Abstract
We have previously reported that two receptor tyrosine kinase inhibitors (RTKIs), called AG879 and tyrphostin A9 (A9), can each block the replication of influenza A virus in cultured cells. In this study, we further characterized the in vitro antiviral efficacies and specificities of these agents. The 50% effective concentration (EC50) of each against influenza A was found to be in the high nanomolar range, and the selectivity index (SI = 50% cytotoxic concentration [CC50]/EC50) was determined to be >324 for AG879 and 50 for A9, indicating that therapeutically useful concentrations of each drug produce only low levels of cytotoxicity. Each compound showed efficacy against representative laboratory strains of both human influenza A (H1N1 or H3N2) and influenza B viruses. Importantly, no drug-resistant influenza virus strains emerged even after 25 viral passages in the presence of AG879, whereas viruses resistant to amantadine appeared after only 3 passages. AG879 and A9 each also exhibited potent inhibitory activity against a variety of other RNA and DNA viruses, including Sendai virus (Paramyxoviridae), herpes simplex virus (Herpesviridae), mouse hepatitis virus (Coronaviridae), and rhesus rotavirus (Reoviridae), but not against Pichinde virus (Arenaviridae). These results together suggest that RTKIs may be useful as therapeutics against viral pathogens, including but not limited to influenza, due to their high selectivity indices, low frequency of drug resistance, and broad-spectrum antiviral activities.
INTRODUCTION
Despite annual vaccination programs, influenza virus continues to be a major public health concern. Due to the highly error-prone nature of the viral RNA polymerase, influenza virus variants resistant to drugs that target viral components arise commonly and spread rapidly. Indeed, viral variants resistant to each of the currently available anti-influenza drugs, i.e., to M2 ion channel inhibitors (amantadine and rimantadine) and neuraminidase inhibitors (zanamivir and oseltamivir), are readily isolated (2, 3, 14, 22, 27). Targeting host signaling pathways or other host cell factors important for influenza virus replication, therefore, represents a potential alternative approach that can minimize the emergence of drug-resistant viruses.
Receptor tyrosine kinases (RTKs) are a group of growth factor receptors that regulate a variety of cellular activities related to growth, metabolism, and differentiation (15). Due to their critical roles in the development and progression of various cancers, RTKs such as epidermal growth factor receptor (EGFR) and erythroblastosis oncogene homolog 2/hergulin receptor 2 (ErbB2/HER2) have been studied extensively as targets for anticancer therapeutics (16). RTKs and other tyrosine kinases have also been shown to play important roles in virus replication. For example, the tyrosine kinase inhibitor genistein was found to block replication of type-1 human immunodeficiency virus (HIV-1), herpes simplex virus type 1 (HSV-1), and certain arenaviruses (24, 25, 28), whereas Src family kinases were shown to be important for the assembly and maturation of dengue virus and West Nile virus (1, 9). Similarly, two of the major signaling pathways (i.e., the Raf/MEK/ERK and PI3K/Akt pathways) that are activated by RTKs were found to play important roles in influenza virus replication (4, 5, 7, 21), and a recent report (6) suggests that EGFR signaling is important to promote influenza A virus uptake by human target cells. Our laboratory recently identified two tyrphostin-type RTK inhibitors (RTKIs), called AG879 and tyrphostin A9 (A9), that each strongly inhibit influenza A virus replication in cultured cells. Specifically, each agent was found to block at least three postentry steps of the influenza virus life cycle, including viral RNA synthesis, Crm1-dependent nuclear export of viral ribonucleoprotein (vRNP), and virus assembly and budding (12). A9 is a selective inhibitor of the platelet-derived growth factor receptor (17), whereas AG879 is known to inhibit tyrosine kinase activity of the nerve growth factor receptor (TrkA) and the heregulin receptor erbB-2 (HER-2) (19). Using additional small-molecule inhibitors and small-hairpin RNA (shRNA)-mediated specific-gene knockdown, we verified that, indeed, the TrkA signaling pathway plays important roles in influenza A virus replication (12).
In the current study, we have extended our previous findings (12) by quantitatively assessing the anti-influenza efficacy and specificity of AG879 and A9, evaluating the evolution of viral drug resistance, and testing the activities of these compounds against several distinct RNA and DNA virus families. Collectively, our studies suggest that RTKIs may have significant potential as broad-spectrum antiviral drugs.
MATERIALS AND METHODS
Compounds, viruses, and cells.
All chemical compounds were purchased from Sigma. A549 cells (human lung epithelial cells) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). Madin-Darby canine kidney (MDCK) cells were maintained in Eagle's minimal essential medium (MEM) supplemented with 5% FBS. Vero (African green monkey kidney epithelial) cells were grown in MEM supplemented with 5% fetal bovine serum. Rhesus monkey kidney MA104 cells were grown in MEM supplemented with sodium pyruvate, sodium bicarbonate, nonessential amino acids, and 10% FBS. Rat lung epithelial (L2) cells were grown in DMEM supplemented with 10% FBS. Baby hamster kidney epithelial (BHK-21) cells were grown in MEM with 10% FBS.
Sendai virus and influenza virus A/Aichi ×31 were obtained from S.-M. Kang and R. Compans (Emory University). Influenza virus B/Victoria was purchased from ATCC. Sendai virus and various influenza virus strains (A/WSN/33, A/PR8/34, A/Aichi ×31, and B/Victoria) were grown in 10-day-old embryonated chicken eggs. The viral titers were determined by plaque assay on MDCK (influenza virus) or Vero (Sendai virus) cells. Herpes simplex virus (HSV) Kos strain was provided by B. Rouse (University of Tennessee) and was grown in Vero cells. Mouse hepatitis virus (MHV) was obtained from D. Brian (University of Tennessee) and was grown in L2 cells. Rhesus rotavirus (RRV) was obtained from M. Vijay-Kumar and A. Gewirtz (Emory University) and was grown in MA104 cells. Pichinde virus was derived from our reverse genetics system (13) and was grown in BHK-21 cells, and viral titers were determined by plaque assay on Vero cells.
After infection with influenza A virus, MDCK cells were grown in low-serum L-15 medium, which consists of 15 mM HEPES (pH 7.5), nonessential amino acids, 0.75 g of sodium bicarbonate per liter, and 0.125% (wt/vol) bovine serum albumin (BSA). With the exception of A/WSN/33, infection of cultured cells with influenza viruses was conducted in the presence of trypsin at a concentration of 2.5 μg per ml. Both HSV and MHV infections were conducted in low-serum DMEM medium supplemented with 0.1% BSA. RRV infection of MA104 cells was conducted by treating RRV with 10 μg/ml of EDTA-free trypsin at 37°C for 30 min followed by the infection of cells in serum-free medium with 10 μg/ml of trypsin. Infections with Sendai virus and Pichinde virus were conducted in serum-free DMEM medium.
CC50 determination.
A549 cells in 96-well plates were treated with 10-fold dilutions of chemicals or dimethyl sulfoxide (DMSO) control, in triplicates, in a total of 100 μl growth medium for 48 h. An amount of 20 μl of freshly made 5 mg/ml MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] solution was added to each well, and cells were incubated at 37°C for 5 h. After removal of the medium, 200 μl of DMSO was added to each well to dissolve the purple formazan product and the plates were incubated at 37°C for another 5 min to remove any air bubbles. MTT signals were measured photometrically at an absorbance of 550 nm. The 50% cytotoxic concentration (CC50) was calculated as the concentration needed to reduce cellular viability to 50%.
EC50 determination.
A549 cells in triplicates were infected with influenza virus A/WSN/33 at a multiplicity of infection (MOI) of 0.1 for 1 h, washed three times with phosphate-buffered saline (PBS), and replaced with fresh L-15 medium containing either DMSO or serial 3-fold dilutions of chemical compounds in duplicates. Viral titers in the supernatants at 24 h postinfection (h.p.i.) were determined by plaque assay on MDCK cells. The 50% effective concentration (EC50) was calculated as the concentration required to reduce viral yield by 50% in the compound-treated cultures when compared to the viral yield in DMSO-treated cultures.
Virucidal activity determination.
Virus suspensions containing approximately 1 × 106 PFU of A/WSN/33 viruses were incubated in serum-free medium containing either DMSO or serial 5-fold dilutions of compounds for 1.5 h at 37°C. The mixed samples were chilled at 4°C and diluted by 104-, 105-, and 106-fold with serum-free medium before being applied onto MDCK cells in 6-well plates for plaque assaying. Plaques were scored at 48 h.p.i. after crystal violet staining, and the results plotted against the concentrations of compounds used. The 50% inactivating concentration (IC50) was calculated as the concentration required to inactivate the cell-free virions by 50%.
Inhibitory effects of RTKIs on influenza virus replication.
MDCK or A549 cells were infected in triplicate with influenza virus strains at the stated molar ratio of infection (MOI) for 1 h, washed with PBS three times, and replaced with fresh L-15 medium with either vehicle control DMSO, 10 μM AG879, 4 μM A9, or 10 μM negative control AG494. Virus production at different time points was quantified by plaque assay.
Selection of drug-resistant influenza virus strains.
Influenza A/WSN/33 was sequentially passaged in MDCK cells at an MOI of 0.01 in the presence of DMSO, 1 to 5 μM AG879, or 50 μM amantadine for 48 to 72 h. After each passage, viruses released into the supernatant were quantified by plaque assay.
Effect of RTKIs on replication of different viruses.
The antiviral effects of RTKIs on different viruses were studied by comparing the levels of viral replication in target cells in the absence or presence of RTKIs. We characterized the effects of RTKIs on the infection of Sendai virus in A549 cells, HSV in Vero cells, MHV in L2 cells, RRV in MA104 cells, and Pichinde virus in A549 cells. To do this, target cells in triplicate were pretreated with DMSO, AG879 (10 μM), A9 (4 μM), or AG494 (10 μM) for 30 min prior to virus infection at an MOI of 0.1 for 1 h. After being washed three times with PBS, the infected cells were grown in serum-free medium containing DMSO or the respective compound. Viral titers in the supernatants at 16 to 24 h.p.i. were quantified by plaque assay. Alternatively, RRV viral particles were analyzed by enzyme-linked immunosorbent assay (ELISA) (26). Briefly, 2-fold serial dilutions of supernatants were added to the 96-well plates coated with anti-RRV polyclonal serum and incubated with guinea pig anti-RRV antibody followed by horseradish peroxidase (HRP)-conjugated anti-guinea pig antibody. After being washed with PBS, the HRP substrates were added to the plates and the samples were measured at an optical density of 490 nm (OD490).
Examination of anti-influenza activity of AG879 in the influenza virus-infected mouse model.
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. Briefly, 6- to 8-week-old female BALB/c mice were housed for 3 to 5 days for acclimation and then infected intranasally with 10 50% mouse lethal doses (MLD50) of A/PR8 virus in a 50-μl volume. DMSO-PBS (16%, vol/vol) or AG879 at a dosage of 5 mg/kg of body weight was given daily for 5 days to the infected animals by intraperitoneal injection. Mice (n = 5 per group) were monitored daily for clinical symptoms and body weight loss until day 21. Mice were euthanized if they reached prespecified terminal points as previously described (18). Three mice per group were euthanized at day 3, and the viral titers in their lungs were analyzed by plaque assay.
Statistical analyses.
Statistical analysis of the survival curve by log-rank (Mantel-Cox) χ2 test was conducted using GraphPad Prism 5 software. Statistical comparison of viral titers among different treatments presented throughout the paper was performed using Student's t test.
RESULTS
In vitro efficacy of AG879 and A9 against influenza A virus.
We previously screened a small library of protein kinase inhibitors for anti-influenza activities and identified two tyrphostin-type RTKI compounds, AG879 and A9 (Fig. 1), that exhibited strong inhibitory effects on influenza A replication in vitro (12). To evaluate their potentials as anti-influenza therapeutics, we therefore set out to quantify more precisely their cytotoxic concentrations (CC50) in cultured A549 human lung epithelial cells and their effective concentrations (EC50) against influenza A viral replication. The CC50 (i.e., the concentration required to produce cytotoxic effects in 50% of target cells) was determined by using an MTT assay to estimate the viability of A549 cells grown in the presence of increasing concentrations (up to 81 μM) of each tested compound. As shown in Fig. 2A, no cytotoxicity was observed even after 48 h of incubation of A549 cells with AG879 at 81 μM (CC50 > 81 μM), whereas cell viability was noticeably affected by exposure to A9 over much of the range of concentrations we tested (CC50 = 8 μM). To determine the half-maximal effective concentration (EC50) of each compound alone, we measured the yield of influenza virus infectious units in the presence of inhibitor concentrations ranging from 0.032 μM to 10 μM. The EC50, defined as the concentration required to inhibit infectious viral yield by 50%, was found to be 250 nM for AG879 and 160 nM for A9 (Fig. 2B). Therefore, the selectivity indices (SI), defined as CC50/EC50, were calculated to be >324 for AG879 and 50 for A9 (Fig. 2D), providing one measure of the potential therapeutic utility of each compound. To determine whether the inhibitory effects of these RTKIs are partially due to direct inactivation of cell-free virions, we incubated infectious virions with increasing concentrations of each compound for 1.5 h and then tested their infectivity on cultured target cells. As shown in Fig. 2C, neither AG879 nor A9 significantly inhibited virion infectivity even at high concentrations (i.e., each showed an IC50 of >81 μM). This supports our earlier conclusion that the anti-influenza activities of AG879 and A9 are due to their inhibitory effects on viral replication within the target cells.
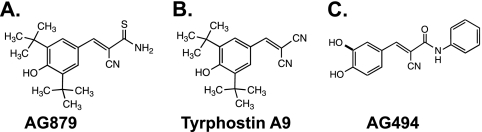
Fig. 1.
Chemical structures of AG879 (A), tyrphostin A9 (B), and AG494 (C).
Fig. 2.
Characterization of AG879 and A9 for cytotoxicity and anti-influenza efficacy. (A) Determination of the 50% cytotoxic concentrations (CC50) of AG879, A9, and AG494. A549 cells were incubated with various concentrations of the compounds for 48 h and measured for cell viability by MTT assay. (B) Determination of the 50% efficacy concentration (EC50) of AG879, A9, or AG494 in blocking influenza A virus replication in vitro. A549 cells were infected with influenza virus at an MOI of 0.1 in the presence of various concentrations of compounds. Viral titers at 24 h.p.i. were quantified by plaque assay. (C) Determination of the virucidal activities of AG879, A9, and AG494. The 50% inactivation concentrations (IC50) of AG879, A9, and AG494 to directly inactivate cell-free influenza virions were determined. (D) Summary of CC50, EC50, IC50, and selective indices (SI) for AG879 and A9. The results shown in panels A to C are the averages of at least three independent experiments, with error bars showing standard deviations.
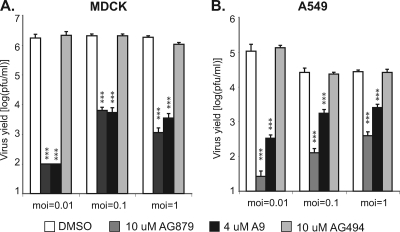
AG879 and A9 block influenza A virus infection in various cell types and virus-to-cell ratios.
To determine to what extent the anti-influenza activity of AG879 and A9 is affected by cell type or virus-to-cell ratio, we conducted viral inhibition assays in two different cell types (A549 lung and MDCK canine kidney epithelial cells) with various multiplicities of infection (MOI, defined as the ratio of input infectious viral particles per target cell). Both cell types were infected with A/WSN virus at an MOI of 0.01, 0.1, or 1 in the presence either of AG879 or A9, of vehicle control (DMSO), or of an unrelated RTKI called AG494 that we had previously shown (12) to lack anti-influenza activity in this assay. AG494 is a potent inhibitor of epidermal growth factor receptor (EGFR) signaling in cell-free kinase assays, but it cannot inhibit EGFR in intact cells (20) and thus served as a negative control. As influenza virus replication peaks at various time points with different MOIs, we quantified viral yield in the supernatants at 48 h (MOI = 0.01), 18 h (MOI = 0.1), or 9 h (MOI = 1) after infection. As shown in Fig. 3, both RTKIs strongly blocked infectious virus production in both cell lines and at all tested virus-to-cell ratios, with the degree of inhibition ranging from 1 to >4 log. These findings imply that the inhibition of influenza A replication by AG879 or A9 is not unique to A549 cells and is effective against relatively high levels of input virus. Additional evidence for the efficacy of these compounds in a wide range of cell types is presented below.
Fig. 3.
AG879 and A9 inhibit influenza virus replication in different cell types and with different virus-to-cell molar ratios. Inhibition of virus production was conducted on MDCK cells (A) or A549 cells (B). Viral yield in the supernatants was quantified at 48 h (for an MOI of 0.01), 18 h (for an MOI of 0.1), or 9 h (for an MOI of 1) after infection. The results shown are the averages of at least three independent experiments, with error bars showing standard deviations. Pairwise statistical comparisons to the DMSO control group were performed using Student's t test. ***, P < 0.001.
AG879 and A9 are effective against diverse strains of influenza virus.
To evaluate the inhibitory effects of these compounds against various influenza virus strains, we infected A549 cells with laboratory strains of H1N1 influenza A (A/WSN/33 or A/PR8/34), H3N2 influenza A (A/Aichi X31), or influenza B (B/Victoria) at an MOI of 0.01 in the presence of the tested compounds. As shown in Fig. 4, each of these four influenza strains replicated to high titers at 48 h.p.i. in the presence of vehicle control (DMSO) or of the inactive control compound AG494. For each of the influenza A and B strains, AG879 and A9 strongly inhibited viral production, by 2 to 3 log (Fig. 4). As A/H1N1, A/H3N2, and influenza B virus are the predominant circulating human influenza strains, our data suggest that RTKIs with activity profiles similar to those of AG879 and A9 could have therapeutic value in treating all of the circulating seasonal influenza viruses that are currently extant.
Fig. 4.
AG879 and A9 inhibit replication of various influenza virus strains. Inhibition of virus production was conducted using the influenza virus strains A/WSN, A/PR8, A/Aichi ×31, and B/Victoria at an MOI of 0.01 as described in Materials and Methods. Viral production at 48 h.p.i. was quantified by plaque assay. The results shown are the averages of at least three independent experiments, with error bars showing standard deviations. Pairwise statistical comparisons to the DMSO control group were performed using Student's t test. ***, P < 0.001.
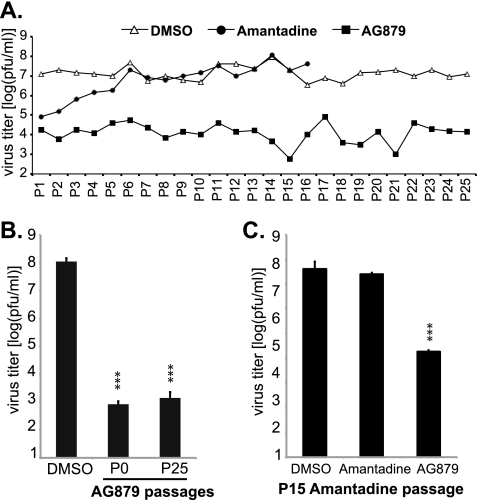
Failure to generate AG879-resistant influenza viruses.
One major theoretical advantage of developing antiviral drugs that target host components compared to antiviral drugs against viral targets is that host-targeting compounds are less likely to favor the evolution of resistant viral strains. This is particularly important for anti-influenza drug development, as drug-resistant influenza strains are known to emerge and spread very quickly. We evaluated the ability of H1N1 influenza virus to develop resistance to RTKIs during long-term in vitro culture (up to 25 passages at an MOI of 0.01) of A/WSN virus in medium containing AG879, vehicle control DMSO, or amantadine, which is an FDA-approved drug that inhibits the viral M2 ion channel protein. Viral supernatants were harvested at 48 h following each passage and were then used to infect fresh A549 cells, and the viral titer in each successive supernatant was quantified, as shown in Fig. 5A. Throughout the long-term culture, DMSO-treated cells consistently supported high levels of virus production, at titers of ∼107 PFU/ml. The presence of amantadine significantly reduced virus production, by ∼2 log, at the earliest passages (first passage [P1] and P2), consistent with its known potent anti-influenza activity. However, viral production began to increase detectably after the third passage in medium containing amantadine and regained the level seen in the DMSO control after the sixth passage, indicating that amantadine-resistant virus strains had successfully emerged. This is consistent with previous reports that amantadine-resistant mutants can be isolated after as few as two passages (8). In contrast, even after 25 passages in AG879, virus production was still significantly lower than that in DMSO (by >2 log), indicating the absence of highly AG879-resistant viruses. Indeed, when we compared the original (P0) and P25 viral stocks for their sensitivity to AG879 inhibition, both showed comparably strong sensitivity to AG879; in particular, AG879 reduced virus production by either stock to a level ∼4 log lower than that of the DMSO control (Fig. 5B). This suggests that the P25 stock does not contain significant quantities of AG879-resistant mutants. Moreover, the 15th amantadine-passaged virus supernatant was found to be completely resistant to amantadine but remained as sensitive to AG879 as the original virus (Fig. 5C), suggesting that AG879 is able to inhibit even those viruses that have already developed resistance to another antiviral drug. Taken together, our data clearly show that the rate at which influenza viruses evolve resistance to RTKIs is extremely low, suggesting that RTKIs might possibly be useful as salvage therapy in clinical settings where the virus has developed resistance to other available drugs.
Fig. 5.
Selection of drug-resistant influenza viruses in vitro. (A) Influenza viruses were passaged in medium containing DMSO, amantadine, or AG879 as described in Materials and Methods. Viral titers in the supernatants at each passage were determined. (B) The viruses collected from either the original (P0) or the 25th (P25) AG879 passage were used to infect A549 cells at an MOI of 0.01 with and without 10 μM AG879. Virus production at 48 h.p.i. was quantified by plaque assay. (C) The 15th amantadine-passaged virus was used to infect A549 cells at an MOI of 0.01 in the presence of DMSO, amantadine (50 μM), or AG879 (10 μM). Virus production at 48 h.p.i. was quantified by plaque assay. The results shown in panels B and C are the averages of at least three independent experiments, with error bars showing standard deviations. Statistical analyses were performed using Student's t test. ***, P < 0.001.
Anti-influenza activity of AG879 in an infected mouse model.
We used a well-established lethal influenza virus-mouse model to assess the antiviral activity of RTKIs (18). Mice were infected with a lethal dosage of influenza virus A/PR8 and then treated with either DMSO-PBS or AG879 at 5 mg per kg of body weight for 5 days, commencing on day 0. As expected, the mice treated with the DMSO-PBS vehicle control all succumbed to infection within 8 days, with 100% mortality (Fig. 6A). In contrast, 60% of mice in the AG879 treatment group survived (Fig. 6A), which is significantly higher than the survival rate in the control group (P < 0.05). To further verify the antiviral activity of AG879 in vivo, we quantified viral titers in the lungs from both groups at day 3, as the peak of viral replication occurs between day 2 and day 4 postinfection (18). Influenza viruses replicated to a mean titer of 4.3 × 105 PFU/mg in the lungs of DMSO-PBS-treated mice, which is nearly 2 log higher than the titers seen in the AG879-treated animals (Fig. 6B), indicating that AG879 can effectively suppress influenza virus replication in vivo. In summary, we have shown that AG879 can significantly reduce influenza virus replication and the associated mortality in infected animals, supporting the potential of RTKIs as antiviral therapeutics.
Fig. 6.
In vivo anti-influenza activity of AG879 in the lethal mouse model. Mice were infected with a lethal dosage of A/PR8 viruses and were treated with either DMSO-PBS (16%, vol/vol) or with AG879 at 5 mg/kg daily for 5 days from day 0. (A) Survival curves of infected mice (n = 5) treated with DMSO-PBS or AG879. (B) Influenza virus titers in the lungs of mice at day 3 postinfection. The results shown are the averages (n = 3), with error bars showing standard deviations. Pairwise statistical comparison was performed using Student's t test. **, P < 0.01.
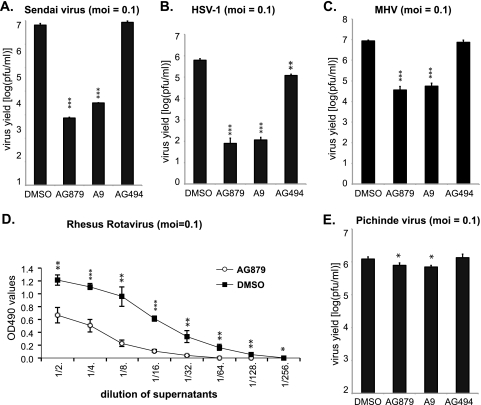
AG879 and A9 have broad-spectrum antiviral activities.
As RTK signaling pathways might in principle play important roles in regulating the life cycles of diverse viruses, we then asked whether AG879 and A9 could inhibit viruses other than influenza. The initial MTT assays verified that neither compound produced cytotoxicity at the tested concentrations in any of the target cell lines used for these studies (data not shown). To examine the effects of the compounds on the replication of Sendai virus, a member of the Paramyxoviridae family, A549 cells were infected with Sendai virus at an MOI of 0.1 in the presence of either the vehicle control DMSO, AG879, A9, or the negative control AG494. Virus production at 24 h.p.i. was compared by plaque assay. As shown in Fig. 7A, both AG879 and A9 strongly reduced Sendai virus replication, by >3 log. We then determined whether the RTKIs could inhibit herpesvirus replication using the HSV-1 Kos strain. Vero cells were infected with HSV-1 at an MOI of 0.1 for 16 h. Viral production was found to be reduced by >3 log in cells treated with either AG879 or A9 (Fig. 7B), suggesting that both RTKIs can strongly inhibit HSV-1 replication. We also noticed that the negative control AG494 slightly reduced the HSV-1 yield, by ∼0.5 log, though the significance of this remains uncertain. Replication of the prototypic coronavirus mouse hepatitis virus (MHV) was then examined in L2 cells treated with either DMSO or RTKIs, revealing that AG879 and A9 could significantly block MHV production, by ∼2 log at 16 h.p.i. at an MOI of 0.1 (Fig. 7C). We also evaluated the effect of AG879 on the replication of the rhesus rotavirus (RRV) in MA104 cells after infection at an MOI of 0.1. RRV viral production in the supernatants was evaluated by ELISA at 48 h.p.i. using anti-RRV polyclonal serum as previously described (26). To ensure that the RRV particles were within the linear detection range of the ELISA, we tested 2-fold serial dilutions of the supernatants. Based on the OD490 values (Fig. 7D), the yield of released RRV particles was significantly lower in cells treated with AG879 than in those treated with DMSO. In contrast, AG879 and A9 did not appreciably affect the in vitro replication of Pichinde virus (Arenaviridae): although the viral titers in A549 cells treated with DMSO or with the tested compounds were statistically different (P < 0.05), the difference between the mean viral titers was only ∼0.2 log (Fig. 7E). In summary, AG879 and A9 show broad-spectrum antiviral activity against various RNA and DNA viruses, including influenza virus, Sendai virus, HSV-1, MHV coronavirus, and rotavirus, with the notable exception of arenavirus.
Fig. 7.
AG879 and A9 have broad-spectrum antiviral activities. (A) A549 cells were infected with Sendai virus at an MOI of 0.1 for 24 h in the presence of either DMSO or compound. Viral yield was determined by plaque assay. (B) Vero cells were infected by HSV-1 at an MOI of 0.1 for 24 h in the presence of either DMSO or compound. Viral yield was determined by plaque assay. (C) L2 cells were infected with MHV at an MOI of 0.1 for 24 h in the presence of either DMSO or compound. Viral yield was determined by plaque assay. (D) MA104 cells were infected with RRV at an MOI of 0.1 for 48 h in the presence of either DMSO or compound. Viral particles in various dilutions of supernatants were quantified by ELISA. (E) A549 cells were infected with Pichinde virus at an MOI of 0.1 for 48 h in the presence of either DMSO or the indicated compound. Viral yield was determined by plaque assay. The results shown are the averages of at least three independent experiments, with error bars showing standard deviations. Pairwise statistical comparisons to the control group were performed using Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Here, we have shown that at least one tyrphostin-class RTKI, AG879, exhibits high potency and a high selectivity index (SI > 324) in blocking influenza A virus replication in vitro (Fig. 2). Moreover, in initial animal studies, we have now shown that AG879 has significant ability to protect mice from lethal infection by influenza A/PR8 (Fig. 6). These findings together underscore the great potential of RTKIs that target specific host-cell kinases to serve as novel therapeutic agents against influenza virus.
Both AG879 and A9 can inhibit the replication of diverse influenza A (H1N1 and H3N2) and B viral strains, which are the seasonal human influenza strains (Fig. 4). More importantly, we did not detect the emergence of resistant viruses even after 25 passages of influenza A virus in AG879-containing medium, whereas amantadine-resistant variants quickly emerged (Fig. 5A). At least two major factors may account for the extremely low frequency of emergence of AG879-resistant influenza strains. First, AG879 targets host RTK signaling rather than viral components themselves. Second, AG879 has previously been shown by us to block several independent steps of the influenza virus life cycle (12). Thus, RTKIs with properties similar to those of AG879 may have great potential as anti-influenza drugs, as they minimize the emergence and spread of drug-resistant mutants. Indeed, our data also indicate that such RTKIs could be used to inhibit the replication of viruses that have already developed resistance to the current FDA-approved drugs, such as amantadine (Fig. 5C), thus enhancing their possible utility and value in clinical settings.
We have recently shown that the anti-influenza activity of AG879 is probably due to its inhibition of TrkA signaling (12), while the precise inhibitory mechanism of A9 remains as yet unknown. In addition to TrkA, other specific RTK signaling pathways may also play important roles in influenza virus replication. Several independent genome-wide small-interfering RNA (siRNA) screens have implicated at least 5 different RTKs, including TGFR (23), FGFR-1 through -4 (11), NTRK2/TrkB (11), EphB6 (10), and EphB2 (11), and many of their downstream targets as important host factors required for influenza virus replication. Therefore, we propose that using RTKIs to target these specific RTK signaling pathways could provide significant therapeutic value against influenza infection.
An expected difficulty of using antivirals targeting host cell functions, however, is their potential toxicity in vivo. Indeed, we observed that, although AG879 at 5 mg per kg significantly reduced viral replication and the associated mortality in a mouse influenza model (Fig. 6), higher dosages of AG879 led to shorter survival than was seen in a PBS-treated control group, indicating toxic effects at the higher dosages in vivo (data not shown). Further studies will be necessary to determine the optimal dosage and duration of RTKI treatment when used for antiviral therapeutics. Due to the high selectivity index (SI > 324) of AG879 in anti-influenza activity (Fig. 2), we expect that a useful range of concentrations can indeed be identified, though the optimal therapeutic strategies may vary for different viruses and in various clinical settings. It is tempting to speculate that antiviral compounds targeting host RTKs might prove especially useful for treating acute, life-threatening infections, such as those caused by the highly pathogenic H5N1 and pandemic influenza viruses, as well as other viruses of biodefense interest.
In summary, our data suggest that certain classes of RTKIs have the potential to be developed as broad-spectrum antiviral drugs that could be used to treat various viral infections, including influenza. In recent years, significant progress has been reported in clinical and preclinical studies of RTKIs as anti-cancer therapeutics, which may facilitate the development of such compounds for use in antiviral therapies.
ACKNOWLEDGMENTS
We thank M. Vijay-Kumar and A. Gewirtz (Emory University) for providing RRV and MA104 cells, S. Kang and R. Compans (Emory University) for Sendai virus and influenza virus A/Aichi, B. Rouse (University of Tennessee) for HSV, and D. Brian (University of Tennessee) for MHV.
This work was supported in part by NIH grants AI067704 to T.G.P. and AI083409 to Y.L.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Chu J. J., Yang P. L. 2007. c-Src protein kinase inhibitors block assembly and maturation of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 104: 3520–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cox N. J., Subbarao K. 1999. Influenza. Lancet 354: 1277–1282 [DOI] [PubMed] [Google Scholar]
- 3. de Jong M. D., et al. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353: 2667–2672 [DOI] [PubMed] [Google Scholar]
- 4. Ehrhardt C., et al. 2006. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol. 8: 1336–1348 [DOI] [PubMed] [Google Scholar]
- 5. Ehrhardt C., et al. 2007. The influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 81: 3058–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eierhoff T., Hrincius E. R., Rescher U., Ludwig S., Ehrhardt C. 2010. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 6: e1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hale B. G., Jackson D., Chen Y. H., Lamb R. A., Randall R. E. 2006. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl. Acad. Sci. U. S. A. 103: 14194–14199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hay A. J., Wolstenholme A. J., Skehel J. J., Smith M. H. 1985. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 4: 3021–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch A. J., et al. 2005. The Src family kinase c-Yes is required for maturation of West Nile virus particles. J. Virol. 79: 11943–11951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karlas A., et al. 2010. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 463: 818–822 [DOI] [PubMed] [Google Scholar]
- 11. Konig R., et al. 2010. Human host factors required for influenza virus replication. Nature 463: 813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar N., Liang Y., Parslow T. G., Liang Y. 2011. Receptor tyrosine kinase inhibitors block multiple steps of influenza A virus replication. J. Virol. 85: 2818–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lan S., et al. 2009. Development of infectious clones for virulent and avirulent Pichinde viruses: a model virus to study arenavirus-induced hemorrhagic fevers. J. Virol. 83: 6357–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Q. M., et al. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437: 1108. [DOI] [PubMed] [Google Scholar]
- 15. Lemmon M. A., Schlessinger J. 2010. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levitzki A., Gazit A. 1995. Tyrosine kinase inhibition: an approach to drug development. Science 267: 1782–1788 [DOI] [PubMed] [Google Scholar]
- 17. Levitzki A., Gilon C. 1991. Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol. Sci. 12: 171–174 [DOI] [PubMed] [Google Scholar]
- 18. Matsuoka Y., Lamirande E. W., Subbarao K. 2009. The mouse model for influenza. Curr. Protoc. Microbiol. Chapter 15: Unit 15G.3 [DOI] [PubMed] [Google Scholar]
- 19. Ohmichi M., et al. 1993. The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry 32: 4650–4658 [DOI] [PubMed] [Google Scholar]
- 20. Osherov N., Gazit A., Gilon C., Levitzki A. 1993. Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J. Biol. Chem. 268: 11134–11142 [PubMed] [Google Scholar]
- 21. Pleschka S., et al. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3: 301–305 [DOI] [PubMed] [Google Scholar]
- 22. Puthavathana P., et al. 2005. Molecular characterization of the complete genome of human influenza H5N1 virus isolates from Thailand. J. Gen. Virol. 86: 423–433 [DOI] [PubMed] [Google Scholar]
- 23. Shapira S. D., et al. 2009. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell 139: 1255–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stantchev T. S., Markovic I., Telford W. G., Clouse K. A., Broder C. C. 2007. The tyrosine kinase inhibitor genistein blocks HIV-1 infection in primary human macrophages. Virus Res. 123: 178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vela E. M., Bowick G. C., Herzog N. K., Aronson J. F. 2008. Genistein treatment of cells inhibits arenavirus infection. Antiviral Res. 77: 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijay-Kumar M., et al. 2008. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 180: 8280–828518523294 [Google Scholar]
- 27. Winquist A. G., Fukuda K., Bridges C. B., Cox N. 1999. Neuraminidase inhibitors for treatment of influenza A and B infections. MMWR Surveill. Summ. 48: 1–9 [Google Scholar]
- 28. Yura Y., Yoshida H., Sato M. 1993. Inhibition of herpes simplex virus replication by genistein, an inhibitor of protein-tyrosine kinase. Arch. Virol. 132: 451–461 [DOI] [PubMed] [Google Scholar]