Abstract
Posaconazole prophylaxis has proven highly effective in preventing invasive fungal infections, despite relatively low serum concentrations. However, high tissue levels of this agent have been reported in treated patients. We therefore hypothesized that the intracellular levels of antifungal agents are an important factor in determining the success of fungal prophylaxis. To examine the effect of host cell-associated antifungals on the growth of medically important molds, we exposed cells to antifungal agents and removed the extracellular drug prior to infection. Epithelial cells loaded with posaconazole and its parent molecule itraconazole, but not other antifungals, were able to inhibit fungal growth for at least 48 h and were protected from damage caused by infection. Cell-associated posaconazole levels were 40- to 50-fold higher than extracellular levels, and the drug was predominantly detected in cellular membranes. Fungistatic levels of posaconazole persisted within epithelial cells for up to 48 h. Therefore, the concentration of posaconazole in mammalian host cell membranes mediates its efficacy in prophylactic regimens and likely explains the observed discrepancy between serum antifungal levels and efficacy.
INTRODUCTION
In the past 2 decades, rates of invasive fungal infections (IFI) in high-risk hematology patients have increased significantly and remain associated with a high rate of mortality (2, 11, 12, 22, 30). This trend has led to renewed interest in prophylactic antifungal strategies to prevent the development of IFI. The most recent prophylactic strategies that have been evaluated are the use of oral formulations of the new broad-spectrum triazoles voriconazole and posaconazole, which have been the subject of four randomized clinical trials. Both triazoles have excellent antifungal activity in vitro; however, there are important pharmacokinetic differences between the two agents in vivo. Voriconazole is a relatively polar molecule, and it concentrates minimally within host cells (1, 5, 8). Serum levels of voriconazole between 1 μg/ml and 5 μg/ml have been reported to correlate well with efficacy both in the treatment of IFI (3, 23–25) and in prophylaxis (31). In contrast, posaconazole is significantly more lipophilic and while its serum levels are lower than those reported with voriconazole, posaconazole has been reported to concentrate to high levels within cells (up to 40- to 50-fold) (5). Despite lower serum levels (Cavg, 0.5 to 1 μg/ml) (17), posaconazole was effective in reducing IFI in two randomized clinical trials of antifungal prophylaxis (6, 7, 28). Of note, the correlation between serum levels and efficacy of posaconazole in antifungal prophylaxis has been the subject of some debate (7, 16).
In light of these observations, we hypothesized that cellular accumulation of antifungal agents may be an important determinant of efficacy in antifungal prophylaxis. The majority of mold infections in immunocompromised patients are acquired by inhalation of fungal conidia or spores. These inhaled fungal elements are believed to be rapidly phagocytosed by pulmonary macrophages or endocytosed by pulmonary epithelial cells, where they must germinate and elongate before emerging from the cell into the serum or extracellular compartment (29). Thus, partitioning of an antifungal agent to the cellular compartment could be an effective mechanism to prevent the initiation of infection by maximizing the early exposure of infecting organisms to antifungals.
To test this hypothesis, we examined the effects of antifungal drug exposure on pulmonary epithelial cell and macrophage resistance to fungal infection in vitro. We found that host cells exposed to posaconazole but not voriconazole were resistant to fungal infection and injury for up to 48 h after antifungal exposure. Further, we determined that posaconazole was concentrated largely within membranes of host cells, rather than the cytosol, leading to extremely high levels of this agent within the host cell membrane. This membrane concentration of posaconazole explains the enhancement of cellular resistance to infection. Collectively, these results may suggest a need to reevaluate therapeutic drug monitoring of posaconazole during prophylaxis and the need to develop a method to measure cellular or membrane levels of this antifungal agent.
MATERIALS AND METHODS
Cell line.
Pulmonary epithelial cells (A549) and macrophages (RAW 264.7) were obtained from ATCC. A549 and RAW 264.7 cells were grown in vitro using F12 Kaighn's (HyClone)/RPMI 1640 (Wisent) medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, respectively. Cells were grown on tissue culture-treated 100-mm dishes, sterile coverslips, and 6- and 24-well dishes as appropriate.
Drug preparation.
Itraconazole (Sigma-Aldrich, Canada), posaconazole (Merck Canada), and voriconazole (Pfizer) were diluted in dimethyl sulfoxide (DMSO), while amphotericin B deoxycholate (Sigma-Aldrich, Canada), liposomal amphotericin B (Astellas, Canada) and caspofungin (Merck Canada) were diluted in sterile deionized H2O. Fresh dilutions were made from these stock solutions just prior to the experiment and diluted further in RPMI 1640 buffered with morpholinepropanesulfonic acid (MOPS) and F12 Kaighn's complete growth medium for use in cell culture experiments. A control stock containing DMSO but without antifungals was also prepared and used in all experiments as a solvent control.
Strains.
Aspergillus fumigatus strain AF293 (a generous gift from P. T. Magee) was used for our initial studies. Clinical isolates of Aspergillus niger, Fusarium spp., Rhizomucor spp., and Mucor indicus were obtained from the mycology culture collection of the McGill University Health Centre. Aspergillus strains were grown on YPD agar (Gibco) at 37°C for 6 days. Other fungal strains were maintained on potato dextrose agar (Gibco) at 30°C for 6 days. For all strains, conidia or spores were harvested by gently washing the plates with phosphate-buffered saline plus 0.1% Tween 80 (PBS-Tween).
Construction of AF-eGFP.
To enhance the visualization of fungal elements by microscopy we constructed a green fluorescent protein-expressing strain of A. fumigatus (AF-eGFP). To accomplish this, an overexpression plasmid (pGFP-Phleo) was generated, containing egfp under the expression of the A. nidulans gpdA promoter. Briefly, the GFP-encoding gene (egfp) was amplified from plasmid p123 using the primers GFP-F and GFP-R (Table 1). After PCR amplification, the PCR product was cloned into the expression plasmid pEYFPC using NcoI and NotI, replacing the N-terminal part of yfp. To insert a transformation selection marker, the phleomycin resistance gene (ble) under the expression of gpdA promoter from A. fumigatus was amplified by fusion PCR. The gpdA promoter was amplified from genomic DNA using primers Af-PgpdA-F and Af-PgpdA-R and the ble gene from plasmid p402 using Phleo-F and Phleo-R. Next, these fragments were fused using hybrid PCR and amplification with the primers Af-PgpdA-F and Phleo-R (32). The subsequent phleomycin resistance cassette was subcloned into the GFP overexpression plasmid using EcoRI and Bsp120I. A. fumigatus transformation with plasmid pGFP-Phleo was carried out according to our previously described protoplasting method (27). Plasmids p123 (26) and pEYFPC (14) were kindly provided by A. Brakhage (Leibniz Institute for Natural Product Research and Infection Biology—HKI, Germany).
Table 1.
Primers used in this study
| Name | Sequence (5′ to 3′) |
|---|---|
| Af-PgpdA-F | ATA ATA GGG CCC GAA TTC ACA TCA TCT GGT ATC TAC GCA |
| Af-PgpdA-R | GAA CGG CAC TGG TCA ACT TGG CCA TTG TGT AGA TTC GTC T |
| Phleo-F | GCT CAG TAC CAG ACG AAT CTA CAC AAT GGC CAA GTT GAC C |
| Phleo-R | ATA ATA GGG CCC TCA GTC CTG CTC CTC GGC CA |
| GFP-F | ATA TCC ATG GAT ATC GCG GCC GCG ATG GTG AGC AAG GGC G |
| GFP-R | AGC AGC ACT AGT TTA CTT GTA CAG CTC GTC CAT GCC G |
Antifungal susceptibility testing.
Microdilution adherence assays were performed in accordance with the CLSI M38-A document for broth dilution antifungal susceptibility testing of filamentous fungi (21). Final drug dilutions were made in RPMI 1640 buffered with MOPS. Drug (100 μl) was serially diluted in 96-well plates, to which 100 μl of 105 conidia/ml solution was added per well. Plates were examined after 24 and 48 h of incubation, and the MIC was determined by visual and microscopic inspection revealing 100% growth inhibition.
Cell-associated antifungal model system.
To test the ability of antifungal exposed cells to resist infection, monolayers of each cell type were grown by inoculating tissue culture-treated plates as follows: 3.5 × 105 A549 cells per well for 6-well plates or 105 cells for 24-well plates; 3.5 × 105 RAW 264.7 cells per well for 6-well plates or 105 cells for 24-well plates. Cells were grown to confluence (approximately 48 h), the growth medium was aspirated, and the cells were washed with Dulbecco's phosphate-buffered saline (dPBS). Next, cells were incubated with the appropriate antifungal in RPMI + MOPS or F12 Kaighn's complete growth medium for 4 h. After incubation, the free drug was removed by aspirating the medium and washing the cells with dPBS (twice). Drug exposed monolayers were then infected with 1 ml of a 5 × 105 conidia/ml stock of A. fumigatus in RPMI-MOPS or F12 Kaighn's complete growth medium and incubated for 48 h. The MIC for each drug exposure was determined via visual inspection and light microscopy. In addition, wells containing no cells and cells incubated with DMSO in RPMI-MOPS alone were included as controls. For confocal microscopy studies, cells were grown on a sterile glass coverslip in a 24-well tissue culture plate before infection with AF-eGFP as described previously. Coverslips were then removed and examined under fluorescence microscopy. For phagocytosis experiments, cells were treated with 70 mM cytochalasin D for 1 h preinfection and 48 h postinfection. Inhibition of phagocytosis was verified by phalloidin staining of microfilaments and fluorescence microscopy as previously described (19). The activities of all antifungal agent stocks were validated by performing standard MIC broth microdilution testing in parallel with cellular inhibition assays.
High-performance liquid chromatography (HPLC).
To determine the cell-associated antifungal concentrations resulting from exposure to voriconazole and posaconazole, monolayers of A549 pulmonary epithelial cells were prepared and exposed to antifungal agents as previously described. The drug was then removed, and the cells were washed with dPBS before being collected in 400 μl of RPMI-MOPS using a cell scraper. Wells were washed with an additional 200 μl of RPMI-MOPS to collect residual cells, and the resulting cell suspension was processed for HPLC.
Concentrations of drugs were determined in samples by high-performance liquid chromatography with UV detection at 260 nm using a validated method (10) with some modifications. All samples were collected in microtubes and stored at −20°C until analysis. Samples were deproteinated with an acetonitrile solution containing 1.25 μg/ml of internal standard (phenacetin for voriconazole analysis and voriconazole for posaconazole analysis). Isocratic separations were carried out on a C18 column (150 by 3 mm [inner diameter] Synergi Fusion-RP 4μ 80 A; Phenomenex). The eluent was a 53:47 (vol/vol) mixture of ammonium phosphate buffer (0.04 M, pH 6.00) and acetonitrile. With a flow rate of 0.8 ml/min the retention times of phenacetin, voriconazole, and posaconazole were 1.7, 2.6, and 6.7 min, respectively. The method proved to be linear, accurate, and precise in the range of 0.5 to 10 μg/ml. For voriconazole analysis, the interassay precision values (coefficient of variation [CV]) were 7.5 and 7.7% at 1.5 μg/ml and 6.0 μg/ml, respectively. The lower limit of quantification for voriconazole was 0.3 μg/ml. For posaconazole analysis, the interassay precision values (CV) were 12.5 and 9.2% at 1.5 μg/ml and 6.0 μg/ml, respectively. The lower limit of quantification for posaconazole was 0.2 μg/ml.
Cell-associated antifungal concentrations were then calculated using the previously published method of Conte et al. (4).
Pharmacokinetics and determination of threshold of inhibition.
To determine posaconazole decay kinetics, A549 epithelial cell monolayers grown in 6-well plates were exposed to various concentrations of posaconazole (1, 2, and 4 μg/ml) for 4 h. Free drug was aspirated, and the cell monolayer was washed with dPBS and then incubated with fresh drug-free F12 Kaighn's complete culture medium. Every 6 h, cells were collected by scraping in RPMI-MOPS and the concentration of cell-associated posaconazole was determined by HPLC. In order to define the cellular threshold concentration that could inhibit A. fumigatus growth, a parallel set of A549 cells was grown in 100-mm dishes and loaded with posaconazole as described above. Directly after drug removal, and at 6-h intervals, monolayers were infected with 106 A. fumigatus conidia. To quantify the kinetics of A. fumigatus hyphal growth, culture supernatants were collected from infected wells at 6-h intervals postinfection and diluted 1:10 to 1:1,000 in RPMI. The galactomannan content was then determined using the Platelia Aspergillus EIA (Bio-Rad), according to the manufacturer's instructions.
Time-kill assays.
To determine the fungistatic activity of posaconazole, A549 cells grown in 6-well culture dishes were exposed to a range of posaconazole (0 to 8 μg/ml) drug concentrations for 4 h. Cells were infected with 5 × 105 A. fumigatus conidia and, in parallel, conidia were inoculated into wells containing free posaconazole. Every 6 h, wells were scraped and conidia were centrifuged and resuspended in PBS with 0.1% Tween 80. Conidial viability was then determined by quantitative culture.
Epithelial cell injury.
To determine the ability of cell-associated antifungal agents to protect epithelial cells from Aspergillus-induced injury, we used a modification of our previously described A549 cell damage assay (21). Briefly, A549 cells were loaded with chromium by incubating monolayers grown in 24-well tissue culture plates with 3 μCi of 51Cr at 37°C in 5% CO2 for 24 h. Excess chromium was removed by washing with Hanks balanced salt solution (HBSS). The labeled A549 cells were then exposed to antifungals for 4 h as described previously and then infected with 5 × 105 conidia in 1 ml serum-free DF12K medium. After 16 h of incubation, the medium above the cells was retrieved. The cells were then lysed with 6 N NaOH, and the lysate was collected. The 51Cr content of the medium and lysates was then measured in a gamma counter, and the degree of epithelial cell damage was calculated. Results are reported as the percentage of total cellular chromium that was released into the supernatant after correction for spontaneous chromium release by uninfected epithelial cells.
Subcellular fractionation.
To determine the compartmentalization of posaconazole within A549 epithelial cells, A549 cells were fractioned as previously described (9, 18). Briefly, A549 epithelial cell monolayers were grown in 100 mm tissue culture-treated dishes and treated with posaconazole as described previously. Drug-exposed cells were then washed with PBS and then resuspended in an equal volume of hypotonic solution containing protease inhibitors (20 mM Tris, pH 7.5, 10 mM KCl, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 0.1 mM Na orthovanadate, 1 μg/ml leupeptin, 1 μg/ml aprotinin). The A549 cells were lysed using a Dounce homogenizer, and lysis was verified with trypan blue staining. Centrifugation at 2,000 × g for 3 min separated the cytoplasmic fraction from the nuclear fraction. Ultracentrifugation (100,000 × g for 1 h) allowed for separation of the membrane fraction from the cytoplasmic fraction. The protein content of each fraction was determined by Bradford assay, and the resulting fractions were assayed by Western blot to ensure their composition. An enolase-specific primary antibody (Santa Cruz) was used to identify the cytosolic fraction, whereas a pan-cadherin antibody (Abcam) was used to identify the membrane fraction. Samples were then diluted for HPLC assay as described above, and the posaconazole content was normalized to the protein concentration of each fraction.
RESULTS
Posaconazole- and itraconazole-exposed cells inhibit the growth of Aspergillus fumigatus.
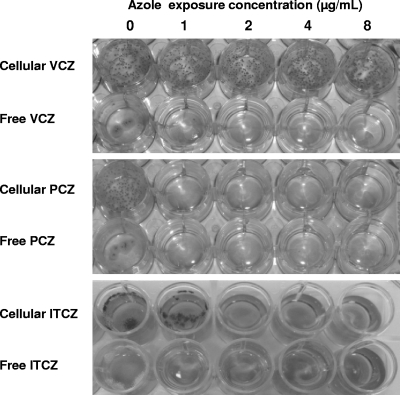
To test if cell-associated antifungal agents were able to inhibit the growth of A. fumigatus, monolayers of the A549 pulmonary epithelial cell line were exposed to a range of concentrations of multiple antifungal agents. These included amphotericin B deoxycholate, liposomal amphotericin B, caspofungin, voriconazole, and posaconazole. After a 4-h exposure to antifungal agents, the free drug was removed and the cells were washed to eliminate any residual drug within the wells. Drug-exposed cells were then infected with conidia of A. fumigatus strain Af293 and examined for fungal growth after 48 h. In the presence of non-drug-exposed epithelial cells, A. fumigatus grew more robustly than in medium alone and produced more floating colonies within the wells. A. fumigatus growth was not inhibited by epithelial cells exposed to voriconazole at concentrations up to 128 μg/ml (Fig. 1) and to the nonazole antifungals, including amphotericin B deoxycholate (0 to 16 μg/ml), liposomal amphotericin B (0 to 8 μg/ml), and caspofungin (0 to 128 μg/ml) (data not shown). However, growth of A. fumigatus was inhibited for at least 48 h by exposure of epithelial cells pretreated with posaconazole at concentrations higher than 1 μg/ml (Fig. 1). No significant fungal growth inhibition was found when posaconazole was incubated in empty tissue culture wells (data not shown), suggesting that there was no significant adsorption of posaconazole to the tissue culture well plastic. These observations strongly suggest that it is the posaconazole present within the epithelial cells that inhibits fungal growth. Since posaconazole is derived from the parent molecule itraconazole, we tested the ability of cell-associated itraconazole to mediate inhibition of A. fumigatus growth. Cells exposed to concentrations of itraconazole ≥2 μg/ml also inhibited growth of A. fumigatus (Fig. 1), suggesting this effect is specific to this class of large lipophilic azoles. In light of their common use in antifungal prophylaxis, we selected posaconazole and voriconazole as representative azoles for further study.
Fig. 1.
Growth inhibition of A. fumigatus by cells exposed to azoles. Wells contained either A549 cells exposed to antifungals for 4 h or free drug at the indicated concentrations. These wells were then infected with AF293 conidia. Photographs were taken 48 h postinfection. No growth inhibition was observed with cells exposed to voriconazole even at higher concentrations up to 128 μg/ml (data not shown). Only posaconazole- and itraconazole-exposed cells were able to inhibit fungal growth.
Since patients may also be exposed to actively germinating conidia or begin antifungal prophylaxis when germinating conidia are present already within their lungs, we examined the effects of cell-associated posaconazole and voriconazole on the growth of germinated conidia. Cells exposed to posaconazole but not voriconazole were also able to inhibit the growth of germinated conidia to the same extent as resting conidia, suggesting that this inhibition is not specific to dormant conidia only (data not shown).
Cellular levels of antifungals after in vitro exposure.
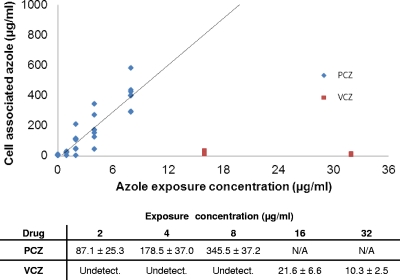
We next confirmed that concentrations of cell-associated posaconazole that were associated with the inhibition of fungal growth were similar to those reported in patients receiving therapy with posaconazole. High-performance liquid chromatography (HPLC) was used to determine the concentration of voriconazole and posaconazole found within epithelial cells after exposure to a range of antifungal concentrations (Fig. 2). Consistent with previous reports (5), we found that voriconazole was only minimally concentrated within the epithelial cells and reached a maximum level at an exposure concentration of 16 μg/ml. In contrast, the cellular concentration of posaconazole was 50-fold higher than the exposure concentration. A linear relationship was observed between exposure concentration and intracellular concentration of posaconazole in any single experiment. Although differences in epithelial cell confluence resulted in absolute interexperiment differences, the linear correlation was conserved in all experiments. In aggregate, these experiments suggested that an in vitro exposure of 2 μg/ml posaconazole resulted in a cellular concentration of 80 to 90 μg/ml, the level previously reported in alveolar cells recovered by bronchoalveolar lavage from patients treated with posaconazole (5). We therefore used this concentration for all further experiments. To control for the higher serum levels reported in patients receiving voriconazole, we used a 4-fold-higher exposure level of voriconazole (8 μg/ml) as a comparator in these experiments.
Fig. 2.
Relationship of exposure concentration to cell-associated concentrations of azoles. A549 cells were exposed to posaconazole and voriconazole at the indicated concentrations for 4 h. Free drug was removed by washing; then the cells were collected and the respective azole drug concentrations determined using HPLC. Individual assay results for each exposure concentration are shown graphically, and the mean drug concentration ± standard error of the mean is shown in the table below. All results are the aggregate of results from at least three independent experiments.
Epithelial cell- and macrophage-associated posaconazole inhibits fungal growth.
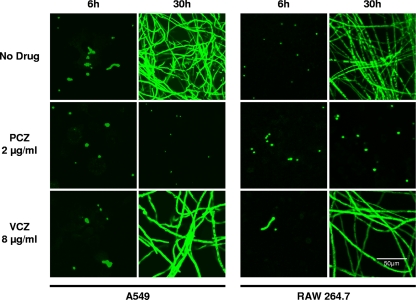
In order to confirm the macroscopic findings of fungal growth inhibition by posaconazole-exposed cells and to examine the level at which posaconazole-loaded cells could inhibit fungal growth, confocal microscopy was performed. To permit visualization of A. fumigatus, we constructed a strain of A. fumigatus with constitutive expression of enhanced green fluorescent protein (Af293-eGFP). Pulmonary epithelial cells exposed to posaconazole at 2 μg/ml but not voriconazole at 8 μg/ml inhibited fungal germination and subsequent growth (Fig. 3). To extend these results, we also examined the ability of cell-associated posaconazole to inhibit the growth of other medically relevant fungi. Pulmonary epithelial cells exposed to 2 μg/ml of posaconazole for 4 h also completely inhibited the growth of a clinical isolate of Aspergillus niger, Fusarium oxysporum, and Rhizomucor sp. In contrast, exposure of epithelial concentrations of voriconazole of 8 μg/ml had no effect on growth of any of these isolates (data not shown).
Fig. 3.
Effects of azoles on germination and growth of A. fumigatus. A549 epithelial cells and RAW 264.7 macrophages were exposed to no drug, 2 μg/ml posaconazole, or 8 μg/ml of voriconazole for 4 h; then the free drug was removed by washing. Drug-loaded cells were then infected with an eGFP-producing strain of A. fumigatus, and images were taken 6 and 30 h postinfection using confocal microscope. Only posaconazole-exposed cells were able to inhibit fungal germination and growth.
To determine if concentration of antifungals within other cell types could also mediate antifungal effects, RAW 264.7 macrophages were exposed to either posaconazole or voriconazole and then infected with A. fumigatus conidia. As was observed with epithelial cells, macrophages exposed to voriconazole were unable to inhibit fungal growth while cells exposed to posaconazole at 2 μg/ml resulted in a marked inhibition of fungal growth (Fig. 3). These results indicate that posaconazole concentrates within both phagocytic and nonphagocytic pulmonary cells to levels sufficient to inhibit the growth of medically relevant fungi.
Transient exposure to therapeutic levels of cell-associated posaconazole inhibits the germination and growth of A. fumigatus conidia for at least 48 h.
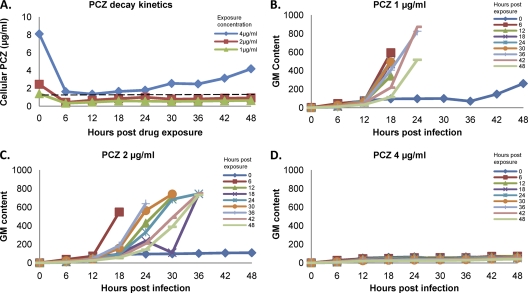
To quantify the kinetics of fungal growth suppression by cell-associated posaconazole, we performed pharmacokinetic studies to determine the decay kinetics of cell-associated posaconazole and the threshold concentration at which cell-associated posaconazole inhibits the germination and growth of A. fumigatus conidia. After removal of extracellular drug, concentrations of cell-associated posaconazole exhibited an initial rapid decline in concentration that was complete by 6 h after drug removal (Fig. 4A). This decay pattern was similar for all exposure concentrations tested. Following the rapid decline phase, levels of posaconazole within cells remained unchanged for at least 48 h, after which the cell cultures became overgrown and further measurement of drug concentrations was not possible.
Fig. 4.
Posaconazole kinetics within epithelial cells and the effects on fungal growth inhibition. (A) A549 cells were exposed to posaconazole for 4 h and then harvested every 6 h after drug removal. The cellular posaconazole concentration at each time point was then determined using HPLC. The dotted line indicates the threshold concentration above which A. fumigatus growth was inhibited (see panels B to D). (B to D) In parallel, A549 cells exposed to the indicated concentration of posaconazole, as in panel A, were infected with A. fumigatus conidia at each of the 6-h intervals. Fungal growth was quantified by serial sampling of the culture supernatant for galactomannan determination. The inset indicates the time between free drug removal and infection of cells with conidia.
To determine the threshold concentration that mediates inhibition of fungal germination and growth, epithelial cell cultures exposed to posaconazole for 4 h were infected at 6-h intervals after drug removal, and the effects on A. fumigatus growth inhibition were quantified by measuring galactomannan production (Fig. 4B to D). Galactomannan is produced only by actively growing hyphae and has been used as a surrogate measure of fungal growth in pharmacodynamic assays (15). Using this experimental approach, we found that even a brief exposure of A. fumigatus to posaconazole cellular concentrations above 80 to 100 μg/ml resulted in a sustained inhibition of fungal growth for at least 48 h, as indicated by visual inspection and galactomannan levels (Fig. 4B to D).
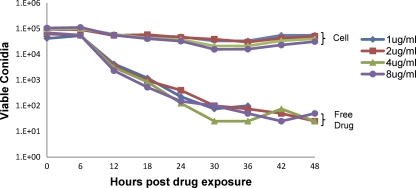
To determine if cell-associated posaconazole was fungicidal under these conditions, we performed time-kill studies comparing cell-associated posaconazole with free drug in solution. Conidia were exposed to cell-associated or free antifungals and were then recovered from wells by scraping. Conidia were then washed to remove antifungal agents and incubated in antibiotic-free medium. Free posaconazole in solution at concentrations greater than 1 μg/ml resulted in near-complete killing of A. fumigatus conidia by 24 h of exposure (Fig. 5). In contrast, when conidia were exposed to cell-associated posaconazole there was only a minor initial reduction in conidial viability, and the majority of conidia remained viable up to 48 h after infection despite the absence of any evident fungal growth within the wells.
Fig. 5.
Time-kill studies of A. fumigatus conidia exposed to free or cell-associated posaconazole. A. fumigatus conidia were incubated with free posaconazole or A549 cells exposed to posaconazole at the indicated concentrations. At the indicated time intervals, conidia were collected, washed to remove posaconazole, and then quantitatively cultured in drug-free medium.
Collectively, these results not only confirm that the clinically reported steady-state cellular posaconazole concentrations are associated with inhibition of fungal growth but also suggest that host cell-associated posaconazole exerts a significant postantifungal effect even after cellular concentrations of the azole have declined below the threshold required to inhibit fungal growth (Fig. 4B to D).
Epithelial cells exposed to posaconazole not only inhibit fungal growth but protect against damage caused by A. fumigatus.
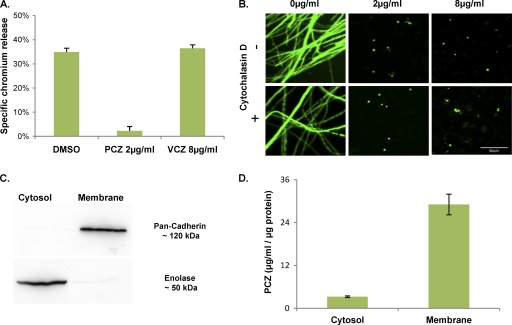
In order to establish whether cell-associated voriconazole or posaconazole is able to protect against cell injury caused by A. fumigatus, we assessed the degree of epithelial cell injury after infection using a chromium release assay. Epithelial cells exposed to posaconazole were completely protected from damage by A. fumigatus infection (Fig. 6A). In contrast, voriconazole failed to reduce epithelial cell injury after infection with A. fumigatus.
Fig. 6.
Effects of posaconazole on A. fumigatus induced cell injury and localization of posaconazole within host cells. (A) A549 cells were loaded with 51Cr and then exposed to the indicated antifungal agents for 4 h before being infected with A. fumigatus conidia. The extent of epithelial cell damage was determined by measuring the amount of 51Cr released by the infected cells. Data are the mean ± standard deviation of results of three experiments, each performed in triplicate. (B) A549 cells were exposed to various concentrations of posaconazole, with or without cytochalasin D. These cells were then infected with eGFP-expressing A. fumigatus conidia and visualized after 30 h growth using confocal microscopy. (C) A549 cells exposed to 8 μg/ml posaconazole for 4 h were separated into membrane and cytosolic fractions by differential centrifugation. Aliquots of each fraction were assayed using Western blotting to confirm the purity of each sample. An anti-pan-cadherin antibody was used for the cell membrane fraction, and an anti-enolase antibody was used for the cytosolic fraction. (D) Subcellular fractions from panel C were then assayed for posaconazole concentrations using HPLC. Posaconazole content was then normalized to total protein concentrations. Results are the mean concentrations obtained from three separate experiments on three independent occasions.
Endocytosis is not required to mediate the inhibition of fungal growth by cell-associated posaconazole.
After adhering to pulmonary epithelial cells, conidia are endocytosed and germinate within the intracellular space. Therefore, we next determined whether endocytosis of conidia is required for inhibition of A. fumigatus growth by cell-associated posaconazole. Posaconazole-loaded cells were exposed to cytochalasin D, which inhibits microfilament formation and endocytosis of conidia by pulmonary epithelial cells (19). Cytochalasin D activity was verified by phalloidin staining, and confocal imaging of microfilaments around endocytosed conidia (data not shown). As described previously, 70 mM cytochalasin D prevented endocytosis of conidia but had no effect on fungal growth inhibition by cell-associated posaconazole (Fig. 6B). These data demonstrate that contact with epithelial cells alone is sufficient to expose conidia to fungistatic concentrations of posaconazole and that exposure to intracellular posaconazole is not required to inhibit fungal growth.
Posaconazole is associated predominately with cellular membranes leading to extremely high local drug concentrations.
The inability of cytochalasin D to prevent the antifungal activity of cell-associated posaconazole combined with posaconazole's lipophilic properties suggests that posaconazole may be concentrated within the cell plasma membrane, rather than within the cytoplasm. To test this hypothesis, cell fractionation experiments were performed using posaconazole-exposed cells in order to separate the cytosol from cellular membranes. Western blotting for cytosolic-specific (enolase) or membrane-specific (cadherin) proteins was performed to confirm the purity of each fraction (Fig. 6C). These fractions were then assayed by HPLC in order to determine the levels of posaconazole. Using this approach, the concentrations of posaconazole were found to be approximately 10-fold greater in the membrane fraction than in the cytosolic fraction (Fig. 6D). Collectively, these results suggest that posaconazole is concentrated within cellular membranes, leading to very high local concentrations of drug that are both fungistatic and cytoprotective.
DISCUSSION
The results of this study provide the first demonstration of the ability of host cell-associated antifungal agents to mediate protection against fungal growth and cellular injury. Of all the antifungal agents tested, only posaconazole was able to inhibit fungal growth and mediate significant cytoprotection after removal of the extracellular drug. Complete and durable fungal inhibition and cellular protection were observed with even transient exposures of fungi to cellular concentrations of posaconazole at or above 80 to 100 μg/ml, the steady-state concentration found in pulmonary epithelial cells isolated from patients treated with posaconazole. Further, this protection was observed for up to 48 h after the removal of extracellular drug, suggesting that the threshold for inhibition of fungal infection can be achieved in patients treated with posaconazole. High cellular levels of posaconazole may therefore provide an explanation for the observation that posaconazole is highly effective in the prevention of invasive mold infections in the face of lower serum levels. In addition, the results of our pharmacokinetic and challenge experiments suggest that a transient exposure to these levels of posaconazole can result in longer-term fungal growth inhibition by a postantifungal effect. Collectively, our results support a model whereby the cell-associated fraction of posaconazole plays an important role in the prevention of fungal infections.
The mechanism and location whereby cell-associated posaconazole mediates fungal growth suppression and cytoprotection remain to be defined. Our fractionation studies suggest that posaconazole partitions strongly to cellular membrane compartments. As conidia of Aspergillus are highly hydrophobic, one hypothesis is that passive diffusion of posaconazole can occur between the conidia and the host cell membrane upon adherence of the organism. Indeed, concentration of posaconazole within fungal cell membranes could explain the postantifungal inhibition of fungal growth observed in our in vitro experiments. Our original hypothesis was that endocytosis exposed conidia to membrane-bound posaconazole within the endocytic vesicle. However, since inhibition of endocytosis does not impair fungal killing, it seems that posaconazole present within the plasma membrane is sufficient to mediate inhibition of fungal growth, although continued exposure also likely continues after endocytosis and during the intracellular phase of fungal growth.
The findings of extremely high membrane concentrations of posaconazole in the face of relatively low exposure concentrations strongly suggest that we may need to reassess our approach to therapeutic drug monitoring for this agent. In particular, the pharmacokinetics of the membrane compartment will need to be better defined, as they likely do not mirror the serum concentration. Although we observed a linear correlation between exposure concentrations and cellular concentrations of posaconazole, this was in a single-dose static system and these results may vary in the host, where serum concentrations vary depending on dosing frequency and absorption. A similar divergence between serum and cellular concentrations of an antimicrobial agent has been reported for the macrolide azithromycin (13). After administration, this weakly basic antibiotic is rapidly taken up into the phagolysosome of cells where it undergoes protonation. This modification enhances cellular retention of the drug in the face of low serum levels, and it is thought to mediate activity against intracellular pathogens. It is unknown if posaconazole undergoes similar protonation; however, the results of our studies would suggest that a similar mechanism of cellular accumulation may be found with this agent. Studies to define the subcellular membrane localization of posaconazole are under way.
In experimental models of posaconazole treatment of invasive aspergillosis, recent reports have identified an AUC/MIC ratio of 167 as being associated with a half-maximal antifungal effect and near maximal survival at an AUC/MIC of 1,000 (15, 20). The authors estimate that serum AUC/MIC levels for patients receiving 800 mg of posaconazole per day are ∼100 to 150 (15). However, the prolonged persistence of high cellular, and by extension, membrane levels of posaconazole identified in our study suggests that the AUC/MIC ratio within epithelial cells may be several hundred-fold higher than that found in serum and well within the maximally effective range. Persistence of membrane-associated posaconazole may therefore impart a protective effect after serum levels decline, although this requires validation in vivo. The persistence of high levels of cell-associated posaconazole and the prolonged fungistatic effect observed in vitro also suggest that less-frequent dosing of posaconazole may be an effective strategy in prophylaxis, although clinical data on such regimens is lacking at this time.
Although the results of our study provide a compelling rationale for the efficacy of posaconazole in antifungal prophylaxis, caution should be exercised in extending our results to the treatment of established infection. During established invasive aspergillosis, hyphae are found within both the extracellular space as well as the intracellular compartment. Further, the extensive tissue necrosis observed around fungal lesions may reduce the exposure of the organism to the plasma membrane of host cells. In this event, serum or extracellular fluid concentrations of antifungals may be a more important predictor of antifungal efficacy.
Collectively, the results of this study suggest that cellular accumulation and membrane partitioning of antifungal agents may present a novel pharmacokinetic paradigm that influences the efficacy of these agents in the prevention of fungal infections. Future studies are required to determine the relative contribution of this mechanism versus extracellular lung fluid and serum levels in the protection against fungal disease. A reinterpretation of serum therapeutic drug monitoring may be required for agents such as posaconazole or itraconazole that exhibit pharmacokinetic differences between the serum and cellular or subcellular compartments, particularly for fungal prophylactic strategies. Further, measurements of the pharmacokinetics of the cellular and membrane compartments may allow us to refine our dosing strategies to maximize the activity of antifungals during prophylaxis while minimizing antifungal exposure.
ACKNOWLEDGMENTS
We thank A. Brakhage (Leibniz Institute for Natural Product Research and Infection Biology—HKI, Germany) for providing the pEYFPC and p123 plasmids which were used to construct the pGFP-Phleo plasmid.
This project was funded by the Career Award in the Biomedical Sciences from the Burroughs Welcome Fund (D.C.S.), United States Public Health Service Grant R01AI073829 (S.G.F.), operating funds from the Canadian Institutes of Health Research and a studentship from McGill University (P.C.).
Footnotes
Published ahead of print on 19 September 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Ballesta S., Garcia I., Perea E. J., Pascual A. 2005. Uptake and intracellular activity of voriconazole in human polymorphonuclear leucocytes. J. Antimicrob. Chemother. 55:785–787 [DOI] [PubMed] [Google Scholar]
- 2. Balloy V., Chignard M. 2009. The innate immune response to Aspergillus fumigatus. Microbes Infect. 11:919–927 [DOI] [PubMed] [Google Scholar]
- 3. Billaud E. M., et al. 2010. Pharmacological considerations for azole antifungal drug management in cystic fibrosis lung transplant patients. Med. Mycol. 48(Suppl. 1):S52–S59 [DOI] [PubMed] [Google Scholar]
- 4. Conte J. E., Jr., Golden J. A., Kipps J., McIver M., Zurlinden E. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conte J. E., Jr., et al. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornely O. A., et al. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359 [DOI] [PubMed] [Google Scholar]
- 7. Cornely O. A., Ullmann A. J. 2011. Lack of evidence for exposure-response relationship in the use of posaconazole as prophylaxis against invasive fungal infections. Clin. Pharmacol. Ther. 89:351–352 [DOI] [PubMed] [Google Scholar]
- 8. Farowski F., et al. 2010. Quantitation of azoles and echinocandins in compartments of peripheral blood by liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 54:1815–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fielhaber J. A., et al. 2009. Inactivation of mammalian target of rapamycin increases STAT1 nuclear content and transcriptional activity in alpha4- and protein phosphatase 2A-dependent fashion. J. Biol. Chem. 284:24341–24353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gage R., Stopher D. A. 1998. A rapid HPLC assay for voriconazole in human plasma. J. Pharm. Biomed. Anal. 17:1449–1453 [DOI] [PubMed] [Google Scholar]
- 11. Groll A. H., et al. 2010. Randomized comparison of safety and pharmacokinetics of caspofungin, liposomal amphotericin B, and the combination of both in allogeneic hematopoietic stem cell recipients. Antimicrob. Agents Chemother. 54:4143–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groll A. H., Tragiannidis A. 2009. Recent advances in antifungal prevention and treatment. Semin. Hematol. 46:212–229 [DOI] [PubMed] [Google Scholar]
- 13. Hand W. L., Hand D. L. 2001. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents 18:419–425 [DOI] [PubMed] [Google Scholar]
- 14. Hoff B., Kuck U. 2005. Use of bimolecular fluorescence complementation to demonstrate transcription factor interaction in nuclei of living cells from the filamentous fungus Acremonium chrysogenum. Curr. Genet. 47:132–138 [DOI] [PubMed] [Google Scholar]
- 15. Howard S. J., et al. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J. Infect. Dis. 203:1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hussaini T., Ruping M. J., Farowski F., Vehreschild J. J., Cornely O. A. 2011. Therapeutic drug monitoring of voriconazole and posaconazole. Pharmacotherapy 31:214–225 [DOI] [PubMed] [Google Scholar]
- 17. Jang S. H., Colangelo P. M., Gobburu J. V. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin. Pharmacol. Ther. 88:115–119 [DOI] [PubMed] [Google Scholar]
- 18. Kim J. E., Chen J. 2000. Cytoplasmic-nuclear shuttling of FKBP12-rapamycin-associated protein is involved in rapamycin-sensitive signaling and translation initiation. Proc. Natl. Acad. Sci. U. S. A. 97:14340–14345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopes Bezerra L. M., Filler S. G. 2004. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood 103:2143–2149 [DOI] [PubMed] [Google Scholar]
- 20. Mavridou E., Bruggemann R. J., Melchers W. J., Mouton J. W., Verweij P. E. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NCCLS 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A (ISBN 1-56238-470-8). NCCLS, Wayne, PA [Google Scholar]
- 22. Pagano L., Caira M., Valentini C. G., Posteraro B., Fianchi L. 2010. Current therapeutic approaches to fungal infections in immunocompromised hematological patients. Blood Rev. 24:51–61 [DOI] [PubMed] [Google Scholar]
- 23. Pascual A., et al. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 24. Pascual A., et al. 2007. Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob. Agents Chemother. 51:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith J., et al. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spellig T., Bottin A., Kahmann R. 1996. Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Mol. Gen. Genet. 252:503–509 [DOI] [PubMed] [Google Scholar]
- 27. Twumasi-Boateng K., et al. 2009. Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 8:104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ullmann A. J., et al. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347 [DOI] [PubMed] [Google Scholar]
- 29. Wasylnka J. A., Moore M. M. 2002. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect. Immun. 70:3156–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson D. T., Drew R. H., Perfect J. R. 2009. Antifungal therapy for invasive fungal diseases in allogeneic stem cell transplant recipients: an update. Mycopathologia 168:313–327 [DOI] [PubMed] [Google Scholar]
- 31. Wingard J. R., et al. 2010. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 116:5111–5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wurch T., Lestienne F., Pauwels P. J. 1998. A modified overlap extension PCR method to create chimeric genes in the absence of restriction enzymes. Biotechnol. Tech. 12:653–657 [Google Scholar]