Abstract
Combination therapy may be required for multidrug-resistant (MDR) Pseudomonas aeruginosa. The aim of this study was to systematically investigate bacterial killing and emergence of colistin resistance with colistin and doripenem combinations against MDR P. aeruginosa. Studies were conducted in a one-compartment in vitro pharmacokinetic/pharmacodynamic model for 96 h at two inocula (∼106 and ∼108 CFU/ml) against a colistin-heteroresistant reference strain (ATCC 27853) and a colistin-resistant MDR clinical isolate (19147 n/m). Four combinations utilizing clinically achievable concentrations were investigated. Microbiological response was examined by log changes and population analysis profiles. Colistin (constant concentrations of 0.5 or 2 mg/liter) plus doripenem (peaks of 2.5 or 25 mg/liter every 8 h; half-life, 1.5 h) substantially increased bacterial killing against both strains at the low inoculum, while combinations containing colistin at 2 mg/liter increased activity against ATCC 27853 at the high inoculum; only colistin at 0.5 mg/liter plus doripenem at 2.5 mg/liter failed to improve activity against 19147 n/m at the high inoculum. Combinations were additive or synergistic against ATCC 27853 in 16 and 11 of 20 cases (4 combinations across 5 sample points) at the 106- and 108-CFU/ml inocula, respectively; the corresponding values for 19147 n/m were 16 and 9. Combinations containing doripenem at 25 mg/liter resulted in eradication of 19147 n/m at the low inoculum and substantial reductions in regrowth (including to below the limit of detection at ∼50 h) at the high inoculum. Emergence of colistin-resistant subpopulations of ATCC 27853 was substantially reduced and delayed with combination therapy. This investigation provides important information for optimization of colistin-doripenem combinations.
INTRODUCTION
Multidrug-resistant (MDR) Pseudomonas aeruginosa is one of several important Gram-negative bacteria emerging as significant pathogens worldwide (8, 50). With a very limited number of therapeutic options against these pathogens remaining and a lack of novel antimicrobial agents in the drug development pipeline (30, 50), particularly those with activity against P. aeruginosa (50), clinicians have been forced to reexamine the use of “old,” previously discarded drugs such as the polymyxins (8, 40). Colistin (also known as polymyxin E) is a multicomponent cationic polypeptide antibiotic largely abandoned in the 1970s due to concerns about the potential for nephro- and neurotoxicity (15, 27). Colistin retains significant in vitro activity against Gram-negative “superbugs” and is often the only therapeutic option available to treat infections caused by these pathogens (1, 27, 35). Several institutions have already experienced outbreaks of infections with MDR Gram-negative bacteria resistant to all commercially available antibiotics except the polymyxins (6, 26, 34). Of particular concern is that with the rapid increase in the use of colistin over the last decade, especially for critically ill patients (8, 27), has come an increase in the number of reports of resistance to colistin (1, 23, 27).
Having entered clinical use in 1959, colistin was never subjected to the scientific rigor required for modern pharmaceuticals before they become available for use in patients. The result has been a dearth of reliable information on pharmacokinetics (PK) and pharmacodynamics (PD) with which to guide therapy, and confusion has surrounded the optimal dosing strategy. It is only very recently that crucial gaps in our knowledge of the PK and PD of colistin have begun to be filled. Recent investigations into the PK of colistin in critically ill patients have revealed low and potentially suboptimal plasma drug concentrations in a substantial proportion of patients receiving currently recommended dosage regimens (16, 47). In addition, both in vitro (3, 4, 48, 52) and in vivo (22, 32) studies have shown the potential for the rapid emergence of colistin resistance with monotherapy, with heteroresistance a likely contributing factor; colistin heteroresistance has been identified in Acinetobacter baumannii (28, 55), Klebsiella pneumoniae (48, 53), and most recently in P. aeruginosa (5a). The potential presence of colistin-resistant subpopulations of heteroresistant strains prior to therapy and the observation of rapid amplification of colistin-resistant subpopulations with colistin monotherapy suggest caution with the use of colistin monotherapy and highlight the importance of investigating rational and novel colistin combinations. The aim of the present study was to systematically investigate the extent of in vitro bacterial killing and the emergence of colistin resistance with colistin alone and in combination with doripenem at both high and low inocula of P. aeruginosa using clinically relevant dosage regimens. This was achieved by simulating, in an in vitro PK/PD model, the PK of colistin formation and doripenem in humans over a range of clinically achievable concentrations in critically ill patients.
(Parts of this study were presented at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Boston, MA, 12 to 15 September 2010.)
MATERIALS AND METHODS
Bacterial isolates.
Two strains of P. aeruginosa were employed in this study: a colistin-heteroresistant reference strain, ATCC 27853 (American Type Culture Collection, Rockville, MD), and a nonmucoid colistin-resistant MDR clinical isolate, 19147 n/m, obtained from a patient with cystic fibrosis; the clinical isolate contained genes encoding IMP-type carbapenemase and CTX-M-type extended-spectrum β-lactamase (ESBL). Heteroresistance to colistin was defined as the ability of subpopulations of a strain to grow in the presence of >2 mg/liter in the population analysis profiles (PAPs; see below) although the colistin MIC for the strain was ≤2 mg/liter. MDR was defined as diminished susceptibility to ≥2-mg/liter concentrations of drugs from the following five classes: antipseudomonal cephalosporins, antipseudomonal carbapenems, β-lactam–β-lactamase inhibitor combinations, antipseudomonal fluoroquinolones, and aminoglycosides (45). MICs of colistin (sulfate) and doripenem were each 1 mg/liter for ATCC 27853 and were 128 and 0.25 mg/liter for 19147 n/m, respectively. MICs of colistin and doripenem for each isolate were determined in three replicates on separate days in cation-adjusted Mueller-Hinton broth (CAMHB; Ca2+ at 23.0 μg/ml, Mg2+ at 12.2 μg/ml [Oxoid, Hampshire, England]) via broth microdilution (13). Resistance to colistin (13) and doripenem (42) was defined as MICs of ≥4 mg/liter. Strains were stored in tryptone soy broth (Oxoid, Basingstoke, Hampshire, England) with 20% glycerol (Ajax Finechem, Seven Hills, New South Wales, Australia) at −80°C in cryovials (Simport Plastics, Beloeil, Quebec, Canada).
Antibiotics and reagents.
For MIC determinations and in vitro PK/PD studies, colistin sulfate (lot 109K1574; 23,251 U/mg) was purchased from Sigma-Aldrich (St. Louis, MO), while doripenem (lot 0137Y01) was kindly donated by Johnson and Johnson (Shionogi and Co., Osaka, Japan). Colistin sulfate was used in the present study as colistin is the active antibacterial agent formed in vivo after administration of its inactive prodrug, colistin methanesulfonate (CMS) (5). Stock solutions of doripenem were prepared using Milli-Q water (Millipore Australia, North Ryde, New South Wales, Australia) immediately prior to each dose, protected from light to minimize loss from degradation, and sterilized by filtration with a 0.22-μm-pore-size Millex-GP filter (Millipore, Bedford, MA). Colistin was similarly prepared at the beginning of each experiment and spiked into the growth medium of the central reservoir (see below) to achieve the desired concentration; preliminary experiments demonstrated that colistin was stable under these conditions for the duration of the experiment. All other chemicals were from suppliers listed previously (24).
Binding of doripenem in growth medium.
The binding of doripenem in CAMHB was measured by equilibrium dialysis using Dianorm equilibrium dialyzer units containing two chambers (with a 1-ml volume in each chamber) separated by a semipermeable membrane (regenerated cellulose membrane; molecular mass cutoff, 10,000 Da [Harvard Apparatus, Holliston, MA]). Doripenem was spiked into CAMHB (in the donor chamber) to achieve a concentration of 25 mg/liter and dialyzed at 37°C against the same volume of isotonic phosphate buffer, pH 7.4 (in the acceptor chamber); samples were prepared in triplicate. Samples of CAMHB and buffer were removed from each reservoir after 4 h (shown in preliminary studies to be the time required for equilibration) and stored at −80°C until being analyzed as described below. The fraction of doripenem unbound in CAMHB (ƒu) was calculated as follows: (acceptor doripenem concentration)/(donor doripenem concentration).
In vitro PK/PD model and colistin-doripenem dosing regimens.
Experiments to examine the microbiological response and emergence of resistance to various dosage regimens of colistin and doripenem alone and in combination were conducted over 96 h at two different starting inocula (∼106 and ∼108 CFU/ml) using a one-compartment in vitro PK/PD model described previously (4) and below. Prior to each experiment, strains were subcultured onto horse blood agar (Media Preparation Unit, The University of Melbourne, Parkville, Australia) and incubated at 35°C for 24 h. One colony was then selected and grown overnight in 10 ml of CAMHB, from which early-log-phase growth was obtained. For a starting inoculum of ∼106 CFU/ml, a 1.0-ml aliquot of this early-log-phase bacterial suspension was inoculated into each compartment at the commencement of the experiment to yield ∼106 CFU/ml. To achieve a starting inoculum of ∼108 CFU/ml, the flow of medium was temporarily halted, a 1.0-ml aliquot of overnight culture was inoculated into each compartment on the morning of the experiment, and the bacteria were allowed to grow until 108 CFU/ml was obtained. The experiment was commenced immediately upon attainment of 108 CFU/ml.
The PK/PD model consisted of eight sealed containers (compartments) each containing 80 ml of CAMHB at 37°C and a magnetic stir bar to ensure adequate mixing. One compartment acted as a control to define growth dynamics in the absence of antibiotic, while colistin and/or doripenem was delivered into the remaining compartments to achieve the desired constant concentration (for colistin) or intermittent (doripenem) dosage regimen (see below). A peristaltic pump (Masterflex L/S; Cole-Parmer) was used to deliver sterile CAMHB from separate central reservoirs into each compartment at a predetermined rate, displacing an equal volume of CAMHB into a waste receptacle. Flow rates were calibrated prior to each experiment and monitored throughout to ensure that the system was performing optimally. For colistin-containing regimens, colistin was delivered as a constant concentration by spiking colistin into the central reservoir prior to initiation of the experiment so that all media flowing through the system (with the exception of that in the growth control compartment) contained a constant concentration of colistin (Table 1); colistin was administered in this way to mimic the flat plasma drug concentration-time profiles of formed colistin at steady state observed in critically ill patients given CMS (16, 47). For colistin-containing regimens at the higher inoculum (∼108 CFU/ml), each compartment was initially filled with sterile drug-free CAMHB to allow bacterial growth up to 108 CFU/ml in the absence of the drug; subsequently, a loading dose of colistin was administered to immediately attain the targeted colistin concentration. For doripenem-containing regimens, doripenem was injected into each treatment compartment following bacterial inoculation to achieve the desired steady-state maximum (peak) concentration (Cmax), with intermittent 8-hourly dosing thereafter (Table 1); as doripenem does not accumulate following multiple intravenous (i.v.) administrations, no loading dose was required to achieve steady-state concentrations. The chosen flow rate simulated a doripenem elimination half-life (t1/2) of 1.5 h, which approximates that in critically ill patients (33).
Table 1.
Colistin and doripenem dosage regimens, PK/PD index values, and sampling times in the in vitro PK/PD modela
| Treatment regimen | Target Cmax/Cmin (mg/liter) | Valuee for ATCC 27853/isolate 19147 n/m |
Sampling times (h) for microbiological measurementsf | ||
|---|---|---|---|---|---|
| AUC/MIC | Cmax/MIC | %T>MIC | |||
| Col monotherapyb | 0.5 | 12.0/0.09 | 0.5/0.004 | 0/0 | 0, 1, 2, 3, 4, 6, 23, 24, 25, 26, 47, 48, 49, 50, 71, 72, 73, 74, 95, 96 |
| 2.0 | 48.0/0.38 | 2.0/0.02 | 100/0 | ||
| 5.0 | 120/0.94 | 5.0/0.04 | 100/0 | ||
| Dor monotherapyc | 2.5/0.062 | 15.8/63.3 | 2.5/10 | 24.8/62.3 | 0, 1, 2, 3, 4, 6, 23, 24, 25, 26, 30, 47, 48, 49, 50, 54, 71, 72, 73, 74, 78, 95, 96 |
| 25/0.62 | 158/633 | 25/100 | 87.1/100 | ||
| 50/1.24 | 317/1,266 | 50/200 | 100/100 | ||
| Combination therapyd | 0, 1, 2, 3, 4, 6, 8, 23, 24, 25, 26, 29, 32, 47, 48, 49, 50, 53, 56, 71, 72, 73, 74, 77, 80, 95, 96 | ||||
Dosage regimens were tested with ∼106 and ∼108 CFU/ml starting inocula.
Colistin (Col) dosage regimens involved a constant concentration of colistin simulating continuous infusion. For the colistin-resistant isolate (19147 n/m), only colistin at 5.0 mg/liter was used as monotherapy. Values shown for isolate 19147 n/m at other dosages of colistin are those for combination therapy with the indicated concentration of colistin.
Doripenem (Dor) dosage regimens involved intermittent administration (every 8 h) to achieve the targeted Cmax/Cmin.
Combination therapy was carried out with the following concentrations: colistin at 0.5 mg/liter plus doripenem at 2.5 mg/liter, colistin at 0.5 mg/liter plus doripenem at 25 mg/liter, colistin at 2.0 mg/liter plus doripenem at 2.5 mg/liter, and colistin at 2.0 mg/liter plus doripenem at 25 mg/liter.
Values shown are target values for PK/PD indices. For combination therapy, the PK/PD indices for each drug were the same as those for equivalent monotherapy. AUC/MIC, area under the concentration-time curve over 24 h in the steady state divided by the MIC; %T>MIC, cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state PK conditions.
The number of CFU per milliliter was determined at all time points. Full PAPs were generated at 0 and 96 h; mini-PAPs were generated at 6, 24, 48, and 72 h.
Three constant concentrations of colistin and three intermittent doripenem dosage regimens were simulated for monotherapy (Table 1). For combination therapy against both isolates, colistin at a constant concentration of 0.5 or 2.0 mg/liter was used in combination with intermittent doripenem at a concentration of 2.5 or 25 mg/liter, yielding four combination regimens (Table 1); combination dosage regimens mimicked the PK profiles of each drug achieved in critically ill patients (16, 31, 47). As we have demonstrated previously that colistin (3) and in the present study doripenem are almost entirely unbound in CAMHB, the specified concentrations represent unbound (free) concentrations.
Microbiological response and emergence of resistance to colistin.
Serial samples (0.6 ml) were collected aseptically at the times shown in Table 1 from each reservoir for viable-cell counting and real-time PAPs, as well as determination of colistin and doripenem concentrations. Viable-cell counts and PAPs were obtained immediately after sampling by using a WASP 2 spiral plater (Don Whitley Scientific Ltd., United Kingdom) to plate 50 μl of a sample appropriately diluted (using 0.9% saline) onto either nutrient agar (for viable-cell counting) or Mueller-Hinton agar (for PAPs) and incubating the plates at 35°C for 24 h (48 h for plates with small colonies). Serial dilution and plating with the spiral plater, which further dilutes the sample, helped reduce the possibility of antibiotic carryover. PAP plates were impregnated with colistin (sulfate) at 0, 0.5, 1, 2, 3, 4, 5, 6, 8, and 10 mg/liter; these concentrations were chosen after consideration of the MICs and the colistin concentrations typically achievable in plasma after intravenous CMS administration to patients (16, 31, 47). Full PAPs incorporating all colistin concentrations were determined at 0 and 96 h; mini-PAPs (for 0, 2, 4, and 8 mg/liter) were determined at 6, 24, 48, and 72 h. Colonies were counted using a ProtoCOL colony counter (Don Whitley Scientific Ltd., United Kingdom); the limit of detection was 20 CFU/ml (equivalent to 1 colony per plate), and the limit of quantification was 400 CFU/ml (equivalent to 20 colonies per plate), as specified in the ProtoCOL manual.
PK validation.
Samples (100 μl) collected in duplicate from the in vitro PK/PD experiments were placed in 1.50-ml microcentrifuge tubes (Greiner Bio-One, Frickenhausen, Germany) and immediately stored at −80°C until analysis; all samples were assayed within 4 weeks. Concentrations of colistin were measured using high-performance liquid chromatography (HPLC) (25) with an assay range for colistin sulfate of 0.10 to 6.00 mg/liter. Doripenem concentrations were assayed at ambient temperature using a validated reversed-phase HPLC method. The HPLC system consisted of a Shimadzu LC-20AD Prominence liquid chromatograph, a SIL-20AC HT Prominence autosampler, and an SPD-M20A Prominence diode array detector (Shimadzu, Columbia, MD). To a 100-μl sample, 100 μl of 3-(N-morpholino)propanesulfonic acid (MOPS) buffer and 400 μl of methanol were added, and the mixture was subjected to a vortex and centrifuged at 10,000 rpm for 10 min. An aliquot of the sample (50 μl) was injected onto a PhenoSphere-NEXT 5-μm C18 column (250 by 4.6 mm; Phenomenex, Torrance, CA). A gradient elution procedure involving 100% methanol and 0.1% trifluoroacetic acid as the mobile phases was used, the proportion of methanol increasing from 5 to 80% over 4 min and then returning to 5% over 0.5 min; the flow rate was 0.7 ml/min, with detection at 311 nm. The run time was 10 min. The assay range for doripenem was 0.5 to 32 mg/liter; samples were diluted when the expected doripenem concentrations were higher than the upper limit of quantification. Analysis of quality control (QC) samples with nominal concentrations of 0.40 and 4.0 mg/liter for colistin and 1.2, 12, and 48 mg/liter for doripenem (the latter QC sample requiring dilution) demonstrated accuracy of >90% and coefficients of variation of <10.2% for both colistin and doripenem.
PD analysis.
Microbiological responses to monotherapy and combination therapy were examined using the log change method, calculating the change in log10 CFU per milliliter from 0 h (CFU0) to time t (6, 24, 48, 72 or 96 h; CFUt) as shown: log change = log10(CFUt) − log10(CFU0).
Single-antibiotic or combination regimens causing a reduction of ≥1 log10 CFU/ml below the initial inoculum at 6, 24, 48, 72, or 96 h were considered active. We considered synergy to be a ≥2-log10-lower number of CFU per milliliter for the combination than for its most active component at the specified time (46); additivity was defined as a 1- to <2-log10-lower number of CFU per milliliter for the combination.
RESULTS
PK validation and doripenem binding.
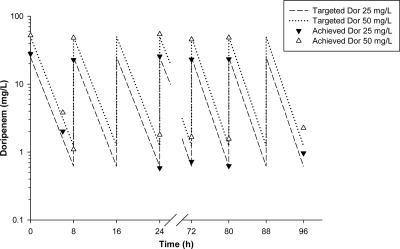
The colistin drug concentrations achieved (means ± standard deviations [SD]) were 0.45 ± 0.07 mg/liter (n = 22), 1.76 ± 0.17 mg/liter (n = 26), and 4.58 ± 0.02 mg/liter (n = 6) for the targeted concentrations of 0.5, 2.0, and 5.0 mg/liter, respectively. Measured doripenem Cmax and minimum (trough) concentration (Cmin) values were 51.47 ± 3.96 mg/liter (n = 30) and 1.24 ± 0.42 mg/liter (n = 30) for the targeted values of 50.0 and 1.24 mg/liter and 25.60 ± 2.53 mg/liter (n = 50) and 0.80 ± 0.26 mg/liter (n = 50) for the targeted values of 25.0 and 0.62 mg/liter. For the targeted doripenem Cmax of 2.5 mg/liter, the measured Cmax was 2.45 ± 0.32 mg/liter (n = 50), with all Cmin values below the limit of quantification (0.5 mg/liter) of the HPLC assay. Typical simulated PK profiles for doripenem dosage regimens of 25 and 50 mg/liter every 8 h are shown in Fig. 1. The observed mean t1/2 for the simulated intermittent doripenem dosage regimens was 1.55 ± 0.17 h (n = 71) for the targeted value of 1.5 h; as the Cmin for some dosage regimens was below the lower limit of quantification of the HPLC assay, t1/2 was not directly measured in all experiments. The ƒu at equilibrium was 0.95, indicating practical equivalence of total and unbound concentrations.
Fig. 1.
Targeted doripenem (Dor) PK profiles for 25- and 50-mg/liter 8-hourly regimens with measured Dor concentrations.
Microbiological response.
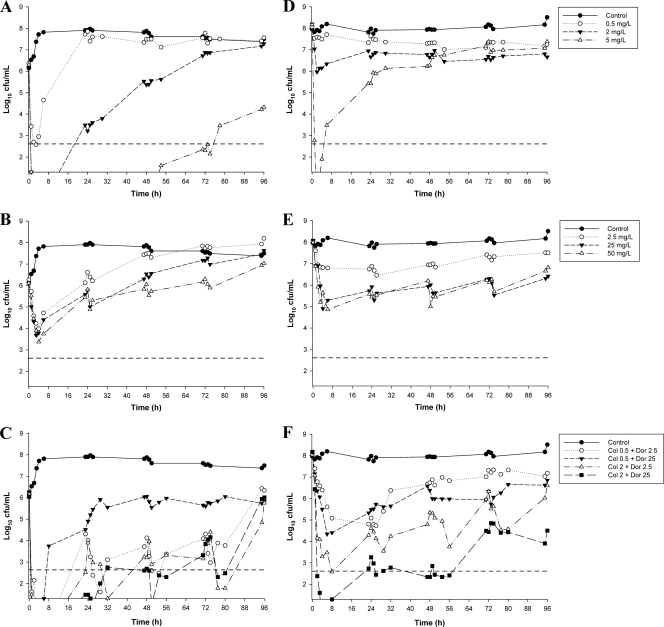
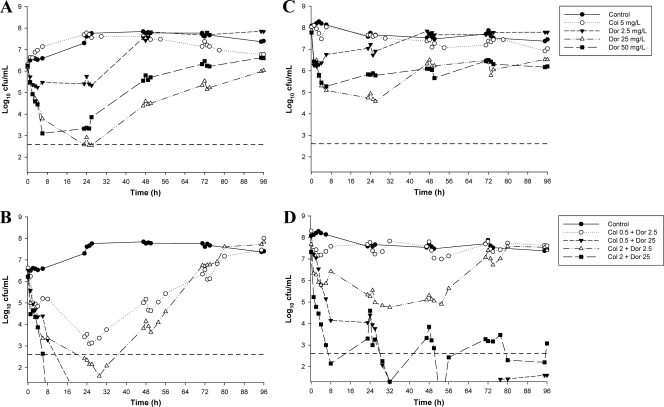
The initial inocula (means ± SD) were 6.20 ± 0.10 log10 CFU/ml (n = 11) and 8.09 ± 0.08 log10 CFU/ml (n = 11) for ATCC 27853 and 6.30 ± 0.16 log10 CFU/ml (n = 9) and 7.88 ± 0.28 log10 CFU/ml (n = 9) for 19147 n/m for the targets of 106 and 108 CFU/ml, respectively. The time course profiles of bacterial numbers achieved with all dosage regimens at both inocula are shown in Fig. 2 (for ATCC 27853) and Fig. 3 (for 19147 n/m). Log changes in viable-cell counts at each inoculum with mono- and combination therapy are presented in Table 2.
Fig. 2.
Time-kill curves for colistin monotherapy (A and D), doripenem monotherapy (B and E), and combination therapy (C and F) against ATCC 27853 at the 106-CFU/ml inoculum (left panels) and the 108-CFU/ml inoculum (right panels). The y axis starts from the limit of detection, and the limit of quantification (LOQ) is indicated by the horizontal broken line.
Fig. 3.
Time-kill curves for colistin and doripenem monotherapy (A and C) and combination therapy (B and D) against 19147 n/m at the 106-CFU/ml inoculum (left panels) and the 108-CFU/ml inoculum (right panels). The y axis starts from the limit of detection, and the limit of quantification (LOQ) is indicated by the horizontal broken line.
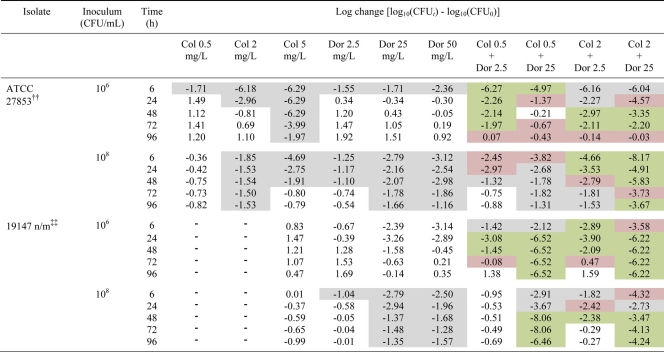
Table 2.
Log changes in CFU/mla
Log changes in CFU/ml at 6, 24, 48, 72, and 96 h at an inoculum of 106 or 108 CFU/ml with colistin (Col) and/or doripenem (Dor) against P. aeruginosa. The gray background indicates activity (a reduction of ≥1-log10 CFU/ml below the initial inoculum); the green background indicates synergy (a ≥2-log10 decrease in the number of CFU per milliliter with the combination compared to its most active component); the red background indicates additivity (a 1.0- to <2-log10 decrease in the number of CFU per milliliter with the combination compared to its most active component). ††, colistin-heteroresistant reference strain. Heteroresistance to colistin was defined as the ability of subpopulations of a strain to grow in the presence of >2 mg/liter although the colistin MIC for the strain was ≤2 mg/liter. ‡‡, nonmucoid MDR colistin-resistant clinical isolate (colistin monotherapy was performed with 5 mg/liter only).
Colistin monotherapy.
With ATCC 27853 at the 106-CFU/ml inoculum, colistin monotherapy produced rapid and extensive initial killing at all concentrations, with colistin at 2 and 5 mg/liter resulting in undetectable bacterial counts at 2 h (Fig. 2A). Substantial regrowth was evident at 6 h with colistin at 0.5 mg/liter and at 24 h with colistin at 2 mg/liter, with regrowth approaching that of the control by 24 h (for 0.5 mg/liter) and 72 h (for 2 mg/liter). No viable colonies were detected until 54 h with colistin at 5 mg/liter, with subsequent regrowth to ∼4 log10 CFU/ml observed at 96 h. An inoculum effect with colistin monotherapy was observed, with substantially reduced initial bacterial killing at the high compared to the low inoculum with colistin at 0.5 and 2 mg/liter (Fig. 2D). While rapid and extensive initial bacterial killing to below the limit of detection remained at the high inoculum with colistin at 5 mg/liter, substantial regrowth (to ∼3.5 log10 CFU/ml) had occurred by 6 h, with regrowth to above the level of the initial inoculum by 30 h. For the colistin-resistant isolate, bacterial growth in the presence of colistin at 5 mg/liter was essentially no different from that of the control with either inoculum (Fig. 3A and C).
Doripenem monotherapy.
With ATCC 27853 at the 106-CFU/ml inoculum, all doripenem regimens (2.5, 25, or 50 mg/liter every 8 h) produced initial bacterial killing of ∼2.5 log10 CFU/ml, with regrowth beginning by 6 h (Fig. 2B). Regrowth close to control levels had occurred by 48, 72, and 96 h with concentrations of 2.5, 25, and 50 mg/liter, respectively. At the high inoculum, all doripenem concentrations produced similar killing profiles, with the 2.5-mg/liter 8-hourly regimen resulting in bacterial counts consistently ∼0.5 to 1 log below control values and the 25- and 50-mg/liter regimens yielding bacterial counts ∼1.5 to 3 log below control values (Fig. 2E). With the MDR isolate, doripenem at 2.5 mg/liter every 8 h produced only minimal bacterial killing (∼1- to 2-log10 reduction in CFU/ml) at each inoculum, with regrowth close to control values by 24 to 48 h (Fig. 3A and C). Higher doripenem concentrations (25 and 50 mg/liter) produced rapid initial killing of ∼3 log at 6 h, with subsequent regrowth to within ∼1 log of control values at 96 h (Fig. 3A and C). No inoculum effect was observed with doripenem against either strain.
Combination therapy.
With ATCC 27853, the addition of doripenem at 2.5 or 25 mg/liter to colistin at 0.5 mg/liter produced an initial (i.e., up to 8-h) period of additional bacterial killing of ∼2.5 log10 CFU/ml compared with the most active monotherapy (colistin) at the low inoculum and resulted in undetectable bacterial counts no later than 3 h (Table 2). Both combinations resulted in synergy or additivity at most time points across 96 h (Table 2). Synergy was particularly evident with the combination of colistin at 0.5 mg/liter and doripenem at 2.5 mg/liter, with ∼3- to 4-log10-greater killing at most time points. Nevertheless, by 96 h regrowth with this regimen approached that of the growth control. The addition of doripenem (2.5 or 25 mg/liter) to colistin at 2 mg/liter produced synergy at 48 and 72 h, and this combination remained additive at 96 h with regrowth close to the level of the initial inoculum (Fig. 2C and Table 2). At the high inoculum, combinations of colistin at 0.5 mg/liter and doripenem (2.5 or 25 mg/liter) produced only modest increases in bacterial killing across the first 8 to 24 h, with regrowth thereafter similar to that in the presence of the most active single agent (doripenem) (Fig. 2F). With combinations containing colistin at 2 mg/liter, rapid and substantial reductions in bacterial counts were observed, with additional killing of ∼3.5 log10 CFU/ml over that achieved with the most active monotherapy at 8 h for the combination with doripenem at 2.5 mg/liter and additional killing of ∼5 log10 CFU/ml achieved at 4 h for the combination with doripenem at 25 mg/liter; with the latter combination, no viable bacteria were detected at 4 h. Synergy or additivity was maintained with these combinations across 48 and 96 h with doripenem at 2.5 and 25 mg/liter, respectively (Table 2).
Against 19147 n/m at the 106-CFU/ml inoculum, colistin at 0.5 mg/liter plus doripenem at 2.5 mg/liter produced synergy at 24 and 48 h, with regrowth approaching control values by 72 to 96 h (Fig. 3B and Table 2). A similar killing profile was generated with the combination of colistin at 2 mg/liter and doripenem at 2.5 mg/liter, although initial bacterial killing was greater (by ∼3 logs) and lower bacterial counts were maintained across the first ∼60 h (Fig. 3B). With the latter regimen, bacterial counts as low as 1.6 log10 CFU/ml (at 29 h) were observed. With combinations containing colistin (0.5 or 2 mg/liter) and doripenem at 25 mg/liter, the initial rate and extent of killing up to 4 to 6 h were similar to those with doripenem monotherapy (Fig. 3B). By 8 and 24 h, no viable bacteria were observed with the combinations containing colistin at 2 and 0.5 mg/liter, respectively, and no regrowth was subsequently detected. At the high inoculum, the combination of colistin at 0.5 mg/liter and doripenem at 2.5 mg/liter was essentially inactive (Fig. 3D). Increasing the concentration of colistin to 2 mg/liter produced greater bacterial killing at both 24 h (additive) and 48 h (synergistic), with regrowth to control levels by 72 h (Fig. 3D and Table 2). Substantially greater killing was observed with combinations containing doripenem at 25 mg/liter. The addition of doripenem at 25 mg/liter to colistin (0.5 or 2 mg/liter) produced substantial reductions in log10 CFU/ml compared to the equivalent doripenem monotherapy by 8 h (with colistin at 2 mg/liter) and 29 h (with colistin at 0.5 mg/liter) (Fig. 3D). No viable bacteria were detected at ∼50 h with both combinations, with regrowth at 96 h substantially below (by ∼3.5 to 5 log10 CFU/ml) that occurring with equivalent doripenem monotherapy (Fig. 3D).
Emergence of colistin resistance.
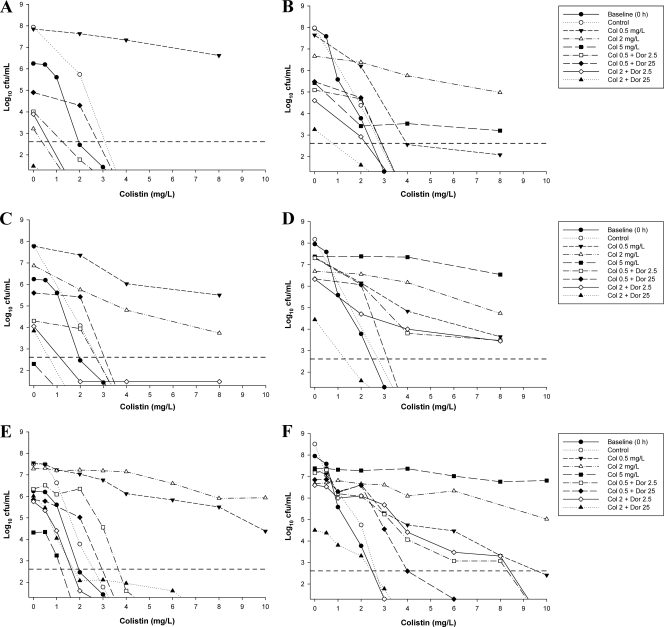
Apart from a small shift to the right from 0 to 96 h at the 106-CFU/ml inoculum, the PAPs for ATCC 27853 at 96 h closely matched those observed at baseline at both inocula. With this strain, a small number of colistin-resistant colonies were detected at baseline at the high inoculum and for both inocula following 96 h of incubation in the model (Table 3). Colistin at 0.5 or 2 mg/liter resulted in substantial increases in the proportion of colistin-resistant subpopulations at both inocula (Fig. 4 and Table 3). With colistin at 5 mg/liter, the substantially lower growth at 96 h (∼4.3 log10 CFU/ml) using an initial inoculum of 106 CFU/ml makes comparison of the PAPs at this time point difficult. However, at the 108-CFU/ml inoculum, a substantial increase in colistin-resistant subpopulations was evident by 24 h with 5-mg/liter colistin monotherapy (Fig. 3 and Table 3). For 19147 n/m, the PAPs at baseline and across the 96 h of incubation did not change, irrespective of the inoculum or colistin treatment (data not shown).
Table 3.
Proportions of colistin-resistant subpopulations of P. aeruginosa ATCC 27853 at various times in the in vitro PK/PD model
| Inoculum (CFU/ml) | Time (h) | Proportion of subpopulations resistant to 4 mg/liter colistin with treatment regimen: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Col at 0.5 mg/liter | Col at 2 mg/liter | Col at 5 mg/liter | Col at 0.5 mg/liter + Dor at 2.5 mg/liter | Col at 0.5 mg/liter + Dor at 25 mg/liter | Col at 2 mg/liter + Dor at 2.5 mg/liter | Col at 2 mg/liter + Dor at 25 mg/liter | ||
| 106 | 0 | NDa | ND | ND | ND | ND | ND | ND | ND |
| 6 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 24 | ND | 3.08 × 10−1 | ND | ND | ND | ND | ND | ND | |
| 48 | ND | 2.82 × 10−1 | ND | ND | ND | ND | 1.12 × 10−3 | ND | |
| 72 | ND | 1.80 × 10−2 | 8.58 × 10−3 | ND | ND | ND | 2.67 × 10−3 | ND | |
| 96 | ND | 3.67 × 10−2 | 7.37 × 10−1 | ND | 1.83 × 10−5 | ND | 1.75 × 10−5 | 8.78 × 10−5 | |
| 108 | 0 | 1.19 × 10−7 | 1.72 × 10−7 | ND | 4.05 × 10−7 | 3.51 × 10−7 | 9.60 × 10−7 | 4.43 × 10−7 | 7.38 × 10−6 |
| 6 | 5.81 × 10−8 | 1.75 × 10−5 | 3.67 × 10−5 | ND | ND | ND | ND | ND | |
| 24 | 1.01 × 10−7 | 8.22 × 10−6 | 1.25 × 10−1 | 1.29 × 10−2 | ND | ND | ND | ND | |
| 48 | 1.83 × 10−7 | 4.90 × 10−3 | 2.65 × 10−1 | 8.74 × 10−1 | 3.70 × 10−6 | 2.51 × 10−5 | 4.57 × 10−5 | ND | |
| 72 | 2.95 × 10−8 | 3.18 × 10−3 | 3.01 × 10−1 | 9.49 × 10−1 | 3.14 × 10−4 | ND | 4.77 × 10−3 | ND | |
| 96 | 8.92 × 10−8 | 3.22 × 10−3 | 2.72 × 10−1 | 9.71 × 10−1 | 7.78 × 10−4 | 5.66 × 10−5 | 6.55 × 10−3 | ND | |
ND, no colistin-resistant subpopulations detected.
Fig. 4.
PAPs of ATCC 27853 with colistin monotherapy, colistin-plus-doripenem combination therapy, or neither antibiotic (control) at the 106-CFU/ml inoculum (left panels) and the 108-CFU/ml inoculum (right panels) at 24 h (A and B), 72 h (C and D), and 96 h (E and F). Baseline (0-h) PAPs are shown in all panels. Colonies growing on ≥4 mg/liter colistin are considered resistant. The y axis starts from the limit of detection, and the limit of quantification (LOQ) is indicated by the horizontal broken line.
Combination therapy against ATCC 27853 substantially reduced the emergence of colistin-resistant subpopulations (Table 3). When doripenem at 2.5 mg/liter was added to colistin (0.5 or 2 mg/liter) at both inocula, a small shift to the right in the PAPs from 72 to 96 h was generally observed (Fig. 4). The emergence of colistin-resistant subpopulations at both inocula was suppressed even further with the addition of doripenem at 25 mg/liter to colistin (0.5 or 2 mg/liter) (Fig. 4). For example, with a starting inoculum of 108 CFU/ml, the combination of colistin at 2 mg/liter plus doripenem at 2.5 mg/liter resulted in substantially fewer colonies growing in the presence of ≥4 mg/liter colistin at 96 h than the equivalent colistin monotherapy (Fig. 4F). The number of resistant colonies was reduced even further with the combination of colistin at 0.5 mg/liter plus doripenem at 25 mg/liter, despite similar levels of growth at this time point with all three regimens. Combination therapy had no effect on colistin resistance of the MDR colistin-resistant isolate (data not shown).
DISCUSSION
Colistin is increasingly used as salvage therapy in critically ill patients for otherwise untreatable MDR infections (15, 27). However, regrowth of colistin-susceptible P. aeruginosa with colistin (or polymyxin B) monotherapy is commonly observed (4, 10, 18, 22, 51), even with colistin concentrations well above those which can be safely achieved clinically. In addition, recent population PK studies employing currently recommended CMS dosage regimens indicate that the plasma colistin concentrations achieved in critically ill patients are in many cases suboptimal (16, 47). Given the potential for the rapid emergence of colistin resistance with monotherapy, combination therapy against P. aeruginosa has been suggested as a possible means by which to increase antimicrobial activity and reduce the development of resistance (29). We systematically investigated the effectiveness of colistin alone and in combination with doripenem against a colistin-heteroresistant strain and an MDR colistin-resistant isolate of P. aeruginosa. Doripenem was chosen because of its high potency against MDR P. aeruginosa (11, 38) and its low potential for selection of carbapenem-resistant P. aeruginosa (19, 37, 49). As some data show that the activity of colistin (10) and carbapenems alone (36) is attenuated at high compared to low inocula, in the present study experiments were conducted at both ∼106 and ∼108 CFU/ml; the latter inoculum mimics the high bacterial densities found in some infections.
The dosage regimens of colistin and doripenem used in the present study were carefully chosen to reflect the plasma drug concentration-time profiles achieved in critically ill patients. Intravenous administration of CMS, the parenteral formulation of colistin, results in average steady-state plasma colistin concentrations of ∼2 to 3 mg/liter, with some patients achieving concentrations up to ∼10 mg/liter (16, 31, 47). As colistin concentrations at steady state remain more or less constant (16, 47), colistin was administered as a constant infusion. We have previously demonstrated that colistin is almost entirely unbound in CAMHB (3). Thus, the colistin concentrations of 0.5 and 2 mg/liter used in our study are clinically achievable, assuming plasma binding of colistin in patients is similar to that in animals (i.e., ∼50% is bound) (25). Unfortunately, although the knowledge of total plasma colistin concentrations achieved in patients is increasing, there is currently no information on unbound plasma colistin concentrations in humans. Though the majority of PK data on doripenem have been obtained with healthy volunteers, plasma drug concentration-time profiles for patients appear to be similar to those for healthy volunteers (39). Doripenem is typically administered intermittently every 8 h, with a standard 500-mg dose achieving a Cmax of ∼25 mg/liter (7, 42). As binding of doripenem in the growth medium was minimal, all doripenem concentrations employed in the combinations are readily achieved in plasma after consideration of protein binding (7, 14, 20, 42).
To our knowledge, this is the first study to investigate the combination of colistin plus doripenem against P. aeruginosa using an in vitro PD model and to utilize colistin PK data recently obtained from critically ill patients (discussed below). An inoculum effect was generally observed for colistin monotherapy, whereas no obvious inoculum effect was present for doripenem (Fig. 2 and 3). The addition of doripenem to colistin resulted in substantial improvements in bacterial killing over equivalent monotherapy against the MDR colistin-resistant isolate at both inocula, particularly with a doripenem concentration of 25 mg/liter. Though the benefits in overall antibacterial activity with the combination were slightly less pronounced against the colistin-susceptible but -heteroresistant strain, combination regimens nevertheless resulted in substantial improvements in bacterial killing, particularly with combinations containing colistin at 2 mg/liter. Overall, our data suggest that the addition of doripenem to even low concentrations of colistin (e.g., 0.5 mg/liter) can substantially improve antibacterial activity. Given the current last-line status of colistin therapy, we have reported not only synergy but also additivity, as even a relatively small increase in activity with clinically achievable concentrations of both antibiotics may be beneficial to patient care.
Previous studies employing static time-kill methods have examined colistin in combination with a carbapenem (imipenem, meropenem, or doripenem) against P. aeruginosa, with mixed results (2, 5a, 12, 43, 44). In these previous studies, investigations were undertaken for no longer than 48 h (usually 24 h) with a single dose of each antibiotic administered at the commencement of treatment. Of these studies, only our previous study employed multiple inocula and investigated the emergence of colistin resistance (5a); that study included both isolates used in the present study. While concentrations of antibiotics in that study and the present study are not directly comparable and the former study examined colistin in combination with imipenem, the activities of colistin combined with either imipenem or doripenem were similar across 48 h (the duration of the former study) at both inocula of ATCC 27853. However, substantial differences against the MDR colistin-resistant isolate were evident. In the static model, combinations with concentrations as high as 32 mg/liter colistin plus imipenem at 16× MIC failed to reduce bacterial numbers to below the limit of detection at any time point. In stark contrast, bacterial eradication was achieved in the PK/PD model with combinations containing colistin (0.5 or 2 mg/liter) and doripenem at 25 mg/liter no later than 24 h at the low inoculum, and bacteria were reduced to below detectable levels at approximately 48 h with the same combinations at the high inoculum. This highlights the importance of simulating PK profiles when examining PD responses.
Though P. aeruginosa can undergo adaptive resistance to polymyxins (17), the report of colistin heteroresistance in P. aeruginosa (5a) and changes in PAPs following treatment with colistin monotherapy (4, 5a, 10) suggest that amplification of preexisting colistin-resistant subpopulations is a contributing factor in the regrowth observed with colistin monotherapy. This pattern was similarly observed in the present study with colistin monotherapy. Though the meaningful interpretation of PAPs is difficult when combination therapy has led to extensive killing, an important finding of the present study is that when bacterial numbers were comparable (within ∼1 to 2 log10 CFU/ml of those achieved with the equivalent monotherapy), combination therapy against the colistin-heteroresistant strain at both inocula substantially reduced and delayed the emergence of colistin-resistant subpopulations. Whereas colistin-resistant colonies emerged rapidly (often within 24 h) with colistin monotherapy, with combination therapy resistant colonies generally emerged later (following 72 to 96 h of treatment) and formed a substantially smaller proportion of the overall bacterial population (Table 3). In addition, the most resistant subpopulations (i.e., those growing in the presence of colistin at 10 mg/liter on the PAP plates) were absent with combination therapy. In contrast, we previously reported that changes in the PAPs with colistin and imipenem combination therapy in a static time-kill model generally mirrored those observed with equivalent exposure to colistin monotherapy (5a). Loss of imipenem due to degradation in the static experiments likely contributed to this result (21). Intermittent dosing of doripenem in the present study replenishes doripenem concentrations and avoids the combination's effectively becoming colistin monotherapy over time. This reported difference highlights once again the importance of PK/PD models in assessing the activity of and the emergence of resistance to antimicrobial therapy.
We have previously suggested two possible reasons for an enhanced PD effect observed with the combination of colistin and a carbapenem (9). Subpopulation synergy involves one drug killing the subpopulation(s) resistant to the other drug and vice versa. ATCC 27853 is colistin heteroresistant, indicating the existence of colistin-resistant subpopulations prior to therapy. Though regrowth of this strain occurred with all combinations, it was considerably reduced with combinations containing each drug at the higher concentration, particularly over the first 48 to 72 h. Interestingly, high-level colistin resistance did not emerge despite the regrowth. While subpopulation synergy may have contributed to an enhanced PD effect against this isolate, it cannot explain the substantially enhanced activity of colistin-doripenem combinations against the MDR colistin-resistant isolate given its near complete resistance to colistin (MIC, 128 mg/liter). This enhanced activity occurred despite the presence of enzymes active against carbapenems. Mechanistic synergy involves colistin and doripenem acting on different cellular pathways to increase the rate or extent of killing by the other drug. It is possible that permeabilization of the outer membrane by colistin (56) resulted in substantially increased concentrations of doripenem in the periplasm, allowing greater access to the critical penicillin-binding proteins located on the cytoplasmic membrane where the carbapenems act (41, 54). Subpopulation and mechanistic synergies are not mutually exclusive, and both may operate simultaneously. Further investigations are ongoing to elucidate the mechanism(s) underpinning the enhanced PD activity observed.
We have shown for the first time that clinically relevant dosage regimens of colistin and doripenem in combination substantially increase killing of both colistin-susceptible (and -heteroresistant) and MDR colistin-resistant P. aeruginosa, even at a high initial inoculum. Combination therapy also substantially reduced and delayed the emergence of colistin resistance. Our data highlight the importance of prospective optimization of colistin combinations using a translational PK/PD approach. Further investigations of colistin combinations in animal infection models and patients are warranted to optimize colistin-doripenem combinations targeting isolates which are resistant to all antibiotics, including the last-line therapy colistin.
ACKNOWLEDGMENTS
The project described herein was supported by award number R01AI079330 from the National Institute of Allergy and Infectious Diseases. D.L.P. has previously received honoraria from Merck for invited lectures and participation in advisory boards. J.L. is an Australian National Health and Medical Research Council Senior Research Fellow.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print on 12 September 2011.
Joint senior authors.
REFERENCES
- 1. Antoniadou A., et al. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J. Antimicrob. Chemother. 59:786–790 [DOI] [PubMed] [Google Scholar]
- 2. Aoki N., et al. 2009. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 63:534–542 [DOI] [PubMed] [Google Scholar]
- 3. Bergen P. J., et al. 2010. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob. Agents Chemother. 54:3783–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergen P. J., et al. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 61:636–642 [DOI] [PubMed] [Google Scholar]
- 5. Bergen P. J., Li J., Rayner C. R., Nation R. L. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a. Bergen P. J., et al. 2011. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob. Agents Chemother. 55:5134–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berlana D., Llop J. M., Fort E., Badia M. B., Jodar R. 2005. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am. J. Health Syst. Pharm. 62:39–47 [DOI] [PubMed] [Google Scholar]
- 7. Bhavnani S. M., Hammel J. P., Cirincione B. B., Wikler M. A., Ambrose P. G. 2005. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob. Agents Chemother. 49:3944–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boucher H. W., et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 9. Bulitta J. B., et al. 2009. Quantifying synergy of colistin combinations against MDR Gram-negatives by mechanism-based models, abstr. A1-573, p. 41. Abstr. 49th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), San Francisco, CA, 12 to 15 September 2009. American Society for Microbiology, Washington, DC. [Google Scholar]
- 10. Bulitta J. B., et al. 2010. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob. Agents Chemother. 54:2051–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castanheira M., Jones R. N., Livermore D. M. 2009. Antimicrobial activities of doripenem and other carbapenems against Pseudomonas aeruginosa, other nonfermentative bacilli, and Aeromonas spp. Diagn. Microbiol. Infect. Dis. 63:426–433 [DOI] [PubMed] [Google Scholar]
- 12. Cirioni O., et al. 2007. Efficacy of tachyplesin III, colistin, and imipenem against a multiresistant Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 51:2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing: 20th informational supplement (M100–S20). CLSI, Wayne, PA. [Google Scholar]
- 14. Crandon J. L., Bulik C. C., Nicolau D. P. 2009. In vivo efficacy of 1- and 2-gram human simulated prolonged infusions of doripenem against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:4352–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falagas M. E., Kasiakou S. K. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333–1341 [DOI] [PubMed] [Google Scholar]
- 16. Garonzik S. M., et al. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilleland H. E., Jr., Champlin F. R., Conrad R. S. 1984. Chemical alterations in cell envelopes of Pseudomonas aeruginosa upon exposure to polymyxin: a possible mechanism to explain adaptive resistance to polymyxin. Can. J. Microbiol. 30:869–873 [DOI] [PubMed] [Google Scholar]
- 18. Gunderson B. W., et al. 2003. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 47:905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huynh H. K., Biedenbach D. J., Jones R. N. 2006. Delayed resistance selection for doripenem when passaging Pseudomonas aeruginosa isolates with doripenem plus an aminoglycoside. Diagn. Microbiol. Infect. Dis. 55:241–243 [DOI] [PubMed] [Google Scholar]
- 20. Ikawa K., et al. 2007. Peritoneal penetration of doripenem after intravenous administration in abdominal-surgery patients. J. Antimicrob. Chemother. 60:1395–1397 [DOI] [PubMed] [Google Scholar]
- 21. Keel R. A., Sutherland C. A., Crandon J. L., Nicolau D. P. 2011. Stability of doripenem, imipenem and meropenem at elevated room temperatures. Int. J. Antimicrob. Agents 37:184–185 [DOI] [PubMed] [Google Scholar]
- 22. Ketthireddy S., et al. 2007. In vivo pharmacodynamics of colistin against Pseudomonas aeruginosa in thighs of neutropenic mice, abstr. A-4, p. 1. Abstr. 47th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), Chicago, IL, 17 to 20 September 2007. American Society for Microbiology, Washington, DC. [Google Scholar]
- 23. Ko K. S., et al. 2007. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J. Antimicrob. Chemother. 60:1163–1167 [DOI] [PubMed] [Google Scholar]
- 24. Li J., et al. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 761:167–175 [DOI] [PubMed] [Google Scholar]
- 25. Li J., et al. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J., Nation R. L., Milne R. W., Turnidge J. D., Coulthard K. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 25:11–25 [DOI] [PubMed] [Google Scholar]
- 27. Li J., et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601 [DOI] [PubMed] [Google Scholar]
- 28. Li J., et al. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lister P. D., Wolter D. J., Hanson N. D. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livermore D. M. 2004. The need for new antibiotics. Clin. Microbiol. Infect. 10(Suppl. 4):1–9 [DOI] [PubMed] [Google Scholar]
- 31. Markou N., et al. 2008. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin. Ther. 30:143–151 [DOI] [PubMed] [Google Scholar]
- 32. Matthaiou D. K., et al. 2008. Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: a matched case-control study. Crit. Care Med. 36:807–811 [DOI] [PubMed] [Google Scholar]
- 33. Matthews S. J., Lancaster J. W. 2009. Doripenem monohydrate, a broad-spectrum carbapenem antibiotic. Clin. Ther. 31:42–63 [DOI] [PubMed] [Google Scholar]
- 34. Michalopoulos A., Kasiakou S. K., Rosmarakis E. S., Falagas M. E. 2005. Cure of multidrug-resistant Acinetobacter baumannii bacteraemia with continuous intravenous infusion of colistin. Scand. . J. Infect. Dis. 37:142–145 [DOI] [PubMed] [Google Scholar]
- 35. Michalopoulos A. S., Karatza D. C. 2010. Multidrug-resistant Gram-negative infections: the use of colistin. Expert Rev. Anti Infect. Ther. 8:1009–1017 [DOI] [PubMed] [Google Scholar]
- 36. Mizunaga S., Kamiyama T., Fukuda Y., Takahata M., Mitsuyama J. 2005. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 56:91–96 [DOI] [PubMed] [Google Scholar]
- 37. Mushtaq S., Ge Y., Livermore D. M. 2004. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Antimicrob. Agents Chemother. 48:3086–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mutters R., et al. 2009. Comparative susceptibility of European Gram-negative rods to doripenem, imipenem and meropenem. The Comparative Activity of Carbapenem Testing Study (COMPACT), abstr. P1034. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. (ECCMID), Helsinki, Finland, 16 to 19 May 2009. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 39. Nandy P., Samtani M. N., Lin R. 2010. Population pharmacokinetics of doripenem based on data from phase 1 studies with healthy volunteers and phase 2 and 3 studies with critically ill patients. Antimicrob. Agents Chemother. 54:2354–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nation R. L., Li J. 2009. Colistin in the 21st century. Curr. Opin. Infect. Dis. 22:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nicolau D. P. 2008. Carbapenems: a potent class of antibiotics. Expert Opin. Pharmacother. 9:23–37 [DOI] [PubMed] [Google Scholar]
- 42. Ortho-McNeil-Janssen Pharmaceuticals. 2007. Doribax (doripenem for injection) package insert. Ortho-McNeil-Janssen Pharmaceuticals, Inc., Raritan, NJ. [Google Scholar]
- 43. Pankuch G. A., Lin G., Seifert H., Appelbaum P. C. 2008. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:333–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pankuch G. A., Seifert H., Appelbaum P. C. 2010. Activity of doripenem with and without levofloxacin, amikacin, and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 67:191–197 [DOI] [PubMed] [Google Scholar]
- 45. Paterson D. L. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43(Suppl. 2):S43–S48 [DOI] [PubMed] [Google Scholar]
- 46. Pillai S. K., Moellering R. C., Eliopoulos G. M. 2005. Antimicrobial combinations, p. 365–440. In Lorian V. (ed.), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 47. Plachouras D., et al. 2009. Population pharmacokinetic analysis of colistin methanesulphonate and colistin after intravenous administration in critically ill patients with gram-negative bacterial infections. Antimicrob. Agents Chemother. 53:3430–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poudyal A., et al. 2008. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 62:1311–1318 [DOI] [PubMed] [Google Scholar]
- 49. Sakyo S., Tomita H., Tanimoto K., Fujimoto S., Ike Y. 2006. Potency of carbapenems for the prevention of carbapenem-resistant mutants of Pseudomonas aeruginosa: the high potency of a new carbapenem doripenem. J. Antibiot. (Tokyo) 59:220–228 [DOI] [PubMed] [Google Scholar]
- 50. Talbot G. H., et al. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657–668 [DOI] [PubMed] [Google Scholar]
- 51. Tam V. H., et al. 2005. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3624–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan C. H., Li J., Nation R. L. 2007. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 51:3413–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turnidge J. D., Bell J. M., Jones R. N. 2007. Emergence of colistin-resistant Klebsiella spp. and Enterobacter spp. in the Asia-Pacific region: a SENTRY antimicrobial surveillance program report, abstr. C2-2054, p. 148.Abstr. 47th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), Chicago, IL, 17 to 20 September 2007. American Society for Microbiology, Washington, DC. [Google Scholar]
- 54. Yang Y., Bhachech N., Bush K. 1995. Biochemical comparison of imipenem, meropenem and biapenem: permeability, binding to penicillin-binding proteins, and stability to hydrolysis by beta-lactamases. J. Antimicrob. Chemother. 35:75–84 [DOI] [PubMed] [Google Scholar]
- 55. Yau W., et al. 2009. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. . J. Infect. 58:138–144 [DOI] [PubMed] [Google Scholar]
- 56. Zhang L., Dhillon P., Yan H., Farmer S., Hancock R. E. 2000. Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3317–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]