Abstract
To truly transform the landscape of tuberculosis treatment, novel regimens containing at least 2 new drugs are needed to simplify the treatment of both drug-susceptible and drug-resistant forms of tuberculosis. As part of an ongoing effort to evaluate novel drug combinations for treatment-shortening potential in a murine model, we performed two long-term, relapse-based experiments. In the first experiment, TMC207 plus pyrazinamide, alone or in combination with any third drug, proved superior to the first-line regimen including rifampin, pyrazinamide, and isoniazid. On the basis of CFU counts at 1 month, clofazimine proved to be the best third drug combined with TMC207 and pyrazinamide, whereas the addition of PA-824 was modestly antagonistic. Relapse results were inconclusive due to the low rate of relapse in all test groups. In the second experiment evaluating 3-drug combinations composed of TMC207, pyrazinamide, PA-824, moxifloxacin, and rifapentine, TMC207 plus pyrazinamide plus either rifapentine or moxifloxacin was the most effective, curing 100% and 67% of the mice treated, respectively, in 2 months of treatment. Four months of the first-line regimen did not cure any mice, whereas the combination of TMC207, PA-824, and moxifloxacin cured 50% of the mice treated. The results reveal new building blocks for novel regimens with the potential to shorten the duration of treatment for both drug-susceptible and drug-resistant tuberculosis, including the combination of TMC207, pyrazinamide, PA-824, and a potent fluoroquinolone.
INTRODUCTION
New drugs with potent activity against Mycobacterium tuberculosis are needed to shorten the duration of treatment for tuberculosis (TB) and facilitate the global implementation of directly observed therapy (25). Several new drugs in clinical development have demonstrated the potential to shorten TB treatment in animal models (13, 20, 23, 24, 29, 35) and even clinical trials (6, 9, 30). To truly transform the TB treatment landscape, novel regimens containing 2 or more new drugs are needed to simplify the treatment of drug-susceptible and multidrug-resistant (MDR) or extensively drug-resistant (XDR) TB alike. Therefore, a new paradigm is needed in which new regimens, rather than new drugs, become the focus of both preclinical and clinical development programs (31).
The Global Alliance for TB Drug Development (TB Alliance) recently launched a phase II study of the extended (to 2 weeks) early bactericidal activity of the novel regimen of PA-824, pyrazinamide (PZA), and moxifloxacin (MXF), a combination, which contains neither rifampin (RIF) nor isoniazid (INH) yet was superior to the current first-line regimen of RIF-PZA-INH in mice (23). While the drugs in this regimen should be active against the majority of MDR-TB isolates, PZA and MXF are unlikely to be active against XDR TB. Moreover, both PZA resistance and fluoroquinolone resistance are on the rise in MDR TB patients (1, 9, 10, 21). Replacement of one or both of these agents with a new drug with a novel mechanism of action may lead to short-course regimens active against drug-susceptible, as well as MDR and XDR, TB.
TMC207 (TMC) is a novel diarylquinoline ATP synthase inhibitor with potent in vitro activity against M. tuberculosis, including strains resistant to commonly used first- and second-line TB drugs (5). It displays bactericidal activity against replicating and nonreplicating organisms in vitro (5, 16, 26). In animal models, treatment with TMC produces impressive reductions in CFU counts (5, 12) and prevents relapse in a manner comparable to that of RIF (38). Furthermore, combinations containing TMC and PZA have demonstrated synergistic bactericidal and sterilizing activities, even exceeding those of RIF-PZA-INH in mice, as manifested by fewer relapses after a given duration of treatment and/or similar numbers of relapses after shorter durations of treatment (12, 13, 34). The addition of rifapentine (RPT), which provides greater rifamycin exposures than RIF at the same mg/kg dose in mice, shortens the duration of treatment needed to prevent relapse even further (4). Together with evidence from a randomized placebo-controlled clinical trial that addition of TMC to standard therapy for MDR TB was well tolerated and led to more rapid sputum culture conversion (9), these data suggest that TMC is an excellent candidate for novel treatment-shortening regimens.
Here we report the results of two long-term, relapse-based chemotherapy studies conducted with mice to determine the potential efficacy of novel drug regimens. The objective of the first experiment was to compare the addition of various agents to the TMC-PZA combination. The objective of the second experiment was to identify the best 3-drug combinations composed of the following 5 drugs: PZA, TMC, PA-824, MXF, and RPT. The results reveal new building blocks for novel regimens with the potential to shorten the treatment duration of both drug-susceptible and MDR or XDR TB.
MATERIALS AND METHODS
Mycobacterial strain.
M. tuberculosis H37Rv was mouse-passaged, frozen in aliquots, and subcultured in Middlebrook 7H9 broth with 10% oleic acid-albumin-dextrose-catalase (OADC; Fisher, Pittsburgh, PA) and 0.05% Tween 80 prior to infection.
Antimicrobials.
INH, RIF, PZA, MXF, RPT, and PA-824 were obtained and formulated for oral administration as previously described (29, 33). TMC, clofazimine (CFZ), and linezolid (LZD) were provided by the Global Alliance for TB Drug Development (New York, NY). TMC was formulated for oral administration in an acidified 20% hydroxypropyl-β-cyclodextrin solution as previously described (19). CFZ was suspended in sterile water with 0.05% agar. LZD was suspended in a solution composed of 5% polyethylene glycol (PEG 200; Sigma) and 95% methylcellulose (0.5%; Fisher, Suwanee, GA) in distilled water (36).
Pharmacokinetics (PK) of TMC alone and in combination with PA-824 and RIF.
The PK of TMC alone and in combination with PA-824 was confirmed in uninfected 4-month-old female BALB/c mice randomized to 1 of 3 groups. The first group received a single oral dose of TMC alone at 25 mg/kg. The second group received TMC-PA-824, with the 2 drugs combined just prior to dosing. The third group received PA-824 4 h after the administration of TMC. The dose of PA-824 was 50 mg/kg. Three mice per group were anesthetized with isoflurane and exsanguinated by cardiac puncture at 1, 2, 8, 24, 48, 72, and 96 h after TMC administration.
To determine whether RIF induces the metabolism of TMC in mice, as it does in humans (17), a single 25-mg/kg dose of TMC was administered by gavage to uninfected 4-month-old female BALB/c mice which had received 4 weeks of daily treatment with RIF at 10 mg/kg or water, administered 5 days per week. Three mice per group were sampled at 1, 2, 4, 8, 16, 24, 48, and 72 h after TMC administration.
Serum harvested from both experiments was frozen at −80°C and shipped to the Peloquin lab, where concentrations of TMC and its active M2 metabolite were determined using a validated high-performance liquid chromatography assay (M. Rouan et al., submitted for publication).
Addition of activated charcoal to solid media to reduce the effects of TMC and CFZ carryover.
Both TMC and CFZ accumulate in animal tissues with repeated dosing, introducing the potential for drug retained in organ homogenates at the time of sacrifice to suppress the growth of viable bacteria when plated on solid media and lead to overestimation of drug activity (14, 15, 18). Use of Lowenstein-Jensen medium (LJ) or supplementation of Middlebrook 7H10 or 7H11 medium with bovine serum albumin (BSA) takes advantage of TMC's high protein binding to limit the effects of drug carryover (18). However, there are no published data on the extent of protein binding by CFZ and LJ is ineffective at preventing the effects of CFZ carryover (15). We studied supplementation of 7H11 agar with activated charcoal as a means of adsorbing both TMC and CFZ in in vitro experiments designed to simulate drug carryover. Initial experiments used Mycobacterium smegmatis before results were confirmed with M. tuberculosis H37Rv. Activated charcoal powder (Sigma, St. Louis, MO) was added to 7H11 agar (0.2 or 0.4%, wt/vol) prior to autoclaving and the addition of OADC and selective antibiotics. CFZ and TMC were dissolved in dimethyl sulfoxide (DMSO) and diluted in phosphate-buffered saline (PBS). Serial 2-fold dilutions ranging from 0.03 to128 μg/ml were prepared in 15-ml conical vials such that the DMSO concentration did not exceed 15%. Drug-free control vials (PBS and 15% DMSO) were also prepared. Vials were then spiked with aliquots of diluted broth culture containing approximately 102 and 103 CFU of M. smegmatis or M. tuberculosis. After mixing, serial 0.4-ml aliquots were plated on 7H11 agar plus OADC (plain agar) and plain agar supplemented with 0.2% or 0.4% charcoal. Plates were incubated at 37°C for 3 days (for M. smegmatis) or 4 weeks (M. tuberculosis) before final CFU counts were determined.
Aerosol infection with M. tuberculosis.
All animal procedures were approved by the Institutional Animal Care and Use Committee. Aerosol infections were performed as previously described (24). Briefly, 5-week-old female BALB/c mice (Charles River, Wilmington, MA) were infected with M. tuberculosis H37Rv using the Inhalation Exposure System (Glas-Col, Terre Haute, IN) and a fresh log-phase broth culture (optical density at 600 nm of 0.8 to 1.0) with the goal of implanting between 3.5 and 4.0 log10 CFU in the lungs of each mouse. Two mice from each of 4 aerosol infection runs were humanely killed 1 day after infection to determine the number of bacteria implanted in the lungs in each run.
Chemotherapy.
Mice were block randomized by run to experimental arms and treatment was initiated 14 days after infection (D0). Treatment was administered 5 days per week (5/7) by gavage. The drug doses (in mg/kg) used were as follows: INH, 10; RIF, 10; PZA, 150; TMC, 25; RPT, 10; MXF, 100; PA-824, 50; CFZ, 20; and LZD, 100 (3, 4, 6, 15, 27, 34). Control mice received 2 months of RIF-INH-PZA, followed by up to 4 months of RIF-INH. In experiment 1 (Table 1), the test regimens were TMC-PZA alone or in combination with RIF, RPT, MXF, PA-824, CFZ, or LZD. A group receiving PA-824-PZA-RIF was added to compare the addition of TMC and PA-824 to RIF-PZA and to compare the addition of TMC and RIF to PA-824-PZA. The experimental scheme, including the duration of treatment for each regimen, is shown in Table 1.
Table 1.
Scheme of experiment 1 to evaluate the addition of a third drug to the TMC-PZA combination
| Drug regimena | No. of mice sacrificed/groupb at: |
|||||||
|---|---|---|---|---|---|---|---|---|
| D-13 | D0 | M1 | M2 (+3) | M3 (+3) | M4 (+3) | M5 (+3) | M6 (+3) | |
| Controls | ||||||||
| No treatment | 6* | 6* | 3 | |||||
| RIF + PZA + INHc | 5 | 5 | 5 | 5 (15) | (15) | (15) | ||
| TMC + PZA | 5 | 5 | (15) | (15) | ||||
| Tests | ||||||||
| TMC + PZA + PA-824 | 5 | 5 | (15) | (15) | ||||
| TMC + PZA + MXF | 5 | (15) | (15) | |||||
| TMC + PZA + LZD | 5 | (15) | (15) | |||||
| TMC + PZA + RIF | 5 | (15) | (15) | |||||
| TMC + PZA + RPT | 5 | (15) | (15) | |||||
| TMC + PZA + CFZ | 5 | 5 (15) | (15) | (15) | ||||
| RIF + PZA + PA-824 | 5 | 5 | (15) | (15) | ||||
| Totald | 6 | 6 | 48 | 25 (30) | 5 (120) | 5 (120) | (15) | (15) |
Drug doses (in mg/kg): RIF, 10; PZA, 150; INH, 10; TMC, 25; PA-824, 50; MXF, 100; LZD, 100; RPT, 10; CFZ, 20.
Time points are shown in days (day −13 [D-13] or day 0 [D0]) or months (e.g., 2 months = M2) of treatment, and (+3) indicates that the mice were held for 3 months after the completion of treatment at the indicated time point.
In the RIF-PZA-INH regimen, PZA was given for the first 2 months only.
n = 380.
In experiment 2 (Table 2), the test regimens included all possible 3-drug combinations of RPT, MXF, PZA, TMC, and PA-824 except TMC-PZA-PA-824 and TMC-RPT-PA-824, which were excluded based on evidence of antagonism between TMC and PA-824 in preceding experiments (data not shown). The experimental scheme, including the duration of treatment for each regimen, is shown in Table 2.
Table 2.
Scheme for experiment 2 to compare the efficacy of 3-drug combinations containing TMC, PZA, RPT, PA-824, and MXF
| Drug regimena | No. of mice sacrificed/groupb at: |
||||
|---|---|---|---|---|---|
| D-13 | D0 | M1 | M2 (+3) | M4 (+3) | |
| Controls | |||||
| No treatment | 6 | 6 | 2 | ||
| RIF + PZA + INHc | 4 | 4 | 4 (15) | ||
| Tests | |||||
| RPT + PZA + MXF | 4 (15) | 4 (15) | |||
| RPT + PZA + PA-824 | 4 (15) | 4 (15) | |||
| TMC + PZA + RPT | 4 (15) | 4 (15) | |||
| TMC + PZA + MXF | 4 (15) | 4 (15) | (15) | ||
| TMC + RPT + MXF | 4 | 4 (15) | (15) | ||
| TMC + PA-824 + MXF | 4 | 4 (15) | (15) | ||
| PA-824 + PZA + MXF | 4 | 4 (15) | (15) | ||
| RPT + PA-824 + MXF | 4 | 4 (15) | (15) | ||
| Totald | 6 | 6 | 38 (60) | 36 (120) | 4 (90) |
Drug doses (in mg/kg): RIF, 10; PZA, 150; INH, 10; RPT, 10; MXF, 100; TMC, 25; PA-824, 50.
Time points are shown in days (day −13 [D-13] or day 0 [D0]) or months (e.g., 2 months = M2) of treatment, and (+3) indicates that the mice were held for 3 months after the completion of treatment at the indicated time point.
In the RIF-PZA-INH regimen, PZA was given for the first 2 months only.
n = 360.
Assessment of treatment efficacy.
Efficacy was assessed on the basis of lung CFU counts at selected time points during treatment (a measure of bactericidal activity) and the proportion of mice with culture-positive relapse after treatment completion (a measure of sterilizing activity). Quantitative cultures of lung homogenates were performed in parallel on 7H11 agar enriched with OADC (basic agar) with and without 0.4% activated charcoal. Plates were incubated for 28 days at 37°C before final CFU counts were determined. The proportion of mice with culture-positive relapse was determined by holding cohorts of 15 mice for 3 additional months after the completion of treatment and then humanely killing them to determine the proportion with positive lung cultures, as defined by ≥1 CFU of M. tuberculosis detected after plating of the entire lung homogenate onto five 7H11 plates. At least 1 plate supplemented with 0.4% charcoal was used to control for drug carryover. The use of 15 mice per group for relapse assessment provides greater than 80% power to detect 40 percentage point differences in the relapse rate, after setting α at 0.01 to adjust for up to 5 simultaneous comparisons. Smaller differences may not be meaningful in terms of shortening the duration of treatment.
Drug susceptibility testing.
Colonies isolated from any mouse relapsing after 3 months of treatment with any TMC-PZA-containing regimen and each of 3 mice relapsing after 4 months of treatment with PA-824-RIF-PZA in experiment 1 were pooled directly from an agar plate, suspended in PBS, and homogenized with glass beads. After settling, the supernatant of a single-cell suspension was plated in serial dilutions on drug-free 7H11 agar or 7H11 supplemented with TMC207 at concentrations of 0.03, 0.06, and 0.125 μg/ml or PA-824 at a 0.125, 0.25, 0.5, 1, and 2 μg/ml. The MIC was defined as the lowest drug concentration preventing at least 99% of the growth observed on drug-free plates. The stock M. tuberculosis H37Rv strain was used as a control.
Statistical analysis.
Mean serum TMC and M2 metabolite concentrations were compared at each time point using paired t tests. CFU counts (x) were log-transformed as (x + 1) before analysis, and group means were compared by one-way analysis of variance with Dunnett's posttest to control for multiple comparisons. Group relapse proportions were compared using Fisher's exact test, adjusting for multiple comparisons. GraphPad Prism version 4 (GraphPad, San Diego, CA) was used for all analyses.
RESULTS
PK of TMC alone and in combination with PA-824 and RIF.
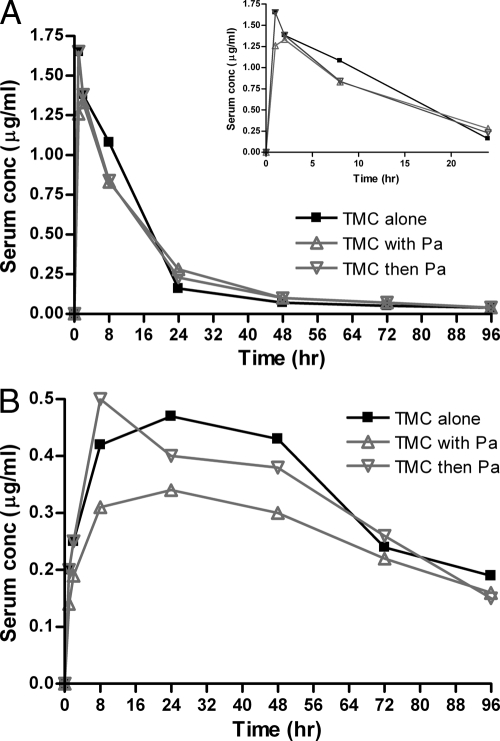
Figure 1 shows the median serum concentration-time curves for TMC (Fig. 1A) and the active M2 metabolite (Fig. 1B) when TMC was administered alone, simultaneously with PA-824, and 4 h before PA-824. No significant differences in the mean TMC concentrations between groups were observed. However, the mean concentrations of the M2 metabolite were significantly lower in the group receiving TMC and PA-824 simultaneously (P = 0.02 versus TMC alone, using paired t test) but not in the group receiving TMC 4 h before PA-824 (P = 0.29). As these results suggested a modest reduction in the rate, and perhaps the extent, of TMC absorption when it was administered simultaneously with PA-824, we elected to administer TMC at least 4 h before PA-824 in the subsequent experiments.
Fig. 1.
Single-dose serum concentration-time profiles for TMC (A) and its M2 metabolite (B) after TMC was administered alone, simultaneously with PA-824 (Pa), or 4 h before Pa. The inset in panel A depicts only the first 24 h only to allow more detailed inspection of the curves at the early time points.
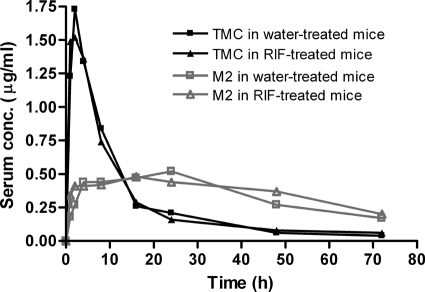
No significant differences in serum concentrations of TMC or its M2 metabolite were observed in mice pretreated with RIF or water for 4 weeks (Fig. 2), indicating no significant induction of TMC metabolism by RIF in mice.
Fig. 2.
Single-dose serum concentration-time profiles for TMC and its M2 metabolite after TMC was administered to mice which had previously received 4 weeks of treatment with water or RIF.
Reduction of drug carryover effect with addition of activated charcoal to 7H11 agar.
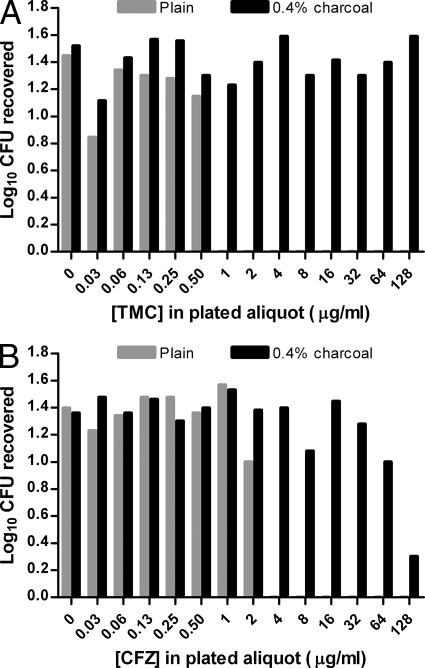
The TMC concentration in broth culture that completely suppressed the growth of M. tuberculosis in the plated aliquot was 1 μg/ml on plain 7H11 plates, while a concentration of 128 μg/ml did not suppress growth on 7H11 plus 0.4% charcoal (Fig. 3A). The CFZ concentration in broth culture that suppressed the growth of M. tuberculosis in the plated aliquot by 90% was 4 μg/ml on plain 7H11 plates and 128 μg/ml on 7H11 plus 0.4% charcoal. The highest CFZ concentration which did not result in any growth suppression was 32 μg/ml on 0.4% charcoal-containing plates (Fig. 3B). Assuming that the drugs diffused evenly throughout the 20 ml of 7H11 agar when the aliquot was plated, the inhibitory concentrations of TMC and CFZ in the agar without charcoal were therefore 0.05 and 0.2 μg/ml, respectively, which is in agreement with the MICs of 0.03 to 0.06 and 0.25 μg/ml, respectively, against M. tuberculosis H37Rv. Charcoal-containing agar was similarly effective at preventing TMC carryover and growth suppression of M. smegmatis, which is more susceptible to TMC than M. tuberculosis is. CFZ suppressed M. smegmatis growth only at concentrations of ≥64 μg/ml, and this suppression was prevented by charcoal in the agar at CFZ concentrations as high as 256 μg/ml (higher concentrations were not tested). These results indicate the effectiveness of activated charcoal in adsorbing TMC and CFZ and reducing the effects of drug carryover after plating of drug-containing organs. Plates containing 0.2% activated charcoal were also effective, but to a lesser extent than plates containing 0.4% activated charcoal (data not shown). Therefore, plates containing 0.4% activated charcoal were used in the subsequent experiments.
Fig. 3.
Effect of supplementing plain 7H11 agar with activated charcoal (0.4%, wt/vol) on bacterial growth when the plated aliquot containing M. tuberculosis also contains the indicated concentration of TMC (A) or CFZ (B) to simulate antibiotic carryover.
Treatment efficacy in experiment 1. (i) Lung CFU counts during treatment.
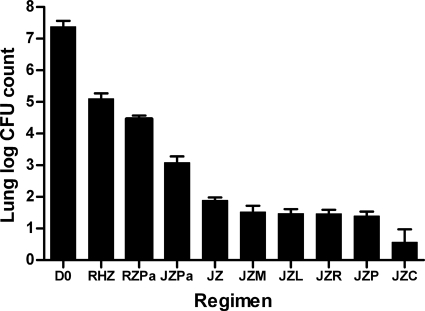
The day after aerosol infection, the mean lung log10 CFU count (± standard deviation [SD]) was 3.67 ± 0.12. At D0, the mean CFU count had increased to 7.38 ± 0.18. Untreated animals became moribund during the fourth week of infection. The lung CFU counts observed after 1 month of treatment are presented in Fig. 4. Compared to the D0 count, 1 month of RIF-INH-PZA reduced the CFU count by just over 2 log10 to 5.16 ± 0.12, whereas 1 month of TMC-PZA reduced the CFU count by 5.5 log10 to 1.88 ± 0.10. The addition of MXF and LZD increased the bactericidal activity of TMC-PZA in a manner that was statistically significant before, but not after, adjusting for multiple comparisons. The addition of either rifamycin (RIF or RPT) was associated with a significant additive effect even after adjusting for multiple comparisons (P < 0.05). The addition of CFZ resulted in a greater bactericidal effect (P < 0.01). Remarkably, the addition of PA-824 antagonized the activity of TMC-PZA, resulting in a mean CFU count (3.00) that was more than 1 log10 higher than that observed with TMC-PZA alone (P < 0.01), yet the combination was still superior to RIF-INH-PZA. Treatment with PA-824-PZA-RIF was more effective than RIF-INH-PZA (P < 0.01) but was not as active as any TMC-PZA regimen.
Fig. 4.
Mean lung log10 CFU counts (±SD) after 1 month of treatment in experiment 1. Abbreviations: R, rifampin; H, isoniazid; Z, pyrazinamide; J, TMC207; Pa, PA-824; M, moxifloxacin; L, linezolid; P, rifapentine; C, clofazimine.
Only selected groups were scheduled for lung CFU assessment after 2 months of treatment (see Table S1 in the supplemental material). TMC-PZA and TMC-PZA-CFZ both rendered the lungs culture-negative, whereas the 5 mice receiving TMC-PZA-PA-824 remained culture-positive, with a mean CFU count of 0.79 ± 0.20. PA-824-PZA-RIF resulted in significantly higher CFU counts than TMC-PZA with or without PA-824 (P < 0.01), but significantly lower CFU counts than RIF-INH-PZA (P < 0.01).
(ii) Relapse after treatment completion.
The relapse results are displayed in Table 3. Relapse occurred in 50%, 14% and 0% of the mice treated for 4, 5 and 6 months with the first-line regimen (2 months of RIF-INH-PZA followed by RIF-INH). In contrast, none of the mice receiving a TMC-PZA-containing regimen relapsed after 4 months of treatment (P < 0.006 for each regimen versus RIF-INH-PZA). Moreover, no more than 7% of the mice receiving a TMC-PZA-containing regimen (including TMC-PZA alone) relapsed when treatment was limited to 3 months of treatment, indicating substantially greater sterilizing activity for any TMC-PZA-containing regimen compared to the current first-line regimen. Finally, just 2 months of treatment with TMC-PZA-RPT and TMC-PZA-CFZ were sufficient to prevent relapse in all mice. Because of the small number of relapses in all groups receiving TMC-PZA-containing regimens, we were unable to establish the superiority of any TMC-PZA-containing regimen over another on the basis of this outcome by Fisher's exact test. Isolates from each mouse relapsing after 3 months of treatment with a TMC-PZA-containing regimen remained fully susceptible to TMC.
Table 3.
Proportions of mice with M. tuberculosis-positive cultures 3 months after completing treatment in experiment 1
| Drug regimen | No. positive/total (%) after treatment for: |
||||
|---|---|---|---|---|---|
| 2 mo | 3 mo | 4 mo | 5 mo | 6 mo | |
| RIF + PZA + INHb | NDa | ND | 7/14 (50) | 2/14 (14) | 0/14 (0) |
| RIF + PZA + PA-824 | ND | 15/15 (100) | 12/13 (92) | ||
| TMC + PZA | ND | 0/15 (0) | 0/15 (0) | ||
| TMC + PZA + PA-824 | ND | 1/15 (7) | 0/13 (0) | ||
| TMC + PZA + MXF | ND | 1/15 (7) | 0/15 (0) | ||
| TMC + PZA + LZD | ND | 0/15 (0) | 0/15 (0) | ||
| TMC + PZA + RIF | ND | 0/15 (0) | 0/15 (0) | ||
| TMC + PZA + RPT | 0/15 (0) | 0/14 (0) | 0/15 (0) | ||
| TMC + PZA + CFZ | 0/14 (0) | 1/14 (7) | 0/15 (0) | ||
ND, not done.
In the RIF-PZA-INH regimen, PZA was given for the first 2 months only.
Treatment with PA-824-PZA-RIF for 3 months did not cure any mouse and was not as active as any TMC-PZA regimen (P < 0.0001 for any TMC-PZA-containing regimen versus PA-824-PZA-RIF). Treatment with PA-824-PZA-RIF for 4 months cured only 1 of 13 mice. This result was inferior to that observed with RIF-INH-PZA before (P = 0.033), but not after, adjusting for multiple comparisons. However, all relapsing mice had similar lung CFU counts and, when the isolates from 3 randomly selected relapsing mice were assessed for susceptibility to PA-824, PA-824 MICs were higher for the relapsing isolates (1 to 2 μg/ml) compared to the parent H37Rv isolate (0.25 μg/ml) (see Table S2 in the supplemental material), suggesting that the selection of PA-824-resistant mutants likely compromised the sterilizing activity of this regimen.
Treatment efficacy in experiment 2. (i) Lung CFU counts during treatment.
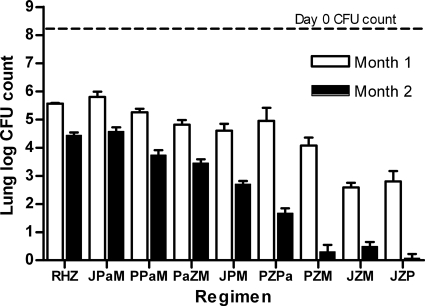
The day after aerosol infection, the mean lung log10 CFU count (±SD) was 4.24 ± 0.06. At D0, the mean CFU count had increased to 8.21 ± 0.14. Untreated animals became moribund during the fourth week of infection. The lung CFU counts are presented in Fig. 5 and Table S3 in the supplemental material. Compared to the D0 count, treatment with RIF-INH-PZA for 1 month reduced the mean CFU count by over 2.5 log10 to 5.56 ± 0.03. All test regimens were significantly better than RIF-INH-PZA, except RPT-PA-824-MXF and TMC-PA-824-MXF. The 2 regimens containing TMC-PZA plus either RPT or MXF were the most effective, reducing CFU counts by at least 5.4 log10 after 1 month of treatment (P < 0.001). After those regimens, RPT-PZA-MXF was statistically significantly superior to the remaining combinations with the exception of TMC-RPT-MXF. However, TMC-RPT-MXF was not statistically significantly superior to PA-824-PZA-MXF, RPT-PZA-PA-824, or RPT-PA-824-MXF.
Fig. 5.
Mean lung log10 CFU counts (±SD) after 1 and 2 months of treatment in experiment 2. Abbreviations: R, rifampin; H, isoniazid; Z, pyrazinamide; J, TMC207; Pa, PA-824; M, moxifloxacin; P, rifapentine.
Rankings on the basis of CFU counts after 2 months of treatment were similar. TMC-PZA-RPT rendered 3 of 4 mice culture negative, and the remaining mouse had just 1 CFU detected. Mice receiving TMC-PZA-MXF and RPT-PZA-MXF had between 1 and 4 CFU detected, except that 1 of 4 mice in the RPT-PZA-MXF group was culture negative. On the basis of statistical comparisons using the month 2 CFU counts, the ranking of the remaining regimens (from strongest to weakest) was as follows: RPT-PZA-PA-824 > TMC-RPT-MXF > PA-824-PZA-MXF = RPT-PA-824-MXF > TMC-PA-824-MXF. The activity of the last regimen was, again, indistinguishable from that of RIF-INH-PZA.
(ii) Relapse after treatment completion.
The relapse results are displayed in Table 4. Relapses occurred in all 15 mice treated with 2 months of RIF-INH-PZA, followed by 2 months of RIF-INH. The higher relapse rate among control animals at this time point in experiment 2 compared to experiment 1 was the result of the higher bacillary burden at the start of treatment and is consistent with previous experiments (23, 28). While 1 month of treatment was insufficient to prevent relapse in any tested group, 2 months of treatment prevented relapse in all mice receiving TMC-PZA-RPT and 67% of those receiving TMC-PZA-MXF. RPT-PZA-MXF did not cure any mice at 2 months. These results indicate that, in combinations containing PZA, TMC had a greater ability to prevent relapse than RPT or MXF. The remaining groups had higher lung CFU counts at the completion of 2 months of treatment and, not surprisingly, had positive cultures after 3 months of follow-up.
Table 4.
Proportions of mice with M. tuberculosis-positive cultures 3 months after completing treatment in experiment 2
| Drug regimeng | No. positive/total (%) after treatment for: |
||
|---|---|---|---|
| 1 mo | 2 mo | 4 mo | |
| RIF + PZA + INH | NDf | ND | 15/15 (100) |
| RPT + PZA + MXF | 15/15 (100) | 15/15 (100)a | |
| RPT + PZA + PA-824 | 15/15 (100) | 15/15 (100) | |
| TMC + PZA + RPT | 15/15 (100) | 0/15 (0) | |
| TMC + PZA + MXF | 15/15 (100) | 5/15 (33)b | 0/14 (0) |
| TMC + RPT + MXF | ND | 15/15 (100) | 7/14 (50) |
| TMC + PA-824 + MXF | ND | 15/15 (100) | 7/14 (50)c |
| PA-824 + PZA + MXF | ND | 15/15 (100) | 10/15 (67)d |
| RPT + PA-824 + MXF | ND | 15/15 (100) | 13/15 (87)e |
One mouse with 2 CFU, two mice with <10 CFU each.
Two mice with 2 CFU each.
Three mice with <12 CFU each.
Two mice with <6 CFU each.
Three mice with <10 CFU each.
ND, not done.
In the RIF-PZA-INH regimen, PZA was given for the first 2 months only.
After 4 months of treatment, the PZA-sparing regimen of TMC-MXF plus either RPT or PA-824 prevented relapse in 50% of the mice, a result significantly better than that obtained with the RIF-INH-PZA regimen (P < 0.05). Four months of treatment with PA-824-PZA-MXF was sufficient to prevent relapse in 33% of the mice, a result which was statistically significantly better than that obtained with RIF-INH-PZA before (P = 0.0421), but not after, adjustment for multiple comparisons.
DISCUSSION
The principal finding of the present study is that several TMC-containing 3-drug combinations prevented relapse more effectively than the standard first-line regimen did in an aerosol infection model of TB in mice. Indeed, the 2-drug backbone of TMC-PZA given for 3 months was superior to 4 months (and equivalent to 5 or 6 months) of the standard regimen in the prevention of relapse. Ibrahim et al. first demonstrated the exceptional bactericidal activity of this combination (12), but its ability to prevent relapse after treatment discontinuation was not reported until recently, when it was shown that 6 months of TMC-PZA and 6 months of the standard regimen produced similarly low relapse rates (34). Neither shorter treatment durations nor selection of drug-resistant organisms was assessed in mice receiving TMC-PZA alone in that study.
The primary objective of experiment 1 was to identify the best companion drugs for TMC-PZA. Addition of RIF and RPT reduced the bacterial load in lungs by a modest, but statistically significant, margin. Because the number of relapses was lower than expected (based on prior results from an intravenous infection-outbred mouse model), we were unable to prove that the addition of a rifamycin decreased the relapse rate in mice receiving TMC-PZA in experiment 1. In experiment 2, TMC-PZA-RPT resulted in no relapse after 2 months of treatment, compared with 33% relapse in the TMC-PZA-MXF group. Although the difference in the relapse rate was not statistically significant, the addition of RPT may have had a greater effect on the sterilizing activity of TMC-PZA than did the addition of MXF. This trend is supported by Andries et al. (4), who also found that TMC-PZA-RPT was more effective than TMC-PZA-MXF and the addition of MXF to TMC-PZA-RPT did not increase the sterilizing activity. It should be noted that both RIF and RPT cause a significant decrease in TMC207 drug concentrations in humans through cytochrome P450 enzyme induction, with an approximately 50% reduction of TMC exposure in plasma (4, 17). As demonstrated in our PK study, this induction does not occur in mice, indicating that TMC exposures in mice receiving concomitant rifamycin treatment may be greater than those observed in humans. Fortunately, the sterilizing activity of the TMC-PZA-RPT regimen is not greatly reduced when the dose of TMC is halved to adjust for such induction (4).
Remarkably, it was the addition of CFZ to TMC-PZA which produced the greatest reduction in CFU counts in experiment 1, suggesting this may be an especially potent combination. CFZ is currently considered a 3rd-line agent for TB but has potent anti-TB activity in mice (14, 27). The mechanism by which it kills M. tuberculosis is not well understood, although a recent study found that CFZ kills M. smegmatis by participating in a redox cycling pathway in which it is reduced by the essential respiratory chain NADH:quinone oxidoreductase-NDH-2 and then oxidized by nonenzymatic means, yielding lethal reactive oxygen species (37). Because ndh, the gene encoding NDH-2, is upregulated by hypoxia, nitric oxide stress, and phagocytosis (37), this mechanism of action may be especially effective against quiescent bacteria in established mouse infection models. CFZ may also inhibit ATP synthesis (37), offering a second mechanism of action with obvious potential for additive or synergistic effects with TMC. Follow-up experiments are under way to determine whether RPT and CFZ add sterilizing activity to the TMC-PZA combination and to evaluate other PZA-sparing combinations including TMC and CFZ.
The addition of PA-824 reduced the bactericidal activity of TMC-PZA in experiment 1. A similar antagonistic effect of PA-824 on TMC and TMC-containing combinations has been observed consistently in our laboratory across multiple experiments, as has antagonism of another nitroimidazole derivative in clinical development, OPC-67683 (data not shown). The mechanism of this interaction remains unclear. However, it does not appear to be due to reduced absorption of TMC, as we have shown with our PK experiment and the occurrence of antagonism even when the drug administration times are at least 4 h apart. Although such antagonism, if mechanistic in nature, would be unfortunate given that these are the two most advanced classes of new drugs in development, it should be emphasized that the TMC-PZA-PA-824 (experiment 1) and TMC-PA-824-MXF (experiment 2) combinations were still significantly more effective than RIF-INH-PZA.
Because resistance to PZA may be found in 50% of MDR TB isolates in some settings (9, 21), the ideal novel regimen would not rely on this sterilizing drug. Attempts to replace PZA with MXF, PA-824, and even RPT in TMC-containing combinations significantly reduced bactericidal and sterilizing activities in experiment 2. These results are consistent with those of Veziris et al., who found higher relapse rates when PZA was omitted from TMC-containing regimens (34). If PZA is to have such a critical treatment-shortening role in novel regimens, concerted efforts are needed to better understand the therapeutic implications of phenotypic and genotypic PZA resistance and improve PZA susceptibility testing methods, including the development of more robust and rapid tests.
Despite lacking PZA, combinations of TMC-MXF with either RPT or PA-824 produced fewer relapses after 4 months of treatment than did the same duration of RIF-PZA-INH. In particular, TMC-PA-824 plus MXF (or another potent fluoroquinolone) may represent an important new backbone for a short-course MDR TB regimen. Indeed, the 4-drug combination of TMC-PZA-MXF-PA-824 may offer potential as a shorter (i.e., ≤4 months) regimen against PZA-susceptible isolates (based on the superior activity of TMC-PZA plus either MXF or PA-824) yet still constitute an effective short-course regimen (i.e., ≤6 months) against isolates resistant to PZA (based on the activity of TMC-PA-824-MXF). Experiments are under way to determine whether CFZ and PNU-100480 can replace the modest antagonistic effect of PA-824 and/or replace MXF in the case of fluoroquinolone resistance to further enhance the potential of such regimens.
Several of the combinations tested in these experiments did not include TMC. In experiment 1, RIF-PZA-PA-824 was more effective than RIF-PZA-INH on the basis of lung CFU counts over the first 2 months of treatment, as described previously (22, 32). Although removal of an antagonistic effect of INH likely contributed to the improved efficacy of this regimen (32), the contribution of PA-824 to the regimen's bactericidal activity is suggested by the selection of PA-824-resistant mutants. We have previously shown that coadministration of PZA with PA-824 may not prevent the selection of PA-824-resistant mutants (32). However, it is surprising that RIF did not prevent the selection of PA-824-resistant mutants in this case. It is also noteworthy that only relatively low-level PA-824 resistance was observed (a 4- to 8-fold shift in the MIC). These findings warrant further investigation. Replacement of RIF and INH with RPT and PA-824, respectively, resulted in even greater CFU count reductions relative to RIF-PZA-INH in experiment 2. However, the RPT-PZA-PA-824 combination was not as effective over the first 2 months as the RPT-PZA-MXF combination, which would appear to limit its potential as a first-line regimen. Finally, the combination of PA-824-PZA-MXF, which was previously shown to be superior to RIF-PZA-INH in mice when the PA-824 dose was 100 mg/kg (23), remained superior in experiment 2 when the PA-824 dose was reduced to 50 mg/kg, which results in free drug concentrations exceeding the MIC for a similar proportion (∼67%) of the dosing interval as a 200-mg daily dose in humans, assuming that the free drug is 7% of the total drug concentration (3). Therefore, this continues to be a promising novel regimen which is currently being studied in an extended early bactericidal activity trial (ClinicalTrials.gov identifier NCT01215851).
The present study was the first to examine the sterilizing activity of TMC-containing drug combinations in mice following high-dose aerosol infection with M. tuberculosis. All previously reported studies of TMC in mice have been conducted in an intravenous infection model (4, 13, 34). Andries et al. previously found that longer durations of therapy with RIF-PZA-INH, RIF-PZA-MXF, and RPT-PZA-MXF were needed to prevent relapse in most intravenously infected, outbred Swiss mice compared to aerosol-infected, inbred BALB/c mice in our lab (4). Our current results extend this observation to TMC-containing combinations. In general, the duration of treatment needed to prevent relapse in all or nearly all mice was 1 to 2 months shorter in aerosol-infected BALB/c mice than in intravenously infected Swiss mice (4, 34), despite higher lung CFU counts at the initiation of treatment in the former model. This was true for treatment with RIF-PZA-INH, RPT-PZA-MXF, TMC-PZA-RIF, TMC-PZA-RPT, and TMC-PZA-MXF. For treatment with TMC-PZA, the difference between the results of experiment 1 and the results of Veziris et al. (34) was even greater, as TMC-PZA produced 0% relapse after 3 and 4 months of treatment in our study while resulting in 22% relapse after 6 months in the latter study. These observations emphasize that results in test mice should not be appreciated at face value but only in comparison with the results obtained in the positive controls. Compared to the positive controls, the relative activities of TMC-PZA combinations are generally similar in intravenously infected Swiss mice and in aerosol infected BALB/c mice. Another group has noted that cure is achieved with shorter durations of treatment with RIF-PZA-INH and RIF-PZA-MXF after aerosol compared to intravenous infection in inbred mice despite similar starting bacterial burdens in the lungs (8), confirming the influence of the route of infection on the duration of treatment necessary to prevent relapse. For the time being, the urge to estimate potential durations of treatment for experimental regimens based on mouse model studies must be tempered by the lack of sufficient clinical outcomes by which to judge their forecasting ability. Whether any one model consistently provides more accurate estimates of treatment-shortening potential or whether the models may themselves define a range of possible clinical outcomes remains a topic for further study.
In addition to using only one route of infection and one mouse strain, our study used only one strain of M. tuberculosis, the H37Rv strain also previously used by other groups. We concur with the recent conclusion of De Groote et al. (8) that the most promising findings in mice warrant confirmation in a second model and/or against a second strain of M. tuberculosis before advancing to human trials. The second model could include another mouse model (e.g., C3HeB/FeJ mice), guinea pigs, or larger species, which all exhibit more human-like pathology (2, 7, 11).
A final limitation of our study was the risk of drug carryover imposed by the significant accumulation of TMC and CFZ in the lungs of mice after repeated dosing. To reduce the risk of carryover when assessing lung CFU counts, we relied primarily on the addition of activated charcoal to the culture medium. Charcoal-containing plates were demonstrably effective in reducing drug carryover. However, there is no way to exclude carryover effects with confidence when few or no CFU are isolated, especially for samples containing high concentrations of CFZ. For this reason, we feel the proper evaluation of the sterilizing activity of combinations containing CFZ and/or TMC requires relapse-based assessments of efficacy made at least 3 months after treatment discontinuation to allow time for tissue drug concentrations to decrease and for regrowth of viable organisms (i.e., persisters). Even then, use of LJ, BSA-supplemented, or charcoal-supplemented medium is recommended to rule out persistent carryover effects. Unfortunately, relapse is a time-consuming and costly outcome to evaluate because its binary nature reduces statistical power and places a premium on the proper (or sometimes fortuitous) selection of time points for assessment.
In conclusion, we have confirmed and extended previous studies describing the substantial treatment-shortening potential of new regimens containing TMC-PZA which may be effective on many drug-susceptible and MDR TB-causing isolates. Novel PZA-sparing combinations may also enable short-course treatment of drug-resistant disease.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the Global Alliance for TB Drug Development.
We thank Simon Lynch for the suggestion to use activated charcoal in 7H11 agar to reduce the effects of antibiotic carryover.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Agrawal D., Udwadia Z. F., Rodriguez C., Mehta A. 2009. Increasing incidence of fluoroquinolone-resistant Mycobacterium tuberculosis in Mumbai, India. Int. J. Tuberc. Lung Dis. 13: 79–83 [PubMed] [Google Scholar]
- 2. Ahmad Z., et al. 2011. Effectiveness of tuberculosis chemotherapy correlates with resistance to Mycobacterium tuberculosis infection in animal models. J. Antimicrob. Chemother. 66: 1560–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmad Z., et al. 2011. PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob. Agents Chemother. 55: 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andries K., Gevers T., Lounis N. 2010. Bactericidal potencies of new regimens are not predictive of their sterilizing potencies in a murine model of tuberculosis. Antimicrob. Agents Chemother. 54: 4540–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andries K., et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307: 223–227 [DOI] [PubMed] [Google Scholar]
- 6. Conde M. B., et al. 2009. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 373: 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis S. L., et al. 2009. Noninvasive pulmonary [18F]-2-fluoro-deoxy-d-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob. Agents Chemother. 53: 4879–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Groote M. A., et al. 2011. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55: 1237–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diacon A. H., et al. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360: 2397–2405 [DOI] [PubMed] [Google Scholar]
- 10. Grimaldo E. R., et al. 2001. Increased resistance to ciprofloxacin and ofloxacin in multidrug-resistant mycobacterium tuberculosis isolates from patients seen at a tertiary hospital in the Philippines. Int. J. Tuberc. Lung Dis. 5: 546–550 [PubMed] [Google Scholar]
- 11. Hoff D. R., et al. 2011. Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PLoS One 6: e17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibrahim M., et al. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob. Agents Chemother. 51: 1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibrahim M., Truffot-Pernot C., Andries K., Jarlier V., Veziris N. 2009. Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis. Am. J. Respir. Crit. Care Med. 180: 553–557 [DOI] [PubMed] [Google Scholar]
- 14. Jagannath C., Reddy M. V., Kailasam S., O'Sullivan J. F., Gangadharam P. R. 1995. Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis. In vitro, intracellular, and in vivo studies. Am. J. Respir. Crit. Care Med. 151: 1083–1086 [DOI] [PubMed] [Google Scholar]
- 15. Ji B., Lounis N., Truffot-Pernot C., Grosset J. 1994. Effectiveness of various antimicrobial agents against Mycobacterium avium complex in the beige mouse model. Antimicrob. Agents Chemother. 38: 2521–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koul A., et al. 2008. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J. Biol. Chem. 283: 25273–25280 [DOI] [PubMed] [Google Scholar]
- 17. Lounis N., Gevers T., Van Den Berg J., Andries K. 2008. Impact of the interaction of R207910 with rifampin on the treatment of tuberculosis studied in the mouse model. Antimicrob. Agents Chemother. 52: 3568–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lounis N., Gevers T., Van Den Berg J., Verhaeghe T., van H. R., Andries K. 2008. Prevention of drug carryover effects in studies assessing antimycobacterial efficacy of TMC207. J. Clin. Microbiol. 46: 2212–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lounis N., et al. 2006. Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob. Agents Chemother. 50: 3543–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsumoto M., et al. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3: e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mphahlele M., et al. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 46: 3459–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nuermberger E., et al. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50: 2621–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nuermberger E., et al. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52: 1522–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nuermberger E. L., et al. 2004. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am. J. Respir. Crit. Care Med. 170: 1131–1134 [DOI] [PubMed] [Google Scholar]
- 25. O'Brien R. J., Nunn P. P. 2001. The need for new drugs against tuberculosis. Obstacles, opportunities, and next steps. Am. J. Respir. Crit. Care Med. 163: 1055–1058 [DOI] [PubMed] [Google Scholar]
- 26. Rao S. P., Alonso S., Rand L., Dick T., Pethe K. 2008. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 105: 11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reddy V. M., Nadadhur G., Daneluzzi D., O'Sullivan J. F., Gangadharam P. R. 1996. Antituberculosis activities of clofazimine and its new analogs B4154 and B4157. Antimicrob. Agents Chemother. 40: 633–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenthal I. M., et al. 2005. Weekly moxifloxacin and rifapentine is more active than the denver regimen in murine tuberculosis. Am. J. Respir. Crit. Care Med. 172: 1457–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenthal I. M., et al. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med 4: e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rustomjee R., et al. 2008. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 12: 128–138 [PubMed] [Google Scholar]
- 31. Spigelman M., Woosley R., Gheuens J. 2010. New initiative speeds tuberculosis drug development: novel drug regimens become possible in years, not decades. Int. J. Tuberc. Lung Dis. 14: 663–664 [PubMed] [Google Scholar]
- 32. Tasneen R., Tyagi S., Williams K., Grosset J., Nuermberger E. 2008. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52: 3664–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tyagi S., et al. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 49: 2289–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Veziris N., Ibrahim M., Lounis N., Andries K., Jarlier V. 2011. Sterilizing activity of second-line regimens containing TMC207 in a murine model of tuberculosis. PLoS One 6: e17556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams K. N., et al. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am. J. Respir. Crit. Care Med. 180: 371–376 [DOI] [PubMed] [Google Scholar]
- 36. Williams K. N., et al. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob. Agents Chemother. 53: 1314–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yano T., et al. 2011. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J. Biol. Chem. 286: 10276–10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang T., Li S. Y., Williams K. N., Andries K., Nuermberger E. L. 2011. Short-course chemotherapy with TMC-207 and rifapentine in a murine model of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 184: 732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.