Abstract
Although vancomycin is often regarded as an agent that concentrates poorly in the lower respiratory tract, as determined from concentrations in epithelial lining fluid (ELF), few data are available. This study sought to determine the profile of vancomycin exposure in the ELF relative to plasma. Population modeling and Monte Carlo simulation were employed to estimate the penetration of vancomycin into ELF. Plasma and ELF pharmacokinetic (PK) data were obtained from 10 healthy volunteers. Concentration-time profiles in plasma and ELF were simultaneously modeled using a three-compartment model with zero-order infusion and first-order elimination and transfer using the big nonparametric adaptive grid (BigNPAG) program. Monte Carlo simulation with 9,999 subjects was performed to calculate the ELF/plasma penetration ratios by estimating the area under the concentration-time curve (AUC) in ELF (AUCELF) and plasma (AUCplasma) after a single simulated 1,000-mg dose. The mean (standard deviation) AUCELF/AUCplasma penetration ratio was 0.675 (0.677), and the 25th, 50th, and 75th percentile penetration ratios were 0.265, 0.474, and 0.842, respectively. Our results indicate that vancomycin penetrates ELF at approximately 50% of plasma levels. To properly judge the adequacy of current doses and schedules employed in practice, future studies are needed to delineate the PK/PD (pharmacodynamics) target for vancomycin in ELF. If the PK/PD target in ELF is found to be consistent with the currently proposed target of an AUC/MIC of ≥400, suboptimal probability of target attainment would be expected when vancomycin is utilized for pneumonias due to MRSA (methicillin-resistant Staphylococcus aureus) with MICs in excess of 1 mg/liter.
INTRODUCTION
Ventilator-associated pneumonia (VAP) continues to be a major source of morbidity and mortality among intensive care unit (ICU) patients (1, 17). In many institutions, methicillin-resistant Staphylococcus aureus (MRSA) accounts for >25% of all VAPs (1, 8). Because MRSA has become such a likely culprit, vancomycin is recommended as first-line empirical therapy for patients with suspected or documented VAP (1, 20). However, several trials have demonstrated suboptimal therapeutic outcomes with this agent against MRSA (7, 19, 21).
The low therapeutic success rates have been associated with a number of factors, including MIC creep, agr phenotype, and emergence of MRSA strains with heteroresistance to vancomycin (20). Poor penetration of vancomycin into the site of infection, as determined from concentrations in epithelial lining fluid (ELF), is also considered a major contributing factor of failure (1, 20). Although low concentration in the lungs is often cited as an attributable factor, the penetration of vancomycin into the ELF remains poorly defined, and the available data on its penetration have notable limitations (3, 6, 9).
The intent of this study was to determine the pharmacokinetics of vancomycin in plasma and ELF among healthy subjects. Population pharmacokinetic (PK) modeling and Monte Carlo simulation were used to estimate the range of ELF concentration-time profiles (exposures) relative to those for plasma, as measured by the ratio of the area under the concentration-time curve (AUC) in the ELF to the AUC in the plasma (AUCELF/AUCplasma ratio).
MATERIALS AND METHODS
Patient population.
Data were collected from 10 healthy, nonsmoking adults ≥18 years of age at presumed steady state for vancomycin pharmacokinetics. Only subjects with clinical laboratory parameters assessed as normal and weight within ±10% of their acceptable range of weight according to the Metropolitan Life Insurance Company were included. Patients were excluded if they had a history or presence of renal impairment (serum creatinine > 2.0 mg/dl and calculated creatinine clearance < 50 ml/min). The study was approved by the Institutional Review Board, and all subjects gave written informed consent.
Pharmacokinetic study design and ELF and blood sampling schedule.
Subjects enrolled in the study received nine doses of 1,000 mg vancomycin intravenously every 12 h (Vancocin HCl [5]). After the ninth dose, subjects underwent one standardized bronchoscopy and bronchoalveolar lavage (BAL) at either 4 or 12 h following the start of the last intravenous infusion of antibiotic to obtain an ELF sample. The 4-h sampling time was selected to approximate the maximum (peak) intrapulmonary concentration, whereas the 12-h sampling time represents the minimum (trough) concentration of vancomycin.
Two percent topical lidocaine was applied to the upper airway to prepare subjects for bronchoscopy. If needed, 1% lidocaine was used in the lower airway. A fiber optic bronchoscope (Olympus P-10; Olympus America Inc., Melville, NY) was inserted into a subsegment of the right middle lobe. The bronchoscope was in place for an average of 5.7 min (range, 4 to 10 min). Four 50-ml aliquots of sterile 0.9% normal saline were instilled into the middle lobe, and each specimen was immediately aspirated and placed in ice. The aspirate from the first 50-ml instillation (BAL fluid 1) was collected separately and discarded because significant contamination with cells from the proximal airways has been reported. The aspirates recovered from the second, third, and fourth instillations were pooled (BAL fluid 2). The volume of BAL fluid 2 was measured and recorded. A 4-ml aliquot was removed from BAL fluid 2 and immediately sent to the laboratory for cell count and differential. The remaining volume of BAL fluid 2 was immediately centrifuged at 400 × g for 5 min. The supernatant and cells were separated and frozen at −70°C until the assays were performed. A single aliquot of supernatant was separated and frozen for the urea assay.
A blood sample to determine drug and urea concentrations were obtained just prior to the scheduled bronchoscopy. In addition, blood samples to determine vancomycin concentrations were obtained prior to the 9th dose and at hours 1 (end of infusion), 1.5, 2, 4, 4.5, 12, and 24 after the 9th dose.
Vancomycin and urea assay.
Vancomycin concentrations in plasma and BAL fluid were quantified by a validated liquid chromatography-tandem mass spectrometry assay. The lower limit of quantification for both plasma and BAL fluid samples was 10 ng/ml. The response from calibration standards was linear from 10 ng/ml to 1,000 ng/ml, and the coefficient of correlation for all measured sequences was at least 0.99. The interday precision and analytical recovery of the vancomycin assay during samples analysis ranged from 4.5 to 6.8% and from 96.8 to 102.8%, respectively. Similar to plasma, the lower limit of quantification for BAL fluid samples was 10 ng/ml. The response from calibration standards was linear from 10 ng/ml to 1,000 ng/ml, and the coefficient of correlation for all measured sequences was at least 0.99. The interday precision and analytical recovery of the vancomycin assay during samples analysis ranged from 2.1 to 4.1% and from 100 to 105.4%, respectively.
Concentrations of urea in plasma and BAL fluid were performed with a commercially available assay kit (urea nitrogen procedure no. 640; Sigma Diagnostics, St. Louis, MO) and measured on a Spectronic 70 spectrophotometer (Analytical Systems Division, Bausch and Lomb). The response from the calibration standards for plasma was linear from 1.50 to 7.50 mg/dl, and the coefficient of correlation for all measured sequences was at least 0.999. The response from the calibration standards for BAL fluid was linear from 0.113 to 4.50 mg/dl, and the coefficient of correlation for all measured sequences was at least 0.999. For both plasma and BAL fluid samples, the coefficient of variation was less than 5%, and relative accuracy was between 96.6 to 112.6%.
Determination of vancomycin concentration in ELF.
To quantify ELF volume recovered by BAL fluid, urea was used as an endogenous marker of ELF. The concentration of vancomycin in ELF (VELF) was determined as follows: VELF = VBAL·BALV/ELFV, where VBAL is the measured vancomycin concentration in the BAL fluid, BALV is the volume aspirated, and ELFV is the volume of ELF sampled by the BAL fluid. ELFV is derived from the following equation: ELFV = BALV·UREAbal/UREAser, where UREABAL is the concentration of urea in BAL fluid (mg/ml) and UREAser is the concentration of urea in serum (mg/ml).
Population pharmacokinetic modeling.
All data were analyzed in a population pharmacokinetic model using the big nonparametric adaptive grid (BigNPAG) with adaptive γ program of Leary et al. (10). The pharmacokinetic model was parameterized as a three-compartment model with zero-order infusion into the central compartment. A three-compartment model with zero-order infusion was selected based on Akaike's information criterion and rule of parsimony (22). Elimination from the central compartment and all intercompartmental distribution processes were modeled as first-order processes.
The general differential equations for the model are as follows: dX(1)/dt = R(t) − [(CL/V) + K12+ K13]·X(1) + K21·X(2) + K31·X(3); dX(2)/dt = K12·X(1) − K21·X(2); dX(3)/dt = K13·X(1) − K31·X(3), where X(1) is the amount of drug in the central compartment (in milligrams), X(2) is the amount of drug in the peripheral compartment (in milligrams), X(3) is the amount of drug in the ELF compartment (in milligrams), CL is clearance from the central compartment (liters per hour), K12, K21, K13, and K31 are first-order intercompartmental transfer rate constants (in hour−1), V is a scalar and represents the volume of the central compartment (in liters), and R(t) is the time-delimited zero-order drug input rate (piecewise input function) into the central compartment (in milligrams per hour). Not shown is VOLELF, which is a scalar term and is the apparent volume of ELF.
The inverse of the estimated assay variance was used as the first estimate for weighting in the pharmacokinetic modeling. Weighting was accomplished by making the assumption that total observation variance was proportional to assay variance. Assay variance was determined on a between-day basis. When convergence was attained, Bayesian estimates for each patient were obtained using the BigNPAG “population of one” utility. The mean, median, and modal values were employed as measures of central tendency for the population parameter estimates and were evaluated in the Bayesian analysis. Scatter plots were examined for individual patients and for the population as a whole. Goodness of fit was assessed by regression with an observed-predicted plot, coefficients of determination, and log likelihood values. Predictive performance evaluation was based on weighted mean error and the bias-adjusted, weighted, mean-squared error.
Monte Carlo simulation.
The mean parameter vector and the major diagonal from the population pharmacokinetic model were embedded in subroutine PRIOR of D'Argenio and Schumitzky's ADAPT II software package (4). The full covariance matrix could not be employed because it did not have symmetric positive definite properties. The population-simulation-without-process-noise option was employed. A Monte Carlo simulation with 9,999 subjects was performed and was used to calculate the ELF/plasma mean and median penetration ratios by estimating the AUCELF and total AUCplasma from zero to infinity (AUCELF,0-∞ and AUCplasma,0-∞) after a single simulated 1,000-mg dose. This is mathematically equivalent to a single dosing interval at steady-state concentrations.
The AUCELF/AUCplasma penetration ratio derived from the mean parameter vector from the population model was also calculated. Both normal and log-normal distributions were evaluated, and these were discriminated on their ability to recreate the mean parameter vector and corresponding standard deviations from the population model.
Monte Carlo simulation was also used to evaluate the predictive performance of the population pharmacokinetic model. The mean parameter vector values were used to simulate the steady-state concentration-time profile of 1,000 mg vancomycin intravenously (IV) every 12 h (q12h) in plasma and ELF. The fidelity with which the concentration-time curves mirrored the raw data was assessed by visual inspection.
RESULTS
Vancomycin pharmacokinetics in plasma and ELF were evaluated in 10 healthy adults (five females and five males). The median age was 24 years (range, 20 to 39 years), and median weight was 84.3 kg (range, 57 to 101 kg). Vancomycin concentrations from 80 plasma samples and 10 BAL fluid samples were available for analysis, and the population parameter estimates are provided in Table 1. Using the population mean parameter values as the measure of central tendency, the overall fit of the model to the data was good, and the observed-predicted plots for plasma and ELF after the Bayesian step were highly acceptable. For plasma, r2 was 0.976, and the observed-predicted plot showed a best-fit regression line of observed = (1.016·predicted) + 0.299. For ELF, r2 was 0.998, and the observed-predicted plot showed a best-fit regression line of observed = (1.021·predicted) − 0.600. Bayesian posterior density results and AUCELF,0-∞/AUCplasma,0-∞ penetration ratios for the 10 subjects included in the analysis are provided in Table 2. The mean (standard deviation) AUCELF,0-∞/AUCplasma,0-∞ penetration ratio for the 10 subjects was 0.85 (1.39). However, this average was skewed because of an outlier value of 4.77. If this value is removed, the mean (standard deviation) AUCELF,0-∞/AUCplasma,0-∞ penetration ratio for the remaining nine subject is 0.41 (0.17).
Table 1.
Overall estimated pharmacokinetic parameter values for vancomycin
| Value type | Vc (liters) | SCL (liters/h) | K12 (h−1) | K21 (h−1) | K13 (h−1) | K31 (h−1) | VELF (liters) |
|---|---|---|---|---|---|---|---|
| Mean | 8.902 | 6.086 | 0.897 | 0.484 | 6.042 | 10.363 | 11.501 |
| Median | 9.070 | 6.220 | 0.872 | 0.318 | 6.181 | 8.032 | 10.398 |
| SD | 2.057 | 1.643 | 0.490 | 0.571 | 3.322 | 4.494 | 5.778 |
Vc, volume of the central compartment; K12, K21, K13, and K31, first-order intercompartmental transfer rate constants; SCL, serum clearance; VELF, volume of ELF.
Table 2.
Bayesian posterior density results and AUCELF,0-∞/AUCplasma,0-∞ penetration ratios for 10 subjects included in the analysis
| Subject | Vc (liters) | SCL (liters/h) | K12 (h−1) | K21 (h−1) | K13 (h−1) | K31 (h−1) | VELF (liters) | ELF/plasma penetration ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 10.12 | 4.404 | 0.34 | 0.22 | 3.77 | 5.65 | 20.49 | 0.33 |
| 2 | 5.51 | 8.64 | 1.72 | 2.19 | 11.00 | 5.90 | 2.15 | 4.77 |
| 3 | 9.09 | 6.84 | 1.00 | 0.28 | 6.36 | 15.98 | 5.82 | 0.62 |
| 4 | 11.57 | 6.41 | 0.98 | 0.38 | 6.31 | 15.03 | 7.62 | 0.64 |
| 5 | 11.72 | 6.25 | 0.90 | 0.35 | 3.72 | 15.81 | 10.38 | 0.27 |
| 6 | 6.07 | 9.22 | 0.37 | 0.25 | 12.50 | 8.19 | 15.72 | 0.59 |
| 7 | 9.02 | 4.78 | 0.76 | 0.30 | 2.52 | 7.57 | 12.46 | 0.24 |
| 8 | 9.02 | 4.79 | 0.76 | 0.30 | 2.52 | 7.58 | 12.46 | 0.24 |
| 9 | 10.09 | 4.40 | 0.34 | 0.21 | 3.80 | 5.72 | 20.40 | 0.33 |
| 10 | 6.79 | 5.11 | 1.77 | 0.36 | 7.88 | 16.17 | 7.48 | 0.44 |
Vc, volume of the central compartment; K12, K21, K13, and K31, first-order intercompartmental transfer rate constants; SCL, serum clearance; VELF, volume of ELF.
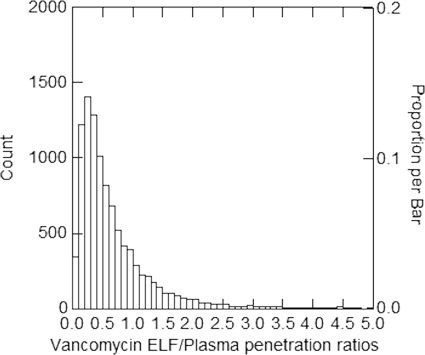
A 9,999-subject Monte Carlo simulation was performed and log-normal distributions were selected based on their abilities to recapitulate the original mean parameter values and corresponding standard deviations. The distribution of AUCELF,0-∞/AUCplasma,0-∞ penetration ratios after a single 1,000-mg dose is shown in Fig. 1. The mean (standard deviation) AUCELF/AUCplasma penetration ratio was 0.675 (0.677). The median AUCELF/AUCplasma penetration ratio was 0.474, and the 25th and 75th percentile ratios were 0.265 and 0.842, respectively. The 10th and 90th percentile ratios were 0.160 and 1.398, respectively. The average value for the Monte Carlo simulation is skewed because of outliers, as is evident when one examines the distribution of AUCELF/AUCplasma penetration ratios (Fig. 1), the median penetration ratio of 0.474, the mean ratio of 0.675, and standard deviation of 0.677. The AUCELF/AUCplasma penetration ratio derived from the mean parameter vector from the population model was 0.451.
Fig. 1.
Distribution of AUCELF,0-∞/AUCplasma,0-∞ penetration ratios after a single 1,000-mg dose.
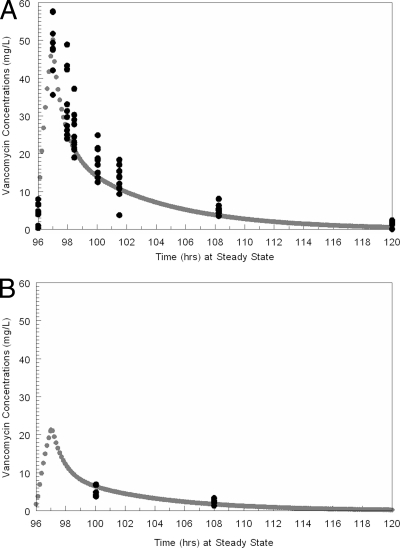
The simulated concentration-time profile of 1,000 mg vancomycin IV q12h from the mean parameter vector values in plasma and ELF after the 9th and last dose are displayed in Fig. 2. The simulated plasma and ELF concentration-time curves mirrored the central tendency of the raw data reasonably well. The vast majority of the data points were evenly distributed around the concentration-time curves in plasma and ELF.
Fig. 2.
Simulated concentration-time profile of 1,000 mg vancomycin IV q12h from the mean parameter vector values in plasma (A) and ELF (B). Black circles are the measured vancomycin concentrations, and the gray line and circles are the pharmacokinetic simulated fit of the concentration-time profile.
DISCUSSION
Despite the widespread use of vancomycin for MRSA VAP, information on its penetration into the ELF is limited. An extensive literature search yielded only three studies which attempted to quantify the exposure profile of vancomycin in the lung in relation to plasma (3, 6, 9). While informative, these studies had notable limitations. Cruciani et al. (3) determined vancomycin concentrations using lung biopsy specimen measurements, which have been shown to be poor markers of lung penetration (2). In the other two analyses, vancomycin penetration was quantified by reporting the ELF-to-plasma concentration ratios at different time points (6, 9). Obtaining ratios of drug concentrations in ELF to drug concentrations determined simultaneously in plasma is problematic due to system hysteresis. In other words, as the drug penetrates from plasma to ELF, these ratios will change as a function of time. As such, estimates of drug penetration based on measurements at a single point in time will strongly depend on the sampling time.
Population pharmacokinetic modeling overcomes this limitation because of its ability to estimate population pharmacokinetics and their associated dispersions for subjects with minimal sampling times. Once the population pharmacokinetics are estimated, Monte Carlo simulation can then be performed to estimate the ability of a drug to penetrate the site of infection and to characterize its AUC at that site. Our results indicate that vancomycin penetrates ELF at approximately 50% of plasma levels, as defined by the AUCELF/AUCplasma ratio. However, considerable variability in penetration was observed. The variability is quite impressive if one considers that a relatively homogenous, noninfected population was studied.
Interestingly, given that plasma protein binding of vancomycin is around 50%, the total AUC for the drug in the ELF approximated what the free or unbound AUC is expected to be in plasma for most patients. This similarity between free-drug plasma AUC and ELF AUC has been noted before with other agents in the glycopeptide family. In our evaluation of telavancin concentrations in ELF, total AUC in ELF approximated the AUC for the free drug in plasma (AUCELF/free AUCplasma penetration ratio [mean ± standard deviation] was 1.01 ± 0.96) (11). Further studies are needed to better quantify the relationship between protein binding and penetration into ELF, but these results suggest that protein binding studies may offer a simple method to estimate concentrations in the site of infection in the absence of quantitative ELF concentration determinations.
Since it is well established that the efficacy of an antibiotic regimen depends largely on its penetration of the infection site, our findings have important implications for practice. Given the distribution of exposure profiles observed in our analysis, our findings highlight the importance of delineating the proper ELF exposure targets for optimal killing and suppression of resistance. The recently published guidelines on therapeutic monitoring of vancomycin advocate an AUC/MIC target of ≥400 for serious MRSA infections, including pneumonia, based in part on animal studies and limited human data (20). For MRSA VAP, the best data available are from a retrospective evaluation of patients with Staphylococcus aureus in a community hospital over a 1-year period (16). There were only a small number of MRSA isolates in the database, and a number of the patients had combination agent chemotherapy. Nonetheless, a number of different analyses identified AUC/MIC ratios of 350 to 400 (total drug) as being related to clinical outcome for patients with staphylococcal nosocomial pneumonia, and this is consistent with in vitro and animal model studies. Although this target is supported from the best available data to date, this pharmacodynamic (PD) index is based entirely on total plasma concentrations (16). We are unaware of any study that has attempted to delineate the PK/PD target in the ELF for vancomycin. Since concentrations in ELF are likely to vary greatly between patients, our findings indicate that further data are sorely needed in order to properly judge the adequacy of current doses and schedules employed in practice.
If the target in ELF is found to be consistent with plasma (AUC/MIC of 400), as observed with other drugs (18), it is unlikely that utilizing vancomycin for pneumonia would be an optimal choice for MRSA infections with MICs of >1 mg/liter. Previous analyses have demonstrated that vancomycin regimens currently used in practice have a low probability of achieving an AUC/MIC ratio of 400 for MICs in excess of 1 mg/liter (15). More intensive dosing schemes may increase the probability of achieving the target. However, it may not be possible to increase the dose without subjecting patients to an unacceptable risk of vancomycin-related toxicities (12, 13). Since vancomycin concentrates to a lesser extent in ELF than in plasma, it is reasonable to infer that probability of target attainment will be limited in ELF, especially in infections with organisms for which MICs are in excess of 1 mg/liter.
A number of limitations to our study exist and should be noted. Our study included only healthy volunteers. Relative to healthy volunteers, infected patients tend to have greater variability in pharmacokinetic parameters and distribution of exposure profiles. Because Monte Carlo simulation explicitly creates a distribution of pharmacokinetic parameters from the embedded population pharmacokinetic model, limited variation surrounding pharmacokinetic parameters will lead to a narrow exposure profile distribution in the resultant Monte Carlo simulation for a given dosing scheme. Of note, considerable variability in pharmacokinetic parameters was observed in our analysis (Tables 1 and 2). Hence, our analysis may be more reflective of the distribution of exposures seen in clinical practice relative to most healthy volunteer pharmacokinetic studies. Since our patients were not infected, our findings are likely conservative estimates, as active infection promotes tissue penetration, at least during the early phases. In a population of patients with VAP, it is probable that these estimates of penetration ratios would be higher. An additional limitation of our study is that sampling of ELF was conducted at just two time points. While the major advantage of population pharmacokinetic modeling is the ability to estimate population pharmacokinetics and their associated dispersions for subjects with sparse sampling times (14), future studies should include infected patients with a more robust sampling scheme. Given the difficulty in obtaining more than one ELF sample in a patient, future studies should consider a greater distribution of sampling times during a dosing interval. Finally, we did not evaluate the relationship between protein binding and penetration into ELF. Most likely, the low protein binding that is often observed in critically ill patients will facilitate greater penetration in the ELF. Future studies should be done to quantify the relationship between protein binding and ELF concentrations to evaluate the effect of protein binding on lung penetration.
In conclusion, we found that vancomycin penetrates the ELF at approximately 50% of plasma levels. Given the distribution of exposure profiles observed in our study and the importance of concentrations at the site of infection, future studies should delineate the pharmacokinetic/pharmacodynamic target in ELF to put the results in proper perspective. If the pharmacokinetic/pharmacodynamic target is found to be consistent with the currently proposed target of an AUC/MIC ≥400, suboptimal pharmacodynamic target attainment would be expected when vancomycin is being used for MRSA pneumonias with MICs in excess of 1 mg/liter. Since this study included only healthy volunteers and limited sampling, further ELF pharmacokinetic studies should include infected patients and more robust sampling early in the dosing scheme to validate the external validity of our findings.
Footnotes
Published ahead of print on 12 September 2011.
REFERENCES
- 1. American Thoracic Society 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171: 388–416 [DOI] [PubMed] [Google Scholar]
- 2. Cars O., Ogren S. 1985. Antibiotic tissue concentrations: methodological aspects and interpretation of results. Scand. J. Infect. Dis. Suppl. 44: 7–15 [PubMed] [Google Scholar]
- 3. Cruciani M., et al. 1996. Penetration of vancomycin into human lung tissue. J. Antimicrob. Chemother. 38: 865–869 [DOI] [PubMed] [Google Scholar]
- 4. D'Argenio D. Z., Schumitzky A. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput. Programs Biomed. 9: 115–134 [DOI] [PubMed] [Google Scholar]
- 5. Eli Lilly Company 2001. Vancocin® HCl prescribing information. Eli Lilly and Company, Indianapolis, IN [Google Scholar]
- 6. Georges H., et al. 1997. Pulmonary disposition of vancomycin in critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 16: 385–388 [DOI] [PubMed] [Google Scholar]
- 7. Kollef M. H., Rello J., Cammarata S. K., Croos-Dabrera R. V., Wunderink R. G. 2004. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 30: 388–394 [DOI] [PubMed] [Google Scholar]
- 8. Kollef M. H., et al. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128: 3854–3862 [DOI] [PubMed] [Google Scholar]
- 9. Lamer C., et al. 1993. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37: 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leary R., Jelliffe R., Schumitzky A., van Guilder M. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p. 389–394. Proceedings, Fourteenth IEEE Symposium on Computer-Based Medical Systems 26–27 July 2001, Bethesda, MD [Google Scholar]
- 11. Lodise T. P., Jr., Gotfried M., Barriere S., Drusano G. L. 2008. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 52: 2300–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lodise T. P., Lomaestro B., Graves J., Drusano G. L. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52: 1330–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lodise T. P., Patel N., Lomaestro B. M., Rodvold K. A., Drusano G. L. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49: 507–514 [DOI] [PubMed] [Google Scholar]
- 14. Mahmood I., Duan J. 2009. Population pharmacokinetics with a very small sample size. Drug Metabol Drug Interact. 24: 259–274 [DOI] [PubMed] [Google Scholar]
- 15. Mohr J. F., Murray B. E. 2007. Point: Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 44: 1536–1542 [DOI] [PubMed] [Google Scholar]
- 16. Moise-Broder P. A., Forrest A., Birmingham M. C., Schentag J. J. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43: 925–942 [DOI] [PubMed] [Google Scholar]
- 17. Muscedere J. G., Martin C. M., Heyland D. K. 2008. The impact of ventilator-associated pneumonia on the Canadian health care system. J. Crit. Care. 23: 5–10 [DOI] [PubMed] [Google Scholar]
- 18. Rodvold K. A., et al. 2009. Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob. Agents Chemother. 53: 3294–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubinstein E., Cammarata S., Oliphant T., Wunderink R. 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin. Infect. Dis. 32: 402–412 [DOI] [PubMed] [Google Scholar]
- 20. Rybak M., et al. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66: 82–98 [DOI] [PubMed] [Google Scholar]
- 21. Wunderink R. G., et al. 2008. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest 134: 1200–1207 [DOI] [PubMed] [Google Scholar]
- 22. Yamaoka K., Nakagawa T., Uno T. 1978. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet Biopharm. 6: 165–175 [DOI] [PubMed] [Google Scholar]