Abstract
To understand the high prevalence of fusB genes in fusidic acid-resistant Staphylococcus epidermidis, analysis of resistance elements in 34 isolates was performed. First, sequence analysis of the aj1-LP-fusB region indicated that at least three types were present. Type I contained full-length aj1, type II contained a partial aj1 truncated from nucleotide position 93 to 421, and type III contained a more truncated aj1 that retained only the last 37 bp. Isolates with type I or type II aj1 displayed slightly higher levels of resistance to fusidic acid (MICs, 8 to 32 μg/ml) than did those with type III aj1 (MICs, 4 to 16 μg/ml). Subsequent sequencing of the flanking regions of fusB from four selected isolates carrying different types of aj1-LP-fusB regions revealed that the fusB genes were all located on phage-related resistance islands (RIs), referred to as SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, and SeRIfusB-7778, respectively. Among them, three islands (SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907) were located downstream of groEL (corresponding to the 44-min position based on Staphylococcus aureus whole genomic sequences), and one (SeRIfusB-7778) was located downstream of rpsR (corresponding to the 8-min position). All of the RIs were inserted into integrase-recognized att sites. Among 34 isolates, the insertion sites of fusB RIs were mostly (28/34, 82%) located downstream of groEL and two were located downstream of rpsR, but four remained unidentified. The pulsotype distribution indicated that fusB-containing S. epidermidis isolates were heterogeneous. In conclusion, the fusB resistance determinant in S. epidermidis was highly associated with phage-related RIs. This is the first report of fusB RI in S. epidermidis.
INTRODUCTION
Staphylococcus epidermidis, a commensal organism, may cause infection in patients whose immune systems are compromised or who have catheters (32). This bacterium is considered a reservoir of antibiotic resistance genes (1). Fusidic acid, the product of Fusidium coccineum, is a steroid antibiotic that is used to treat skin and systemic staphylococcal infections (7). Fusidic acid is usually used as an agent of combination therapy for treating deep-seated infections such as osteomyelitis, septic arthritis, and infective endocarditis caused by staphylococci in our hospital. The combination agents are usually vancomycin, teicoplanin, and linezolid. If the staphylococci are resistant to fusidic acid, the combination agent may be changed to rifampin or fluoroquinolones. Fusidic acid inhibits bacterial protein synthesis by interacting with elongation factor G (EF-G) to sterically influence the release of the EF-G/GDP complex from the ribosome (3, 11, 14). Two major fusidic acid resistance mechanisms have been reported: one is the alternation of the drug target site (fusA and fusE) (2, 17, 18) and the other is the protection of the drug target site by FusB family proteins (fusB, fusC, and fusD) (22, 24).
Acquired fusidic acid resistance genes found in Staphylococcus spp. include fusB, fusC, and fusD. The genes fusB and fusC were found in Staphylococcus aureus and coagulase-negative staphylococci (15, 22, 24, 33), and fusD is an intrinsic factor causing fusidic acid resistance in Staphylococcus saprophyticus (24). The fusB determinant, encoding a 25-kDa protein, was originally found on a transposon-like element of plasmid pUB101 or in a pathogenicity island (PI) in Staphylococcus aureus (21, 23). The FusB protein resulted in resistance to fusidic acid by directly binding to EF-G (the target of fusidic acid) and protecting translation in the presence of fusidic acid in vitro.
A high prevalence of resistance to fusidic acid has been reported for S. epidermidis. Resistance to fusidic acid in S. epidermidis is mostly associated with the fusB determinant (15). The proportion of fusidic acid-resistant S. epidermidis isolates each year ranged from 39% to 46%, higher than that in S. aureus, at the National Taiwan University Hospital (NTUH), Taiwan (4). Our previous study of fusidic acid resistance determinants in S. aureus revealed that most methicillin-susceptible S. aureus isolates carried fusB-containing plasmids. In contrast to the case of S. aureus, our preliminary data indicated that fusB was not located on a plasmid in S. epidermidis. Because fusB is widely distributed in different species, we wanted to determine if there was any correlation between S. aureus and S. epidermidis with respect to fusidic acid resistance. In this study, we identified three types of aj1-leader peptide (LP)-fusB fragments with identical LP and fusB sequences but different sizes of aj1 regions: type I, full-length aj1; type II, truncated aj1 that was translated into a smaller putative protein; and type III, truncated aj1 retaining only the last 37 bp. We further analyzed the genetic structures of fusB resistance islands in four representative S. epidermidis isolates with different aj1-LP-fusB types. Sequence analysis indicated that fusB determinants in the test S. epidermidis isolates were all carried by phage-related resistance islands (RIs). Thus, the fusB-containing RIs were highly associated with the dissemination of fusidic acid resistance in S. epidermidis.
MATERIALS AND METHODS
Bacterial strains.
Thirty-six fusidic acid-resistant S. epidermidis isolates were used from a collection made between January 2003 and January 2007 in the Bacteriology Laboratory of the National Taiwan University Hospital, a 2,500-bed teaching hospital in northern Taiwan. S. epidermidis was initially identified by the Phoenix Automated System and further confirmed by 16S rRNA gene sequencing. The sources of the 36 isolates included blood samples (n = 35) and a central venous catheter (n = 1). Among these isolates, four (NTUH-2793, NTUH-704, NTUH-5907, and NTUH-7778) were used to determine the full-length sequences of the fusB element.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed by standard agar dilution according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (5). Bacterial inocula were prepared by direct colony suspension to a turbidity of a 0.5 McFarland standard. A bacterial density of 104 CFU/spot was inoculated on Mueller-Hinton agar containing various concentrations of fusidic acid (0.03 to 128 μg/ml) using a Steers replicator, and the plates were incubated at 33 to 35°C for 16 to 20 h. S. aureus ATCC 29213 was used as the reference organism. The breakpoint used to indicate fusidic acid resistance was 2 μg/ml (6).
Detection of fusidic acid resistance determinants by PCR.
For detection of fusidic acid resistance determinants, the DNA of the isolates was amplified with primers specific for fusB, fusC, or fusD, as previously described (4). For isolates showing negative results for fusB, fusC, and fusD, sequencing of fusA was also performed (4).
aj1-LP-fusB detection.
To detect the aj1-LP-fusB fragments among the S. epidermidis clinical isolates, DNA was amplified with the primers ri18 250-269F and 7778 LA 410-390R (Table 1). Because this pair of primers could not produce PCR products for two isolates, another pair of primers, aj1 606-577R and fusB 389-361(R) (Table 1), was used. PCRs were carried out with 30 cycles of denaturation (94°C, 30 s), annealing (50°C, 30 s), and extension (72°C, 1.5 min), followed by a final extension step (72°C, 10 min). PCR products were subsequently sequenced.
Table 1.
Primers used in this study
| Description | Primer name | Sequence (5′ to 3′)a | Position | Reference |
|---|---|---|---|---|
| aj1-LP-fusB detection | ri18 250-269F | GTTGCTAAATCTCCTCACGG | ri18 250-269 | This study |
| 7778 LA 410-390R | GGGTAAATCCAGAGTTAATCG | fusB −232 to −252 | ||
| aj1 606-577R | AGTAAAGAATAAGTTTTTAATCGTTAATGC | aj1 606-577 | ||
| fusB 389-361R | TTCCGATTTGATGCAAGTTCATTCCATCC | fusB 389-361 | ||
| LA-PCR for HindIII fragments | fusB 437-465F | GAGAAATTTCTAATCAGGTTGTAAAGGGG | fusB 437-465 | This study |
| fusB 530-558F | CGGATGGTCAATATGTAAAAAAAGGTGAC | fusB 530-558 | ||
| fusB 553-580F | GGTGACTATATATGTCGAGATAGCATTC | fusB 553-580 | ||
| fusB 282-253R | AAGTTTTTGCGGACTAGGTAGTTCAAAAGG | fusB 282-253 | ||
| LA-PCR for PstI fragments | 7778 LA 755-736R | CGATTGAATAACTTTGACGG | fusB 114-95 | This study |
| 7778 upstream detection | S. epi rpsR 6-24F | AGGTGGACCAAGAAGAGGC | rpsR 6-24 | This study |
| 2793 upstream detection | S. epi groEL 1213-1232F | GTKGAAGAAGGTATYGTTGC | groEL 1213-1232 | This study |
| Prediction of insertion sites | int(I) 109-128F | CGTAAATCAGACGCTAAACA | SeRIfusB-2793int 109-128 | This study |
| int(I) 1139-1122R | CTAAACTTGTGGGAAGCG | SeRIfusB-2793int 1139-1122 | ||
| int(II) 541-565F | GCTAAACGTAATAACTATTTAGAAG | SeRIfusB-7778int 541-565 | ||
| int 1061-1042R | GTGTGACGTAATGTGTGCGT | SeRIfusB-7778int 1061-1042 | ||
| S. epi groEL 1582-1599F | GAACAACCTGGAATGGGT | groEL 1582-1599 | ||
| 185 LA 3R | CTCACAGAGGTTCTATAATGTTGG | SeRIfusB-2793 ORF19 419-396 | ||
| fusB LA-2R | AATACTCCTGGATGGCGT | SeRIfusB-7778 ORF19 −216 to −233 |
K = G or T; Y = C or T.
Southern hybridization.
To clone and sequence the fusB and flanking fragment in S. epidermidis, Southern hybridization was used to estimate the fragment sizes after digestion with restriction enzymes and to choose the optimal restriction enzyme. Southern blot analysis was performed with the DNA of NTUH-2793 and NTUH-7778, which were digested with a series of restriction enzymes (BamHI, EcoRI, HindIII, PstI, SalI, and XbaI; New England BioLabs) and detected with a digoxigenin (DIG)-labeled fusB-specific probe prepared by PCR amplification (4). Probe labeling and detection were performed using a commercial kit (Roche Diagnostics GmbH, Penzberg, Germany).
Cloning and sequencing of fusB and flanking regions.
To determine the sequence of the entire fusB gene and its flanking regions, an LA-PCR in vitro cloning kit (Takara Shuzo Co. Ltd., Japan) was used. For S. epidermidis NTUH-2793, the LA-PCR was carried out with the HindIII fragments. After ligating the HindIII-digested DNA fragments with cassette adapters, the amplification was performed with the cassette primers (C1 for the first PCR and C2 for the nested PCR) supplied by the manufacturer and target gene-specific primers (fusB 437-465F paired with C1 and fusB 530-558F paired with C2, Table 1). After determining the downstream sequence of fusB fragments, the insertion site of the island was predicted. The upstream sequence was amplified by the designed primers S. epi groEL 1213-1232F and fusB 282-253R (Table 1). For S. epidermidis NTUH-7778, the LA-PCR was carried out with the HindIII- and PstI-digested DNA fragments hybridized with a fusB-specific probe. After ligating the HindIII- or PstI-digested DNA fragments with cassette adapters, the amplification of the HindIII-digested DNA was performed with four pairs of primers, including C1 and fusB 389-361R, C1 and fusB 282-253R, C2 and fusB 530-558F, and C2 and fusB 553-580F (Table 1). The amplification of PstI-digested DNA fragments was performed by two pairs of primers (C1 and 7778 LA 755-736R, C2 and 7778 LA 410-390R; Table 1). After determining the downstream sequences of the fusB fragments, the insertion site of the island was predicted. The upstream sequence was amplified by the designed primers 7778 LA 410-390R and S. epi rpsR 6-24F (Table 1). The amplified fragments were subsequently sequenced on an Applied Biosystems model 3100 DNA sequencer (Applied Biosystems, Foster City, CA) using the Taq BigDye-Deoxy Terminator cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions. The sequences of two other S. epidermidis isolates, NTUH-5907 and NTUH-704, were determined by PCR amplification based on the sequence of NTUH-2793.
Phylogenetic analysis of integrases.
The phylogenetic relationships among integrases, which are associated with pathogenicity islands found in staphylococci, were analyzed by the neighbor-joining (NJ) method described in the MEGA4 (molecular evolutionary genetic analysis) analytical package (27). For the NJ analysis, the distance between the sequences was calculated using Kimura's two-parameter model. The levels of similarity among species were determined. Bootstrap values were obtained using 500 randomly generated trees.
Prediction of insertion sites in other S. epidermidis isolates.
We designed eight pairs of primers to detect the insertion sites (downstream of groEL or rpsR) in S. epidermidis clinical isolates. The primer pairs int(I) 109-128F/int(I) 1139-1122R and int(II) 541-565F/int 1061-1042R (Table 1) were used to detect the integrase genes recognizing the sites of groEL or rpsR, respectively. The primer pairs S. epi groEL 1582-1599F/int(I) 109-128F and S. epi rpsR 6-24F/int(II) 541-565F were used to amplify the junction of the chromosomal genes and the integrase genes, groEL-integrase and rpsR-integrase, respectively. The junctions of the fusB gene and the downstream chromosomal genes were detected by fusB 530-558F/185 LA 3R and fusB 530-558F/fusB LA-2R. The junctions of two chromosomal genes without the insertion of resistance islands were amplified using S. epi groEL 1582-1599F/185 LA 3R and S. epi rpsR 6-24F/fusB LA-2R. The primers used in this study are listed in Table 1.
PFGE.
The genotypes of the fusidic acid-resistant S. epidermidis isolates were determined by pulsed-field gel electrophoresis (PFGE). Genomic DNA was prepared as described previously (16). The DNA was digested with SmaI (New England BioLabs, Ipswich, MA) and then separated in a CHEF-DRII apparatus (Bio-Rad Laboratories). PFGE was carried out at 200 V and 13°C for 18.5 h, with pulse times ranging from 5 to 60 s. The pulsotypes were analyzed by BioNumerics software version 4.0 (Applied Maths, Sint-Martens-Latem, Belgium). The dendrogram of pulsotype relationships was produced by the unweighted pair group method using arithmetic averages (UPGMA) based on Dice similarity indices.
Nucleotide sequence accession numbers.
The SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, and SeRIfusB-7778 sequences from the S. epidermidis clinical isolates NTUH-2793, NTUH-704, NTUH-5907, and NTUH-7778, respectively, were deposited in GenBank under accession numbers JF777505, JF808725, JF777506, and JF808726, respectively.
RESULTS
Fusidic acid resistance determinants.
FusB family resistance determinants were detected by PCR. Amplification with primers specific for fusB, fusC, and fusD revealed that 33 of the 36 S. epidermidis isolates possessed fusB (92%), one isolate carried fusC, one isolate carried both fusB and fusC, and one isolate carried none of the FusB family resistance determinants but contained a fusA point mutation (V90I). The presence of fusB was also confirmed by Southern hybridization with a fusB-specific probe (data not shown).
Variation of aj1-LP-fusB fragments.
To test the correlation between aj1-LP-fusB structures and the levels of resistance, the aj1-LP-fusB fragments of clinical isolates were amplified and sequenced. The results revealed that at least three different types of aj1-LP-fusB fragments were present. Different sizes of the aj1 regions were detected among the fusB-carrying isolates (Fig. 1). Structures with full-length aj1 were classified as type I. The type II aj1 region had a partial aj1 gene truncated from nucleotide (nt) 93 to 421 that was translated into a putative protein smaller than full-length aj1. The type III fragment possessed a more truncated aj1 gene that retained only the last 37 bp. Among 34 isolates (33 clinical isolates carrying fusB alone and one isolate carrying both fusB and fusC), 4 type I, 11 type II, and 17 type III isolates were identified, but two remained unidentified. The correlation between the different structures of the aj1-LP-fusB fragment and the resistance level is shown in Table 2. Low-level resistance (MIC < 8 μg/ml) was detected mostly in isolates with the type III aj1-LP-fusB structure. Most isolates carrying type I or type II aj1-LP-fusB showed relatively higher levels of resistance (MIC = 16 or 32 μg/ml). One isolate with an unidentified aj1-LP-fusB fragment also had an MIC of 32 μg/ml, possibly due to the presence of both the fusB and fusC determinants and/or unknown mechanisms.
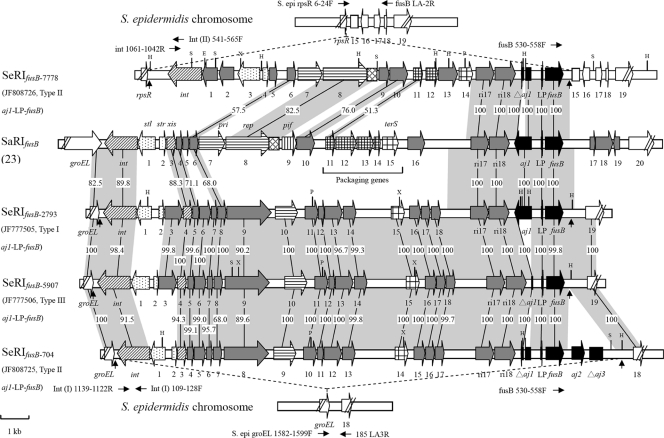
Fig. 1.
Genetic organization of SeRIfusB-2793 (GenBank accession no. JF777505), SeRIfusB-704 (GenBank accession no. JF808725), SeRIfusB-5907 (GenBank accession no. JF777506), and SeRIfusB-7778 (GenBank accession no. JF808726) compared with SaRIfusB (GenBank accession no. AM292600). Genes are drawn according to their sequences and function: int and xis are ; transcription regulators are ; replication genes (including the primase gene [pri] and the replication initiator gene [rep]) are ; the replication origin (ori) is ; of encapsidation genes, the terminase small subunit gene (terS) is and other genes are ; pif (phage interference) is ; aj1-LP-fusB regions are ; chromosome genes adjacent to SeRIfusB are ; other genes encoding hypothetical proteins are . The predicted direct repeats are indicated by vertical arrows. The horizontal arrows represent the PCR primers used to determine the insertion sites. Regions homologous between resistance islands are shown in shadow, and the numbers in shadow show percent homology between the corresponding sequences. The restriction sites are also shown: H, HindIII; P, PstI; X, XbaI; E, EcoRI; S, SpeI.
Table 2.
Distribution of fusidic acid resistance determinants and MICs among fusidic acid-resistant S. epidermidis isolates
| Resistance determinant | aj1-LP-fusB type | No. of bacterial isolates | No. of isolates with different MICs (μg/ml): |
|||
|---|---|---|---|---|---|---|
| 4 | 8 | 16 | 32 | |||
| fusB | I | 4 | 0 | 0 | 2 | 2 |
| II | 11 | 0 | 1 | 7 | 3 | |
| III | 17 | 1 | 5 | 11 | 0 | |
| Ua | 1 | 0 | 1 | 0 | 0 | |
| fusC | 1 | 0 | 0 | 1 | 0 | |
| fusB and fusC | Ua | 1 | 0 | 0 | 0 | 1 |
| fusA point mutation | 1 | 1 | 0 | 0 | 0 | |
U, undetermined aj1-LP-fusB type.
Sequence analysis of fusB and flanking regions.
Based on aj1-LP-fusB types and Southern blot hybridization patterns, we chose four isolates to further analyze the flanking regions of fusB elements. Sequence data revealed that the fusB elements of four representative isolates were all located on phage-related resistance islands (RIs) (Table 3 and Fig. 1). The sizes of the RIs ranged from 15 kb to 17.5 kb, which were shown as follows. The sizes of the RIs in NTUH-2793 (type I aj1-LP-fusB), referred to as SeRIfusB-2793 (S. epidermidis resistance island carrying fusB in NTUH-2793); NTUH-704 (type II aj1-LP-fusB), referred to as SeRIfusB-704; NTUH-5907 (type III aj1-LP-fusB), referred to as SeRIfusB-5907; and NTUH-7778 (type II aj1-LP-fusB), referred to as SeRIfusB-7778, were 16,913 bp, 17,554 bp, 16,695 bp, and 15,307 bp, respectively. SeRIfusB-2793 was composed of 24 putative open reading frames (ORFs), SeRIfusB-5907 had 23 putative ORFs and one truncated gene (aj1), SeRIfusB-704 was made up of 23 putative ORFs and two truncated genes (aj1 and aj3), and SeRIfusB-7778 was composed of 19 putative ORFs and truncated aj1, as shown in Fig. 1. The G+C contents of SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, and SeRIfusB-7778 were 29.9%, 29.3%, 30.2%, and 29.5%, respectively. Three islands, SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907, were flanked by groEL, which encodes a heat shock protein on the left side, and another chromosomal gene encoding a cell wall surface anchor family protein on the right side. SeRIfusB-7778 was flanked by rpsR, which encodes the 30S ribosomal protein S18, and another chromosomal gene encoding a hypothetical protein, followed by three other chromosomal genes encoding two hypothetical proteins and lysE, which encodes an efflux protein. The chromosomal genes flanking the resistance islands were arranged in the same order as in S. epidermidis ATCC 12228 (GenBank accession no. AE015929) and RP62A (GenBank accession no. CP000029). Taken together, the resistance islands containing fusB determinants were inserted into at least two different sites of the chromosome.
Table 3.
Comparison of genetic content of resistance islands carrying fusB
| Annotation or function | ORF no. or gene name of RIa |
Orientation | ||||
|---|---|---|---|---|---|---|
| SeRIfusB-7778 | SaRIfusB | SeRIfusB-2793 | SeRIfusB-5907 | SeRIfusB-704 | ||
| Integrase | int | int | int | int | int | − |
| Hypothetical protein | 1, 2 | Absent | Absent | Absent | Absent | − |
| PI master repressor | 3 | 1 | 1 | 1 | 1 | − |
| Regulatory protein | 4 | 2 | 2 | 2 | 2 | + |
| Prophage antirepressor | Absent | Absent | 3 | 3 | Absent | + |
| Excisionase | Absent | 3 | 4 | 4 | 3 | + |
| Hypothetical protein | Absent | 4 | 5 | 5 | 4 | + |
| Hypothetical protein | Absent | 5 | Absent | Absent | Absent | + |
| Hypothetical protein | 5 | Absent | Absent | Absent | Absent | + |
| SaPIbov ORF-17-like protein | Absent | Absent | 6 | 6 | 5 | + |
| Hypothetical protein | Absent | Absent | 7 | 7 | 6 | + |
| Hypothetical protein | 6 | 6 | 8 | 8 | 7 | + |
| Bacteriophage resistance protein | Absent | Absent | 9 | 9 | 8 | + |
| Similar to DNA primase | 7 | 7 | Absent | Absent | Absent | + |
| Primase-polymerase | Absent | Absent | 10 | 10 | 9 | + |
| Replication initiator | 8 (rep) | 8 (rep) | Absent | Absent | Absent | + |
| Replication origin | ori | ori | Absent | Absent | Absent | |
| Phage interference | Absent | 9 | Absent | Absent | Absent | + |
| Hypothetical protein | 9 | Absent | Absent | Absent | Absent | + |
| Hypothetical protein | 10 | 10 | Absent | Absent | Absent | + |
| Capsid size determinant | 11 | 11 | Absent | Absent | Absent | + |
| Capsid size determinant | 12 | 12–14 | Absent | Absent | Absent | + |
| Hypothetical protein | Absent | Absent | 11–14 | 11–14 | 10–13 | + |
| Hypothetical protein | 13 | Absent | Absent | Absent | Absent | + |
| Bacteriophage terminase small subunit | 14 (terS) | 15 (terS) | 15 (terS) | 15 (terS) | 14 (terS) | + |
| Hypothetical protein | Absent | 16 | Absent | Absent | Absent | + |
| Hypothetical protein | Absent | Absent | 16, 17 | 16, 17 | 15, 16 | + |
| Pathogenicity island protein | Absent | Absent | 18 | 18 | 17 | + |
| Hypothetical protein | ri17 | ri17 | ri17 | ri17 | ri17 | + |
| Hypothetical protein | ri18 | ri18 | ri18 | ri18 | ri18 | + |
| Hypothetical protein | Δaj1 | aj1 | aj1 | Δaj1 | Δaj1 | − |
| fusB leader peptide | LP | LP | LP | LP | LP | + |
| Fusidic acid resistance gene | fusB | fusB | fusB | fusB | fusB | + |
| Hypothetical protein | Absent | 17 | Absent | Absent | Absent | + |
| Hypothetical protein | Absent | 18 | Absent | Absent | Absent | + |
| Transposase | Absent | 19 | Absent | Absent | Absent | − |
| Hypothetical protein | Absent | Absent | Absent | Absent | aj2 | + |
| Hypothetical protein | Absent | Absent | Absent | Absent | Δaj3 | + |
SaRI, Staphylococcus aureus resistance island (23); SeRI, Staphylococcus epidermidis resistance island.
Comparison of the sequences of SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, SeRIfusB-7778, and SaRIfusB (GenBank accession no. AM292600) showed that SeRIfusB-7778 was more similar to SaRIfusB found in S. aureus than to the other three islands in S. epidermidis, but the other three islands were similar to each other (Table 3 and Fig. 1). Three genes near the fusB gene (ri17, ri18, and LP) were very conserved in the above five islands. Besides, all five islands carried genes encoding integrase (int) at the left end, two divergently oriented putative transcriptional regulators, primase, and terminase small subunit (Table 3 and Fig. 1). Four islands (SaRIfusB, SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907) had genes encoding excisionase. SeRIfusB-7778 and SaRIfusB carried the rep encoding replication initiator (ORF 8) and replication origin (ori), which could not be found within SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907 (Table 3 and Fig. 1). Comparison of packaging genes showed that all had the terminase small subunit. But unlike SaRIfusB, carrying four other genes associated with capsid size determinant, SeRIfusB-7778 carried two capsid size determinant genes, and no putative capsid size determinants were detected in SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907.
attc site sequences and locations.
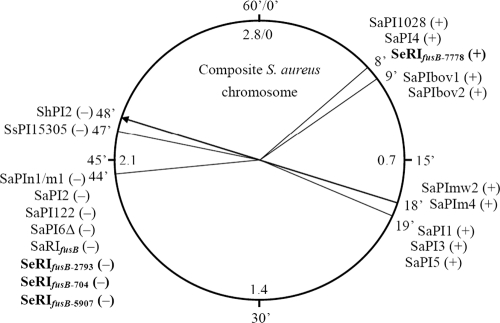
The 21-nt direct repeats flanking SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907, known as attc core sites, ATGCCAGGTATGATGTAAAAA and ATGCCAGG(T/A)ATGATGTAAATA, were located downstream of the groEL gene. The attc site was located at 44 min based on the whole genomic sequences of S. aureus (Fig. 2). SeRIfusB-7778 was inserted 99 nt downstream of the rpsR gene, and the first 18 nt of the sequence (AAAGAAGAACAATAATAT) were predicted to be an attc core site located at 8 min related to the sequence of the S. aureus genome (Fig. 2).
Fig. 2.
Locations of PI attc core sites based on S. aureus whole genomic sequence. The circle presents the composite of the staphylococcal genome with average locations of 60 min (outer scale) to assign 2.8-Mb sequences (inner scale) (modified from reference 20 with permission). Plus or minus in the parentheses indicates the insertion orientation of PIs in clockwise or counterclockwise orientation with respect to the chromosome, respectively.
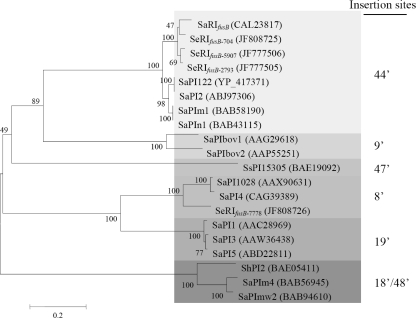
Phylogenetic relationships among integrases.
The insertion sites of the pathogenicity islands in the bacterial chromosome are recognized by integrases. An unrooted phylogenetic tree constructed from the amino acid sequences of integrases collected from GenBank and determined in this study is presented in Fig. 3. The phylogenetic analysis revealed that the amino acid sequences of the integrases were divided into six major clusters consistent with the insertion sites of the pathogenicity islands (Fig. 2). These results indicate that the integrases recognize the attc sites specifically.
Fig. 3.
Phylogenetic relationships among integrases based on amino acid sequences collected from PIs. The phylogenetic tree was generated by using the neighbor-joining method in the MEGA4 package. Numbers at nodes are confidence levels expressed as percentages of occurrence in 500 bootstrapped resamplings. The scale bar indicates the evolutionary distance between sequences determined by measuring the lengths of the horizontal lines connecting two organisms. GenBank accession numbers for integrases are shown in the parentheses. Different degrees of gray indicate PI insertions into different sites based on the S. aureus chromosome (Fig. 2).
Determination of insertion sites in the remaining S. epidermidis isolates.
Based on the obtained sequences from four representative fusB resistance islands, we designed eight pairs of primers to determine the insertion sites of resistance islands in the remaining 30 fusB-carrying isolates. For isolates with a resistance island inserted downstream of the groEL gene, the left junction (groEL-integrase) and the right junction (fusB and a downstream chromosomal gene such as ORF19 in SeRIfusB-2793 [Fig. 1]) could be amplified by different pairs of the primers listed in Materials and Methods. Moreover, the chromosomal genes could not be amplified due to the insertion of the resistance island (Fig. 1). Among the isolates tested, the majority (28/34, 82%) had insertions downstream of groEL, regardless of the aj1-LP-fusB types. Resistance islands in two isolates with type II aj1-LP-fusB were inserted downstream of rpsR, and the insertion sites in the remaining four isolates (two carrying type I aj1-LP-fusB and two carrying undetermined aj1-LP-fusB) were unknown (Table 4).
Table 4.
Insertion sites of fusB elements in S. epidermidis clinical isolates
| Type of aj1-LP-fusB (no. of isolates) | No. of isolates with insertion site: |
||
|---|---|---|---|
| groEL | rpsR | Unknown | |
| I (4) | 2 | 0 | 2 |
| II (11) | 9 | 2 | 0 |
| III (17) | 17 | 0 | 0 |
| Ua (2) | 0 | 0 | 2 |
| Total (34) | 28 | 2 | 4 |
U, undetermined aj1-LP-fusB type.
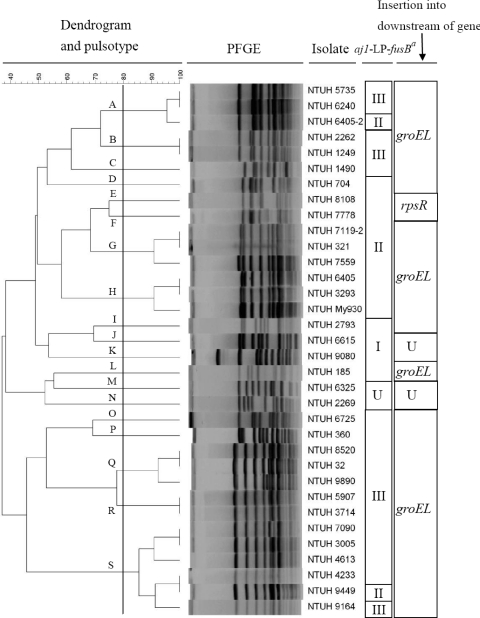
PFGE analysis.
PFGE analysis was used to determine the genetic relatedness among the 34 fusB-containing S. epidermidis isolates, and the pulsotypes were analyzed by BioNumerics software version 4.0. The 34 isolates were divided into 19 clusters with >80% similarity based on the Dice similarity index in the dendrogram created by the UPGMA algorithm (Fig. 4). Isolates with type II or type III aj1-LP-fusB regions were distributed in different pulsotypes. Two isolates (NTUH-8108 and NTUH-7778) with rpsR insertion were closely related. No particular pulsotype was predominant among these isolates.
Fig. 4.
Dendrogram produced by BioNumerics software, showing distances calculated by Dice similarity index of SmaI-digested DNA fragments among 34 fusB-carrying S. epidermidis isolates. The degree of similarity is shown in the scale. Different degrees of gray indicate aj1-LP-fusB types. a, aj1-LP-fusB types; b, insertion sites of resistance islands; U, undetermined.
DISCUSSION
In agreement with previous studies, the predominant fusidic acid resistance determinant in our S. epidermidis isolates was the fusB gene, which has been reported in Leeds General Infirmary in the United Kingdom and around Europe (15). In the present study, among the 34 fusB-positive S. epidermidis clinical isolates, 33 carried fusB only and 1 carried both fusB and fusC. All fusB-positive isolates contained the aj1-LP-fusB fragments but with different sizes of the aj1 region, which can be divided into at least three types. In a previous study in S. aureus, fusB constructs carrying partial aj1 fragments of different sizes conferred different levels of resistance to fusidic acid (22). In this study, a slightly higher level of resistance (MICs, 16 to 32 μg/ml) to fusidic acid was found for isolates carrying type I (full-length aj1) and type II (truncated aj1 gene from 93 to 421 nt) aj1-LP-fusB fragments than for isolates carrying the type III fragment (truncated aj1, only the last 37 bp retained) (MICs, 4 to 16 μg/ml). Although it has been demonstrated that the fusB gene alone can mediate full resistance to fusidic acid (22), the role of aj1 in fusidic acid resistance is still unknown.
Sequence analysis of flanking regions of fusB in four representative S. epidermidis isolates revealed that the resistance elements containing fusB were PI-like structures (9). The sizes of the four RIs (SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, and SeRIfusB-7778) ranging from 15,307 to 17,554 bp fit the criteria of a PI. PIs in staphylococci usually comprise genomic regions of approximately 10 to 200 kb. The G+C contents of the four RIs ranged from 29.3% to 30.2%, which were lower than that of the published whole genome of S. epidermidis (∼32%). The four resistance islands were all flanked by directed repeats and carried conserved core genes, including integrase at one end and other proteins associated with staphylococcal PIs. Several PIs in S. aureus, including SaPI1 to SaPI4 (12, 26), SaPIn1 to SaPIn3 (10), and SaPIbov1 to SaPIbov3 (30, 31), usually carry genes encoding toxic shock syndrome toxin 1 (TSST-1) and other superantigen toxins. Phage-related chromosomal islands containing antibiotic resistance genes, including aad, fosB, or fusB, have been reported in Staphylococcus (19). SaRIfusB, containing the fusB resistance determinant, was identified from a European fusidic acid-resistant impetigo clone (EEFIC) of S. aureus that did not harbor any virulence gene but only the fusB resistance gene (23). Mobile genetic elements (MGEs), including plasmids, transposons, phages, and pathogenicity islands (PIs), have been reported to be mobile carriers of antibiotic resistance or virulence factors. We believe that RIs of S. epidermidis should play an important role in horizontal gene transfer for fusidic acid resistance within species and/or between species (9).
Two major insertion sites of SeRIfusB in the chromosome were found: one (from the majority of isolates) was downstream of groEL (for SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907) and the other one was at rpsR (SeRIfusB-7778). The same site of insertion downstream of groEL (44 min based on the whole genomic sequences of S. aureus) was also found in SaRIfusB (21). However, the direct repeats at the right end of the flanking resistance islands were slightly different, ATGCCAGG(T/A)ATGATGTAAATA in SeRIfusB-2793, SeRIfusB-704, and SeRIfusB-5907 and ATGCCAGGTATGATGTAAAAC in SaRIfusB (23). The other insertion site, downstream of the rpsR gene, found in SeRIfusB-7778, was located at the 8-min position of the S. aureus genome, the same site as that in SaPI4 and SaPI1028 (19). The integration sites, known as the att sites of the classical temperate phage, contain core sequence (15 to 22 nt), plus essential sequences depending on the PIs and the chromosome (8, 31). In the present study, SeRIfusB-7778 was flanked by 99-nt directed repeats comprised of an 18-nt putative conserved core sequence, which was identical to that in SaPI4 and SaPI1028, and an 81-nt essential flanking sequence.
Integrase is responsible for the insertion of PIs into specific sites. Phylogenetic analysis of integrases in staphylococcal PIs and RIs revealed that those islands that were inserted into the same sites grouped together. Accordingly, PIs with closely related integrases were located at integrase-recognized specific sites. For example, SeRIfusB-2793, SaRIfusB (23), and SaPI2 (25) with the same integrases were all inserted at the same chromosome site, the 44-min site.
Although we did not test the transferability of SeRIfusB, the mobility of PIs in S. aureus has been studied (28, 29). The excision and replication of SaPIbov1 were induced after SOS induction in the presence of temperate phage, and then SaPIbov1 was packed into phage-like particles to infect more bacteria (30). In the present study, resistance islands can be found in different clones and the pulsotypes of fusB-containing S. epidermidis isolates were very heterogeneous, suggesting the possibility that RIs are easily transferred. In addition, some integrases can be found in S. epidermidis and S. aureus, which means that they recognize the same core att sequences. Thus, it is possible that the RIs have the ability to spread among different species (13). Since S. epidermidis has been considered to be a reservoir of antibiotic resistance genes (1), the high prevalence of fusB in S. epidermidis may raise the chance for spreading fusidic acid resistance to other species.
The four different types of fusB-containing resistance islands (SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, and SeRIfusB-7778) identified in S. epidermidis isolates showed many similarities but with some variations. Preliminary identification of SeRIfusB-2793, SeRIfusB-704, SeRIfusB-5907, or SeRIfusB-7778 can be determined based on the aj1-LP-fusB type, restriction hybridization with fusB probes, and the insertion sites.
In summary, the fusB genes in S. epidermidis isolates were mostly located in phage-related resistance islands. The resistance islands may be responsible for the dissemination of fusidic acid resistance in S. epidermidis through horizontal gene transfer. This is the first report that fusB resistance islands are found in S. epidermidis.
ACKNOWLEDGMENT
This work was supported by grant NSC 98-2320-B-002-015-MY3 from the National Science Council of Taiwan.
Footnotes
Published ahead of print on 3 October 2011.
REFERENCES
- 1. Archer G. L., Johnston J. L. 1983. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob. Agents Chemother. 24:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Besier S., Ludwig A., Brade V., Wichelhaus T. A. 2003. Molecular analysis of fusidic acid resistance in Staphylococcus aureus. Mol. Microbiol. 47:463–469 [DOI] [PubMed] [Google Scholar]
- 3. Bodley J. W., Zieve F. J., Lin L., Zieve S. T. 1969. Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem. Biophys. Res. Commun. 37:437–443 [DOI] [PubMed] [Google Scholar]
- 4. Chen H. J., et al. 2010. Fusidic acid resistance determinants in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 54:4985–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2009. M07-A8 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Coutant C., Olden D., Bell J., Turnidge J. D. 1996. Disk diffusion interpretive criteria for fusidic acid susceptibility testing of staphylococci by the National Committee for Clinical Laboratory Standards method. Diagn. Microbiol. Infect. Dis. 25:9–13 [DOI] [PubMed] [Google Scholar]
- 7. Dobie D., Gray J. 2004. Fusidic acid resistance in Staphylococcus aureus. Arch. Dis. Child. 89:74–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitzgerald J. R., et al. 2001. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J. Bacteriol. 183:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hacker J., Kaper J. B. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641–679 [DOI] [PubMed] [Google Scholar]
- 10. Kuroda M., et al. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225–1240 [DOI] [PubMed] [Google Scholar]
- 11. Laurberg M., et al. 2000. Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J. Mol. Biol. 303:593–603 [DOI] [PubMed] [Google Scholar]
- 12. Lindsay J. A., Ruzin A., Ross H. F., Kurepina N., Novick R. P. 1998. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol. Microbiol. 29:527–543 [DOI] [PubMed] [Google Scholar]
- 13. Maiques E., et al. 2007. Role of staphylococcal phage and SaPI integrase in intra- and interspecies SaPI transfer. J. Bacteriol. 189:5608–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martemyanov K. A., Liljas A., Yarunin A. S., Gudkov A. T. 2001. Mutations in the G-domain of elongation factor G from Thermus thermophilus affect both its interaction with GTP and fusidic acid. J. Biol. Chem. 276:28774–28778 [DOI] [PubMed] [Google Scholar]
- 15. McLaws F., Chopra I., O'Neill A. J. 2008. High prevalence of resistance to fusidic acid in clinical isolates of Staphylococcus epidermidis. J. Antimicrob. Chemother. 61:1040–1043 [DOI] [PubMed] [Google Scholar]
- 16. Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagaev I., Bjorkman J., Andersson D. I., Hughes D. 2001. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol. Microbiol. 40:433–439 [DOI] [PubMed] [Google Scholar]
- 18. Norstrom T., Lannergard J., Hughes D. 2007. Genetic and phenotypic identification of fusidic acid-resistant mutants with the small-colony-variant phenotype in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:4438–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Novick R. P., Christie G. E., Penades J. R. 2010. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 8:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novick R. P., Subedi A. 2007. The SaPIs: mobile pathogenicity islands of Staphylococcus. Chem. Immunol. Allergy 93:42–57 [DOI] [PubMed] [Google Scholar]
- 21. O'Brien F. G., Price C., Grubb W. B., Gustafson J. E. 2002. Genetic characterization of the fusidic acid and cadmium resistance determinants of Staphylococcus aureus plasmid pUB101. J. Antimicrob. Chemother. 50:313–321 [DOI] [PubMed] [Google Scholar]
- 22. O'Neill A. J., Chopra I. 2006. Molecular basis of fusB-mediated resistance to fusidic acid in Staphylococcus aureus. Mol. Microbiol. 59:664–676 [DOI] [PubMed] [Google Scholar]
- 23. O'Neill A. J., Larsen A. R., Skov R., Henriksen A. S., Chopra I. 2007. Characterization of the epidemic European fusidic acid-resistant impetigo clone of Staphylococcus aureus. J. Clin. Microbiol. 45:1505–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Neill A. J., McLaws F., Kahlmeter G., Henriksen A. S., Chopra I. 2007. Genetic basis of resistance to fusidic acid in staphylococci. Antimicrob. Agents Chemother. 51:1737–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruzin A., Lindsay J., Novick R. P. 2001. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol. Microbiol. 41:365–377 [DOI] [PubMed] [Google Scholar]
- 26. Subedi A., Ubeda C., Adhikari R. P., Penades J. R., Novick R. P. 2007. Sequence analysis reveals genetic exchanges and intraspecific spread of SaPI2, a pathogenicity island involved in menstrual toxic shock. Microbiology 153:3235–3245 [DOI] [PubMed] [Google Scholar]
- 27. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 28. Tormo-Mas M. A., et al. 2010. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature 465:779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ubeda C., et al. 2008. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol. Microbiol. 67:493–503 [DOI] [PubMed] [Google Scholar]
- 30. Ubeda C., et al. 2005. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol. Microbiol. 56:836–844 [DOI] [PubMed] [Google Scholar]
- 31. Ubeda C., et al. 2003. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol. Microbiol. 49:193–210 [DOI] [PubMed] [Google Scholar]
- 32. Worthington T., Lambert P. A., Elliott T. S. 2000. Is hospital-acquired intravascular catheter-related sepsis associated with outbreak strains of coagulase-negative staphylococci? J. Hosp. Infect. 46:130–134 [DOI] [PubMed] [Google Scholar]
- 33. Yazdankhah S. P., Asli A. W., Sorum H., Oppegaard H., Sunde M. 2006. Fusidic acid resistance, mediated by fusB, in bovine coagulase-negative staphylococci. J. Antimicrob. Chemother. 58:1254–1256 [DOI] [PubMed] [Google Scholar]