Abstract
Fluconazole is a widely used antifungal agent that is extensively reabsorbed in patients with normal renal function. However, its reabsorption can be compromised in patients with acute kidney injury, thereby leading to altered fluconazole clearance and total systemic exposure. Here, we explore the pharmacokinetics of fluconazole in 10 critically ill anuric patients receiving continuous venovenous hemodiafiltration (CVVHDF). We performed Monte Carlo simulations to optimize dosing to appropriate pharmacodynamic endpoints for this population. Pharmacokinetic profiles of initial and steady-state doses of 200 mg intravenous fluconazole twice daily were obtained from plasma and CVVHDF effluent. Nonlinear mixed-effects modeling (NONMEM) was used for data analysis and to perform Monte Carlo simulations. For each dosing regimen, the free drug area under the concentration-time curve (fAUC)/MIC ratio was calculated. The percentage of patients achieving an AUC/MIC ratio greater than 25 was then compared for a range of MIC values. A two-compartment model adequately described the disposition of fluconazole in plasma. The estimate for total fluconazole clearance was 2.67 liters/h and was notably 2.3 times faster than previously reported in healthy volunteers. Of this, fluconazole clearance by the CVVHDF route (CLCVVHDF) represented 62% of its total systemic clearance. Furthermore, the predicted efficiency of CLCVVHDF decreased to 36.8% when filters were in use >48 h. Monte Carlo simulations demonstrated that a dose of 400 mg twice daily maximizes empirical treatment against fungal organisms with MIC up to 16 mg/liter. This is the first study we are aware of that uses Monte Carlo simulations to inform dosing requirements in patients where tubular reabsorption of fluconazole is probably nonexistent.
INTRODUCTION
Acute kidney injury (AKI) often develops in critically ill patients and is associated with a mortality rate as high as 60% (3, 37). Renal replacement therapy (RRT) is therefore used routinely as a therapeutic measure in a large proportion of patients admitted to intensive care (18, 33). Continuous venovenous hemodiafiltration (CVVHDF) is a commonly used form of RRT, in which solute removal occurs via diffusion gradients through a countercurrent dialysate flow (11, 36). This dialysis modality is effective at removing solutes during a 24- to 48-h period and is better tolerated by hemodynamically unstable patients than intermittent hemodiafiltration (13). However, CVVHDF can modify the clearance of some drugs, thereby necessitating dose adjustment to achieve therapeutic targets (32, 33). CVVHDF is thought to be of clinical relevance only when extracorporeal elimination exceeds 25 to 30% of total body clearance (28).
In addition to RRT, pathophysiological changes can alter the pharmacokinetics (PK) of drugs in the critically ill (25, 30). Suitable drug dosage guidelines are therefore required in the treatment of critically ill patients receiving CVVHDF or other continuous RRT (6). This information is particularly relevant to drug dosing in patients with AKI, for which mortality rates are higher than those in other critical care settings (37).
Fluconazole is a triazole antimycotic agent used for the treatment of superficial and systemic fungal infections (10). It is recommended for patients with candidemia due to its concentration-dependent activity, favorable safety profile, and proven efficacy (23). Furthermore, a free drug area under the concentration-time curve from 0 to 24 h (fAUC0-24)/MIC ratio greater than 25 has been suggested as the appropriate pharmacodynamic endpoint for fluconazole efficacy (1, 7, 21). In patients with normal renal function, fluconazole has an elimination half-life of 25 to 35 h, with 80% of the drug excreted unchanged in the urine (10, 31). It is normally extensively reabsorbed by the kidneys (9), and it has previously been validated as a marker for renal reabsorption (35). However, in patients with severely impaired kidney function, the ability for tubular reabsorption is unlikely, and it could considerably alter the clearance of fluconazole when combined with continuous RRT (8, 20, 26, 38, 39). As a consequence, this could significantly influence the fAUC0-24/MIC ratio of fluconazole, thereby compromising its desired therapeutic response.
In this study, we developed a population PK model to describe the disposition of fluconazole in anuric patients receiving CVVHDF. We then performed Monte Carlo dosing simulations to demonstrate that dose adjustment is required to maximize the opportunity for therapeutic outcomes in these patients.
MATERIALS AND METHODS
Patients.
This study was conducted at the Royal Brisbane and Women's Hospital (Queensland, Australia) over a 28-month period. Ethical approval was obtained from the Royal Brisbane Hospital Research Ethics Committee and the Medical Research Ethics Committee of the University of Queensland. Informed consent was obtained from the patient or next of kin. Patients were enrolled if they were >17 years of age, critically ill, required CVVHDF for renal failure of any cause, and were prescribed fluconazole for a suspected infection. Patients were excluded if informed consent was declined or could not be obtained.
Dialysis prescription.
For all patients, CVVHDF was performed with predilution filtration solution (2 liters h−1) and dialysate (1 liters h−1), giving a 3 liters h−1 dialysis effluent. Fluid input and effluent rates were set at 999 ml h−1 and were controlled using IMED PC4 volumetric pumps (Alaris Medical Systems Inc., San Diego, CA). Blood was pumped (200 ml min−1) using a Gambro BMM-10 pump (Gambro AB, Stockholm, Sweden) through an extracorporeal circuit containing a Hospal AN69HF hemofilter (Hospal AG, Lyon, France). With the exception of patient 4, all patients had filters in use <48 h at the start of CVVHDF treatment. For all patients (except 1 and 5), dialysis commenced prior to fluconazole dosing.
Dosing and sample collection.
A standard 200 mg dose of fluconazole was administered twice daily as a 60-min intravenous (i.v.) infusion. Blood was obtained from an indwelling arterial cannula during the infusion (30 min), at the end of the infusion (1 h), and after 2, 3, 4, 6, 8, and 12 h. The blood samples were collected in heparinized tubes and chilled on ice and then centrifuged (2,000 × g for 5 min at 4°C) within 1 h to obtain plasma. CVVHDF effluent was sampled every hour over 12 h from all patients. All samples were subsequently stored at −80°C until analysis.
For patients 3, 4, and 8, blood and CVVHDF effluent sampling was performed on the first day of fluconazole treatment to obtain an initial profile. In all other patients, blood and CVVHDF effluent were collected on day 3 or day 5 to provide a steady-state profile. In the latter group of patients, a 12-h sample was obtained immediately prior to administration of the next dose. No data from both initial and steady-state profiles were available for any of the patients enrolled in the study.
Determination of plasma and CVVHDF concentrations.
The concentrations of fluconazole in plasma were measured by high-performance liquid chromatography, as reported previously (19). Analysis of drug concentrations in the CVVHDF effluent was performed by adapting the same assay for urine. Standard curves were prepared at 0.2 to 20 mg/liter (plasma) and 2.0 to 200 mg/liter (effluent). The limit of quantitation was 0.2 mg/liter for plasma and 2.0 mg/liter for CVVHDF samples. In both matrices, intraday and interday precision were below 12%.
Population PK modeling.
The concentration-time data for fluconazole in plasma were fitted to one-, two-, or three-compartment models by nonlinear mixed-effects modeling (NONMEM version 6.1; Globomax LLC, Hanover, MD) (5). A Digital Fortran compiler was used, and the runs were executed using Wings for NONMEM (http://wfn.sourceforge.net). Data were analyzed using the first-order conditional estimation method with interaction and ADVAN6 to solve the differential equations. Between-subject variability (BSV) was calculated using an exponential variability model and was assumed to follow a lognormal distribution. Residual unexplained variability (RUV) was modeled using a combined exponential and additive random error. Visual inspection of diagnostic scatter plots and the NONMEM objective function value (OBJ) were used to evaluate goodness of fit. Statistical comparison of nested models was undertaken using the NONMEM program on the basis of a χ2 test of the difference in OBJ. A decrease in the OBJ of 3.84 units (P < 0.05) was considered statistically significant.
Covariate screening.
A forwards and backwards stepwise approach was used to include the following covariates: age, total body weight, sex, and APACHE II score into the model. Covariates were included if they were biologically plausible and if the decrease in OBJ was at least 3.84 units.
CVVHDF model.
A CVVHDF model was developed by partitioning total fluconazole clearance from the central compartment into CVVHDF (CLCVVHDF) and non-CVVHDF (CLNCVVHDF) routes. CLCVVHDF was then modeled by simultaneously fitting cumulative amounts of fluconazole in the CVVHDF effluent with its concentration-time data in plasma.
Model evaluation.
The final CVVHDF model was evaluated by performing a visual predictive check (VPC) and by nonparametric bootstrapping with resampling and replacement (24). For the VPC, 1,000 data sets were simulated from the final parameter estimates using the original data as a template. The median, 10th, and 90th percentiles of simulated concentrations were then computed and plotted against observed values. A nonparametric bootstrap method was used to assess the uncertainty of all parameter estimates in the final model. The 2.5th, 50th, and 97.5th percentiles for all parameters were calculated from the empirical posterior distribution of 1,000 bootstrap replicates. Eta shrinkage was evaluated to identify model suitability for performing dose simulations and for the addition of covariates into the model.
Dosing simulations.
Monte Carlo simulations for fluconazole dosing with CVVHDF treatment were undertaken using NONMEM. The simulations included a standard dose of 200 mg twice daily, a loading dose (LD) of 400 mg, 800 mg, and 1,600 mg, followed by a standard dose of 200 mg twice a day and 400 mg twice daily. For each dosing schedule, the Monte Carlo simulations generated concentration-time profiles, fAUC0-24 values, and fAUC/MIC ratios for 1,000 subjects, using the parameters from the final model. The MICs utilized ranged from 0.0625 mg/liter to 32 mg/liter, as this is likely to represent the maximal spectrum of candidemia treated with fluconazole in critical care (Clinical and Laboratory Standards Institute [CLSI] breakpoints for antifungal agents). For each dosage regimen, the probability of target attainment (PTA) was calculated as the percentage of patients achieving a fAUC/MIC ratio of >25 for a given MIC. An additional analysis was also performed using a fAUC/MIC ratio of >100 based on clinical experience, as defined by European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology (http://www.srga.org/eucastwt/MICTAB/index.html). For both analyses, the PTA was plotted against the range of MICs.
RESULTS
Population PK analysis.
A total of 10 anuric patients were enrolled in this study, requiring i.v. fluconazole for a suspected fungal infection while receiving CVVHDF. Eight of the 10 patients had normal liver function. For the two patients with abnormal liver function, one patient had four times the upper limit of alanine aminotransferase (ALT) (145 u/liters), and the other patient had four times the upper limit of aspartate aminotransferase (AST) (168 u/liters). Albumin concentrations were low in all patients, ranging from 11 to 30 g/liter. Demographic information for the 10 patients is provided in Table 1.
Table 1.
Demographic and clinical information of enrolled patients
| Patient | Age (yr) | wt (kg)a | Sex | APACHE II scoreb | Diagnosis on admission | Site of infection | Causative organism |
|---|---|---|---|---|---|---|---|
| 1 | 66 | 55 | F | 22 | Medical | Urinary tract | Candida albicans |
| 2 | 68 | 50 | F | 43 | Medical | Lung | Nonfungal |
| 3 | 70 | 80 | M | 38 | Medical | Lung | Candida albicans |
| 4 | 69 | 80 | M | 19 | Emergency; surgery | Intraabdominal | Candida albicans |
| 5 | 62 | 80 | M | 20 | Emergency; surgery | Intraabdominal | Candida albicans |
| 6 | 51 | 104 | F | 17 | Emergency; surgery | Intraabdominal | Candida albicans |
| 7 | 72 | 80 | M | 44 | Emergency; surgery | Blood | Candida albicans |
| 8 | 67 | 100 | M | 30 | Medical | Indwelling vascular catheter | Candida parapsilosis |
| 9 | 76 | 75 | F | 28 | Medical | Urinary tract | Candida albicans |
| 10 | 59 | 72 | F | 31 | Medical | Urinary tract | Candida albicans |
Estimated weight at time of treatment.
APACHE II score, acute physiology and chronic health evaluation II score on admission.
The time course of fluconazole in plasma was best described by a two-compartment model with combined residual error, BSV on clearance, central volume of distribution, and infusion duration. Input into the central compartment was fitted by zero-order kinetics.
Covariate screening.
After screening all biologically plausible covariates on clearance and volume of distribution, no statistically significant improvements in the base model were found. None of the covariates tested were therefore incorporated into the model for fluconazole in plasma.
CVVHDF model.
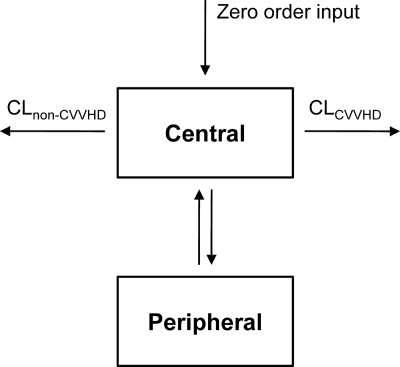
Cumulative amounts of fluconazole in the CVVHDF effluent were combined with the plasma concentration-time data to allow for estimation of CLCVVHDF (Fig. 1). The residual variability for the amount of fluconazole in the CVVHDF effluent was best described by an additive error model. When the effect of filter age was included as a covariate, the BSV on CLCVVHDF was reduced from 34.4% to 19.8%, with a significant drop in objective function (ΔOBJ = 11.46). This model predicted that the efficiency of CLCVVHDF decreased to 36.8%, when filters were in use >48 h. Table 2 summarizes the parameter estimates for the final model, together with their median, 2.5th, and 97.5th percentiles from all bootstrap replicates. The population estimate for CLCVVHDF (1.66 liters/h) represented 62% of total fluconazole clearance from the central compartment, where CLNCVVHDF was 1.01 liters/h. For the latter clearance (CLNCVVHDF), the estimate for BSV (77.1%) was relatively high.
Fig. 1.
Structural model for the estimation of fluconazole clearance by CVVHDF (CLCVVHD) and non-CVVHDF (CLnon-CVVHD) pathways. The time course of fluconazole in plasma was best described by a two-compartment model with zero order input.
Table 2.
Population parameter estimates for the final fluconazole CVVHDF model and the 1,000 bootstrap runs
| Parameter | Description | Unit | CVVHDF model estimate | 1,000 bootstrap replicates |
|
|---|---|---|---|---|---|
| Median | 95th percentileb | ||||
| CLCVVHDF | Clearance of fluconazole by CVVHDF | Liters/h | 1.66 | 1.65 | 1.45–1.92 |
| CLNCVVHDF | Non-CVVHDF clearance of fluconazole | Liters/h | 1.01 | 0.95 | 0.42–1.56 |
| Vc | Central vol of distribution | Liters | 31.7 | 29.8 | 9.3–47.0 |
| Q | Intercompartmental clearance | Liters/h | 27.6 | 30.8 | 5.4–70.1 |
| Vp | Peripheral vol of distribution | Liters | 21.9 | 23.9 | 15.0–38.5 |
| D1 | Duration of intravenous infusion | h | 0.689 | 0.701 | 0.570–0.885 |
| ffCLCVVHDF | Effect of filter in use >48 h on CLCVVHDF | 0.368 | 0.368 | 0.326–0.426 | |
| BSV CLCVVHDF | Between-subject variability in CLCVVHDF | CVa | 19.8 | 18.6 | 9.90–26.3 |
| BSV CLNCVVHDF | Between-subject variability in CLNCVVHDF | CV | 77.1 | 78.0 | 20.5–156 |
| BSV Vc | Between-subject variability in VC | CV | 22.9 | 23.8 | 8.40–93.5 |
| BSV D1 | Between-subject variability in D1 | CV | 23.0 | 20.3 | 0–55.1 |
| RUVSDP | Additive residual error in plasma | mg/liters | 0.239 | 0.233 | 0–0.410 |
| RUVCVP | Exponential residual error in plasma | CV | 3.67 | 3.73 | 0–6.67 |
| RUVSDC | Additive residual error in CVVHDF | mg/liters | 2.84 | 2.77 | 1.31–3.71 |
CV, coefficient of variation (%).
2.5th and 97.5th percentile range.
Model evaluation by VPC and bootstrapping.
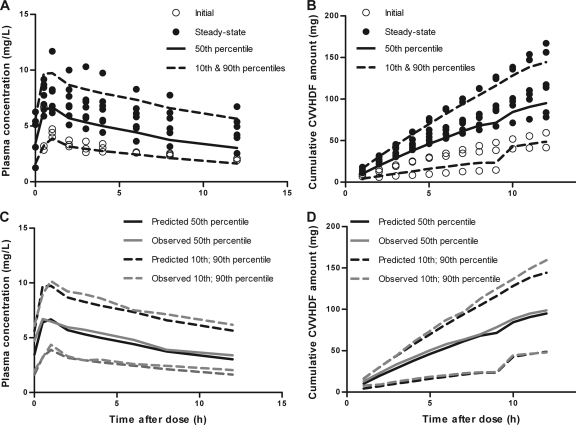
The VPC for fluconazole in plasma and in CVVHDF effluent is presented in Fig. 2. Approximately 15 to 20% of the data lie outside the 10th and 90th percentiles, with the majority of observed concentrations evenly distributed around the median (Fig. 2, top). In addition, prediction percentiles closely match corresponding observation percentiles (Fig. 2, bottom). Furthermore, median values for all parameters from the bootstrap analysis were similar to those in the final model (Table 2) and were within their respective 95th percentile range. Evaluation of eta shrinkage on clearance (0.03), volume of distribution (0.1), and infusion duration (0.08) suggested that none of the parameters were poorly estimated. These results confirm the suitability of the model for describing fluconazole disposition in this population, making it suitable for simulating new potential dosing regimens in this group.
Fig. 2.
Visual predictive check for fluconazole in plasma (A and C) and in the CVVHDF effluent (B and D). Top panels show the observations for initial (○) and steady-state (•) profiles, with model-predicted median (solid lines) and 10th and 90th percentiles (dashed lines). Bottom panels illustrate that predicted percentiles (black lines) closely match corresponding observed percentiles (gray lines).
Dosing guidelines.
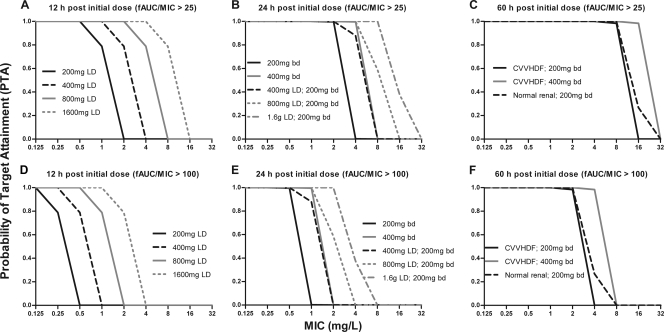
Multiple dosing regimens were assessed on the basis of the final model and by using MIC values ranging from 0.0625 mg/liter to 32 mg/liter. The abilities of the different dosing schedules to achieve a predefined pharmacodynamic endpoint (fAUC/MIC > 25) were then calculated and compared. Figure 3A shows that a 200 mg LD is ineffective for a MIC of >1 mg/liter for the first 12 h after initialization of fluconazole treatment. A 400 mg and 1,600 mg LD is required to achieve attainment for MIC of 1 mg/liter and 4 mg/liter, respectively. Similar PTA is obtained for a dose of 400 mg twice daily and a 400 mg LD with subsequent dosing of 200 mg twice daily (Fig. 3B). At steady state (60 h), the current dose (200 mg twice daily) performs better in subjects with normal renal function than in anuric patients receiving CVVHDF (Fig. 3C). For the CVVHDF patients, a dose of 400 mg twice daily provides similar or slightly better PTA for the treatment of fungal infection, with MIC values up to 16 mg/liter. For all dosing regimens, a 4-fold dose increase is required to achieve the pharmacodynamic endpoint of a fAUC/MIC ratio of >100 (Fig. 3D to F) compared to a fAUC/MIC ratio of >25.
Fig. 3.
Simulation data for the probability of target attainment (PTA) following fluconazole dosing in patients receiving CVVHDF. The dosing schedules included loading doses (LD) ranging from 200 mg to 1,600 mg (A and D), a standard dose of 200 mg twice daily following the range of LD (B and E), and the effect of CVVHDF treatment relative to that of patients with normal renal function (C and F). A PTA of 1.0 was defined when fAUC/MIC ratios exceeded 25 (A to C) and 100 (D to F). Parameters for patients with normal renal function were obtained from reference 31.
DISCUSSION
The present study develops a population PK model that describes the disposition of fluconazole in critically ill anuric patients receiving CVVHDF. This model is then used to identify dosing schedules that achieve the pharmacodynamic endpoints that should be associated with optimal fluconazole activity. We show that a significant dose increase (doubling) is required for the treatment of fungal infection in anuric patients for whom CVVHDF with the stated settings is also prescribed. This is the first study we are aware of that uses Monte Carlo simulations in this patient population to inform dosing requirements of fluconazole.
In the final covariate model, the estimate for total fluconazole clearance by CVVHDF and non-CVVHDF routes (2.67 liters/h) is considerably faster than that previously reported (1.18 liters/h) for healthy patients (31). Differences in clearance are expected with renal insufficiency, particularly for drugs that are predominantly eliminated via the kidneys (17, 35). A possible explanation for this relatively faster clearance in people receiving CVVHDF is that tubular reabsorption is probably nonexistent in anuric patients, and there is no ability for reabsorption via the CVVHDF process. This assertion is not unreasonable, given that fluconazole is extensively reabsorbed in subjects with normal renal function (9) and is therefore used as a marker of reabsorption in renal cocktails (35).
Extracorporeal elimination by CVVHDF accounted for approximately 62% of total fluconazole clearance, with a BSV of 19.8% when the effect of filter age was included in the model (Table 2). The corresponding BSV for non-CVVHDF clearance was relatively high (77%), which we suggest is expected due to the disease status of these critically ill patients. Of note was our finding that filters in use >48 h considerably reduced the efficiency of dialysis to 37% of total fluconazole clearance, which could explain some of the random BSV associated with CVVHDF clearance. However, while this finding appears of clinical relevance, caution must be applied, since only 1 patient received CVVHDF treatment with a 48-h filter. Further data are required to confirm the long-term effects of filter age on fluconazole clearance. Alternatively, a cautious approach would be to recommend filter changes more frequently than every 48 h to enable more predictable clearance of fluconazole.
The steady-state volume of distribution (Vc plus Vp) for fluconazole in this cohort of patients (53.6 liters) was consistent with that previously reported (55.7 liters) in healthy volunteers (31). However, the disposition of fluconazole was best described by a one-compartment model in the latter study, unlike the two-compartment model presented here. One possible reason for the existence of a peripheral compartment is drug redistribution, which could arise due to leaky capillaries and other pathophysiological changes in critically ill patients (25, 30).
Monte Carlo dosing simulations were performed to calculate the probability of achieving pharmacodynamically relevant fAUC/MIC ratios following fluconazole treatment. These were necessary as the enhanced fluconazole clearance in this CVVHDF group will result in considerably smaller AUC values than those in healthy subjects. Two different fAUC/MIC breakpoints were applied to reflect potentially more severe infections, as well as the uncertainty in PK/PD target values required for successful therapy. This also accounts for the different types of data that were utilized to determine PK/PD target values by CLSI and EUCAST. The simulations include the first 12 h following dosing, since early attainment of therapeutic target concentrations is essential to improve efficacy during antimicrobial therapy (15, 16, 27). At 12 h postinitialization of treatment, a standard loading dose of 200 mg is effective only for MICs up to 1 mg/liter when using the target of a fAUC/MIC ratio of >25 (Fig. 3A). For early attainment beyond this 1 mg/liter MIC value, higher loading doses are required and can be directly obtained from Fig. 3A. At steady state (60 h post initial dose), a dose of 400 mg twice a day maximizes treatment against Candida with MIC values up to 16 mg/liter (Fig. 3C). For attainment of fAUC/MIC values of >100, a 4-fold dose increase is required compared to the fAUC/MIC target of >25 (Fig. 3D to F). Several studies have previously recommended a daily dose of 800 mg with CVVHDF (4, 8, 12, 14, 20, 22, 26, 36, 39). However, due to study design and analysis methods, these recommendations do not accurately identify between-subject variability, the effect of covariates such as filter age, or the likely probability of attainment at steady state. The study presented here is the first and largest study to date that considers fluconazole dosing in patients that are likely to lack renal tubular reabsorption. Furthermore, the analysis method utilized (NONMEM), appropriately handles the issues of between-subject variability and covariate inclusion. Clearly, the current dose of 200 mg twice daily performs worse in anuric patients compared to that in healthy volunteers.
Several potential limitations of using Monte Carlo simulations must be considered. First, a small sample size (10 subjects) was used in the current study, and this may not represent the true PK variability in a population of critical care patients (29, 34). However, the recruitment of large numbers of critically ill patients is challenging, and simulations based on small cohorts are instructive for assessing altered dosing strategies, especially when it is clear that a large change in dosage from the usual is required to attain suitable concentrations. A second limitation is the use of published MIC data, given that the susceptibilities of Candida species can vary over time, between countries and between hospitals (29, 30). For this reason, a range of MIC values (0.0625 to 32 mg/liter) was used in the present study, such that suitable dosing schedules for an organism resistance patterns can be determined from data shown in Fig. 3. Falsely high PTA could arise if modeling free drug concentration in healthy subjects (plasma protein binding, ∼10%) as opposed to that in critical care patients. However, this is unlikely to confound the results presented here, since the plasma protein binding of fluconazole is only slightly higher (22%) in critically ill patients (2). Finally, the dosing simulations presented here are representative of patients receiving adequate or average filtration. On this basis, target concentration intervention (TCI) could be considered to further optimize fluconazole dosing if subtherapeutic exposure is suspected due to organisms with high MICs or if dialysis filter clotting is an issue.
Conclusions.
Fluconazole is an important antimycotic agent that is used for the treatment of fungal infections across a wide range of patient populations. However, when used in anuric patients receiving CVVHDF, the total clearance of fluconazole is significantly increased. In this study, we show that doubling the current dose of 200 mg twice daily (to 400 mg twice daily) better achieves the pharmacodynamic endpoint associated with optimal antifungal activity. A dose increase is therefore highly recommended in similar patient cohorts prescribed both CVVHDF and fluconazole treatment.
ACKNOWLEDGMENTS
We thank K. Avent for performing the fluconazole assays.
This study was supported by institutional departmental funds only.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Andes D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47: 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arredondo G., Martinez-Jorda R., Calvo R., Aguirre C., Suarez E. 1994. Protein binding of itraconazole and fluconazole in patients with chronic renal failure. Int. J. Clin. Pharmacol. Ther. 32: 361–364 [PubMed] [Google Scholar]
- 3. Bellomo R., et al. 2009. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 361: 1627–1638 [DOI] [PubMed] [Google Scholar]
- 4. Bergner R., et al. 2006. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): need for a high daily dose of 800 mg. Nephrol. Dial. Transplant. 21: 1019–1023 [DOI] [PubMed] [Google Scholar]
- 5. Boeckmann A. J., Sheiner L. B., Beal S. 1994. NONMEM users guide—part V: introductory guide. NONMEM Project Group, University of California at San Francisco, San Francisco, CA [Google Scholar]
- 6. Choi G., et al. 2009. Principles of antibacterial dosing in continuous renal replacement therapy. Crit. Care Med. 37: 2268–2282 [DOI] [PubMed] [Google Scholar]
- 7. Clancy C. J., Yu V. L., Morris A. J., Snydman D. R., Nguyen M. H. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49: 3171–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cousin L., Berre M. L., Launay-Vacher V., Izzedine H., Deray G. 2003. Dosing guidelines for fluconazole in patients with renal failure. Nephrol. Dial. Transplant. 18: 2227–2231 [DOI] [PubMed] [Google Scholar]
- 9. Debruyne D. 1997. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 33: 52–77 [DOI] [PubMed] [Google Scholar]
- 10. Debruyne D., Ryckelynck J. P. 1993. Clinical pharmacokinetics of fluconazole. Clin. Pharmacokinet. 24: 10–27 [DOI] [PubMed] [Google Scholar]
- 11. Dufour G., Montravers P. 2009. Pharmacokinetics of antibiotics or antifungal drugs in intensive care units. Curr. Infect. Dis. Rep. 11: 14–20 [DOI] [PubMed] [Google Scholar]
- 12. Heintz B. H., Matzke G. R., Dager W. E. 2009. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy 29: 562–577 [DOI] [PubMed] [Google Scholar]
- 13. Joy M. S., Matzke G. R., Armstrong D. K., Marx M. A., Zarowitz B. J. 1998. A primer on continuous renal replacement therapy for critically ill patients. Ann. Pharmacother. 32: 362–375 [DOI] [PubMed] [Google Scholar]
- 14. Kishino S., et al. 2001. Effective fluconazole therapy for liver transplant recipients during continuous hemodiafiltration. Ther. Drug Monit. 23: 4–8 [DOI] [PubMed] [Google Scholar]
- 15. Kollef M. H., Sherman G., Ward S., Fraser V. J. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115: 462–474 [DOI] [PubMed] [Google Scholar]
- 16. Kumar A., et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34: 1589–1596 [DOI] [PubMed] [Google Scholar]
- 17. Lam Y. W., Banerji S., Hatfield C., Talbert R. L. 1997. Principles of drug administration in renal insufficiency. Clin. Pharmacokinet. 32: 30–57 [DOI] [PubMed] [Google Scholar]
- 18. Levy E. M., Viscoli C. M., Horwitz R. I. 1996. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494 [PubMed] [Google Scholar]
- 19. McLachlan A. J., Gross A. S., Beal J. L., Minns I., Tett S. E. 2001. Analytical validation for a series of marker compounds used to assess renal drug elimination processes. Ther. Drug Monit. 23: 39–46 [DOI] [PubMed] [Google Scholar]
- 20. Muhl E., Martens T., Iven H., Rob P., Bruch H. P. 2000. Influence of continuous veno-venous haemodiafiltration and continuous veno-venous haemofiltration on the pharmacokinetics of fluconazole. Eur. J. Clin. Pharmacol. 56: 671–678 [DOI] [PubMed] [Google Scholar]
- 21. Pai M. P., Turpin R. S., Garey K. W. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51: 35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48: 503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pappas P. G., et al. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38: 161–189 [DOI] [PubMed] [Google Scholar]
- 24. Parke J., Holford N. H., Charles B. G. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Computer Methods Programs Biomed. 59: 19–29 [DOI] [PubMed] [Google Scholar]
- 25. Patel K., Kirkpatrick C. M. J. Pharmacokinetic concepts revisited—basic and applied. Curr. Pharm. Biotechnol., in press [DOI] [PubMed] [Google Scholar]
- 26. Pittrow L., Penk A. 1999. Dosage adjustment of fluconazole during continuous renal replacement therapy (CAVH, CVVH, CAVHD, CVVHD). Mycoses 42: 17–19 [DOI] [PubMed] [Google Scholar]
- 27. Playford E. G., Lipman J., Sorrell T. C. 2010. Management of invasive candidiasis in the intensive care unit. Drugs 70: 823–839 [DOI] [PubMed] [Google Scholar]
- 28. Reetze-Bonorden P., Bohler J., Keller E. 1993. Drug dosage in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clinical Pharmacokinet. 24: 362–379 [DOI] [PubMed] [Google Scholar]
- 29. Roberts J. A., Kirkpatrick C. M. J., Lipman J. 2010. Monte-Carlo simulations: maximizing antibiotic pharmacokinetic data to optimise clinical practice for critically ill patients. J. Antimicrob. Chemother. 66: 227–231 [DOI] [PubMed] [Google Scholar]
- 30. Roberts J. A., Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37: 840–851 [DOI] [PubMed] [Google Scholar]
- 31. Roos J. F., Kirkpatrick C. M. J., Tett S. E., McLachlan A. J., Duffull S. B. 2008. Development of a sufficient design for estimation of fluconazole pharmacokinetics in people with HIV infection. Br. J. Clin. Pharmacol. 66: 455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schetz M., Ferdinande P., Van den Berghe G., Verwaest C., Lauwers P. 1995. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 21: 612–620 [DOI] [PubMed] [Google Scholar]
- 33. Schetz M., Lauwers P., Ferdinande P. 1989. Extracorporeal treatment of acute renal failure in the intensive care unit: a critical view. Intensive Care Med. 15: 349–357 [DOI] [PubMed] [Google Scholar]
- 34. Tam V. H., Kabbara S., Yeh R. F., Leary R. H. 2006. Impact of sample size on the performance of multiple-model pharmacokinetic simulations. Antimicrob. Agents Chemother. 50: 3950–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tett S. E., Kirkpatrick C. M. J., Gross A. S., McLachlan A. J. 2003. Principles and clinical application of assessing alterations in renal elimination pathways. Clin. Pharmacokinet. 42: 1193–1211 [DOI] [PubMed] [Google Scholar]
- 36. Trotman R. L., Williamson J. C., Shoemaker D. M., Salzer W. L. 2005. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin. Infect. Dis. 41: 1159–1166 [DOI] [PubMed] [Google Scholar]
- 37. Uchino S., et al. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study JAMA 294: 813–818 [DOI] [PubMed] [Google Scholar]
- 38. Valtonen M., Tiula E., Neuvonen P. J. 1997. Effect of continuous venovenous haemofiltration and haemodiafiltration on the elimination of fluconazole in patients with acute renal failure. J. Antimicrob. Chemother. 40: 695–700 [DOI] [PubMed] [Google Scholar]
- 39. Yagasaki K., et al. 2003. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med. 29: 1844–1848 [DOI] [PubMed] [Google Scholar]