgium), and Gustavo Viana (Janssen, Buenos Aires, Argentina) for providing FLBZ used in the present experimental work. The CD was kindly supplied by Cargill Inc. (Hammond, IN).
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Alvarez L., et al. 2009. Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet. Parasitol. 164:211–216 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez L., Mottier L., Lanusse C. 2007. Drug transfer into target helminth parasites. Trends Parasitol. 23:97–104 [DOI] [PubMed] [Google Scholar]
- 3. Alvarez L., Sánchez S., Lanusse C. 1999. In vivo and ex vivo uptake of albendazole and its sulphoxide metabolite by cestode parasites: relationship with their kinetics behaviour in sheep. J. Vet. Pharmacol. Ther. 22:77–86 [DOI] [PubMed] [Google Scholar]
- 4. Alvarez L., Saumell C., Sánchez S., Lanusse C. 1996. Plasma disposition kinetics of albendazole metabolites in pigs fed different diets. Res. Vet. Sci. 60:152–156 [DOI] [PubMed] [Google Scholar]
- 5. Casado N., Rodríguez-Caabeiro F., Hernandez S. 1986. In vitro survival of Echinococcus granulosus protoscoleces in several media, at 42°C and 37°C. Z. Parasitenkd. 72:273–278 [DOI] [PubMed] [Google Scholar]
- 6. Ceballos L., et al. 2006. Preliminary kinetic data on flubendazole formulated as a hydroxypropyl-β-cyclodextrin aqueous solution for use in sheep. J. Vet. Pharmacol. Ther. 29:85–86 [Google Scholar]
- 7. Ceballos L., et al. 2008. Albendazole treatment in cystic echinococcosis: pharmacokinetic and clinical efficacy of two different aqueous formulations. Parasitol. Res. 103:355–362 [DOI] [PubMed] [Google Scholar]
- 8. Ceballos L., et al. 2009. Flubendazole in cystic echinococcosis therapy: pharmaco-parasitological evaluation in mice. Parasitol. Int. 58:354–358 [DOI] [PubMed] [Google Scholar]
- 9. Davis A., Pawlowski Z. S. H., Dixon H. 1986. Multicenter trials of benzimidazole-carbamates in human echinococcosis. Bull. World Health Organ. 64:383–388 [PMC free article] [PubMed] [Google Scholar]
- 10. Eckert J., Deplazes P. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17:107–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elissondo C., Dopchiz M. C., Brasesco M., Denegri G. 2004. Echinococcus granulosus: first report of microcysts formation from protoscoleces of cattle origin using the in vitro vesicular culture technique. Parasite 11:415–418 [DOI] [PubMed] [Google Scholar]
- 12. Elissondo M., et al. 2006. In vitro effects of flubendazole on Echinococcus granulosus protoscoleces. Parasitol. Res. 98:317–323 [DOI] [PubMed] [Google Scholar]
- 13. Evrard B., et al. 2002. Oral bioavailability in sheep of albendazole from a suspension and from a solution containing hydroxypropyl-β-cyclodextrin. J. Control. Release 85:45–50 [DOI] [PubMed] [Google Scholar]
- 14. Filice C., Brunetti E. 1997. Use of PAIR in human cystic echinococcosis. Acta Trop. 64:95–107 [DOI] [PubMed] [Google Scholar]
- 15. Gibaldi M., Perrier D. 1982. Pharmacokinetics, 2nd ed., revised and expanded Marcel Dekker, New York, NY [Google Scholar]
- 16. Gyurik R., et al. 1981. Metabolism of albendazole in cattle, sheep, rat, and mice. Drug Metab. Dispos. 9:503–508 [PubMed] [Google Scholar]
- 17. Kern P. 2006. Medical treatment of echinococcosis under the guidance of Good Clinical Practice (GCP/ICH). Parasitol. Int. 55(suppl.):S273–S282 [DOI] [PubMed] [Google Scholar]
- 18. Lacey E. 1988. The role of the cytoskeletal protein tubulin in the mode of action and mechanism of drug resistance to benzimidazole. Int. J. Parasitol. 18:885–936 [DOI] [PubMed] [Google Scholar]
- 19. Lacey E. 1990. Mode of action of benzimidazoles. Parasitol. Today 6:112–115 [DOI] [PubMed] [Google Scholar]
- 20. Lacey E., Brady R., Prichard R. K., Watson T. 1987. Comparison of inhibition of polymerisation of mammalian tubulin and helminth ovicidal activity by benzimidazole carbamates. Vet. Parasitol. 23:105–119 [DOI] [PubMed] [Google Scholar]
- 21. Lanusse C., Gascon L., Prichard R. K. 1995. Comparative plasma disposition kinetics of albendazole, fenbendazole, oxfendazole and their metabolites in adult sheep. J. Vet. Pharmacol. Ther. 18:196–203 [DOI] [PubMed] [Google Scholar]
- 22. Lubega G., Prichard R. K. 1991. Interaction of benzimidazole anthelmintics with Haemonchus contortus tubulin: binding affinity and anthelmintic efficacy. Exp. Parasitol. 73:203–209 [DOI] [PubMed] [Google Scholar]
- 23. Marriner S., Bogan J. 1980. Pharmacokinetic of albendazole in sheep. Am. J. Vet. Res. 41:1126–1129 [PubMed] [Google Scholar]
- 24. McKellar Q. A., Sanchez Bruni S. F., Jones D. G. 2004. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 27:503–514 [DOI] [PubMed] [Google Scholar]
- 25. Moreno L., et al. 2004. Integrated pharmacological assessment of flubendazole potential for use in sheep: disposition kinetics, liver metabolism and parasite diffusion ability. J. Vet. Pharmacol. Ther. 27:299–308 [DOI] [PubMed] [Google Scholar]
- 26. Mottier L., et al. 2003. Transtegumental diffusion of benzimidazole anthelmintics into Moniezia benedeni: correlation with their octanol-water partition coefficients. Exp. Parasitol. 103:1–7 [DOI] [PubMed] [Google Scholar]
- 27. Petersen M., Friis C., Bjørn H. 1997. A new in vitro assay of benzimidazole activity against adult Oesophagostomum dentatum. Int. J. Parasitol. 27:1333–1339 [DOI] [PubMed] [Google Scholar]
- 28. Prichard R. K., Hennessy D., Steel J., Lacey E. 1985. Metabolite concentrations in plasma following treatment of cattle with five anthelmintics. Res. Vet. Sci. 39:173–178 [PubMed] [Google Scholar]
- 29. Recco P., et al. 1984. Hydatidose pleurale disseminee et hydatidoses hepatiques. Traitment post-operatoire par flubendazole a proposde 3 cas. Bull. Soc. Fr. Parasitol. 3:115–118 [Google Scholar]
- 30. Rogan M., Hai W., Richardson R., Zeyhle E., Craig P. 2006. Hydatid cysts: does every picture tell a story? Trends Parasitol. 22:431–438 [DOI] [PubMed] [Google Scholar]
- 31. Sánchez S., Sallovitz J., Savio E., McKellar Q. A., Lanusse C. 2000. Comparative plasma bioavailability of two dosage forms of albendazole in dogs. Vet. J. 160:150–153 [DOI] [PubMed] [Google Scholar]
- 32. Smego R. A., Sebanego P. 2005. Treatment options for hepatic cystic echinococcosis. Int. J. Infect. Dis. 9:69–76 [DOI] [PubMed] [Google Scholar]
- 33. Stamatakos M., et al. 2009. Anthelmintic treatment: an adjuvant therapeutic strategy against Echinococcus granulosus. Parasitol. Int. 58:115–120 [DOI] [PubMed] [Google Scholar]
- 34. Thompson R. C. 1995. Biology and systematics of Echinococcus, p. 1–37 In Thompson R. C., Lymbery A. J. (ed.), Echinococcus and hydatid disease. CAB International, London, United Kingdom [Google Scholar]
- 35. Xiao S. H., et al. 2002. Augmented bioavailability and cysticidal activity of albendazole reformulated in soybean emulsion in mice infected with Echinococcus granulosus or Echinococcus multilocularis. Acta Trop. 82:77–84 [DOI] [PubMed] [Google Scholar]
- 36. Xiao S. H., et al. 1994. Effects of benzimidazole compounds on mice infected with secondary cysts of Echinococcus granulosus. Chin. Med. J. 107:521–532 [PubMed] [Google Scholar]