Abstract
The World Health Organization (WHO) recently issued revised first-line antituberculosis (anti-TB) drug dosage recommendations for children. No pharmacokinetic studies for these revised dosages are available for children <2 years. The aim of the study was to document the pharmacokinetics of the first-line anti-TB agents in children <2 years of age comparing previous and revised WHO dosages of isoniazid (INH; 5 versus 10 mg/kg/day), rifampin (RMP; 10 versus 15 mg/kg/day), and pyrazinamide (PZA; 25 versus 35 mg/kg/day) and to investigate the effects of clinical covariates, including HIV coinfection, nutritional status, age, gender, and type of tuberculosis (TB), and the effect of NAT2 acetylator status. Serum INH, PZA, and RMP levels were prospectively assessed in 20 children <2 years of age treated for TB following the previous and the revised WHO dosage recommendations. Samples were taken prior to dosing and at 0.5, 1.5, 3, and 5 h following dosing. The maximum drug concentration in serum (Cmax), the time to Cmax (tmax), and the area under the concentration-time curve (AUC) were calculated. Eleven children had pulmonary and 9 had extrapulmonary TB. Five were HIV infected. The mean Cmax (μg/ml) following the administration of previous/revised dosages were as follows: INH, 3.19/8.11; RMP, 6.36/11.69; PZA, 29.94/47.11. The mean AUC (μg·h/ml) were as follows: INH, 8.09/20.36; RMP, 17.78/36.95; PZA, 118.0/175.2. The mean Cmax and AUC differed significantly between doses. There was no difference in the tmax values achieved. Children less than 2 years of age achieve target concentrations of first-line anti-TB agents using revised WHO dosage recommendations. Our data provided supportive evidence for the implementation of the revised WHO guidelines for first-line anti-TB therapy in young children.
INTRODUCTION
Isoniazid (INH), rifampin (RMP), and pyrazinamide (PZA) are routinely used to treat tuberculosis (TB) in children (23, 44). Recommendations for pediatric dosages are based on a small number of pharmacokinetic studies, few of which included children younger than 2 years of age. During early life, children experience significant changes in the relative sizes of their body compartments and their ability to absorb, metabolize, and excrete drugs (5, 17). These changes are greatest within the first 2 years of life (4). Most published studies on first-line anti-TB drugs in children have not analyzed differences between older and younger children or the effect of HIV coinfection. The pharmacokinetics of INH are further complicated by genetic polymorphisms of N-acetyltransferase type 2 (NAT2) in the metabolic pathway of INH, which influences INH concentrations (18, 26, 46).
In the absence of pharmacodynamic data for children and therefore data that demonstrate an association between serum drug concentration and clinical outcome, optimal anti-TB therapy should aim to produce the targeted serum drug concentrations that have been determined in adult pharmacokinetic and pharmacodynamic studies. For INH, the proposed optimal maximum serum drug concentration (Cmax) for therapy is 3 to 5 μg/ml (15, 27). Target serum RMP concentrations in adults after a standard oral dose of 600 mg are in the range of 8 to 24 μg/ml; serum RMP concentrations below 8 μg/ml are considered low, and those below 4 μg/ml are considered very low (28, 29). There is more uncertainty regarding the optimal therapeutic serum PZA concentration. In adults, serum PZA concentrations are targeted at 20 to 60 μg/ml (11, 28). However, in a recent study of adults, poor treatment outcome of pulmonary TB was associated with serum PZA concentrations of <35 μg/ml (8).
Optimal anti-TB therapy is important in all children but particularly in young children (<2 years of age) and those HIV infected, where there is a high risk of progression to severe forms of TB. Recent studies have demonstrated that children require higher mg/kg body weight doses of anti-TB drugs to achieve the same serum drug concentrations as in adults (6, 10, 34, 38, 39, 41, 45). Consequently, the World Health Organization (WHO) has recently issued revised dosage recommendations (44) for the treatment of children with first-line drugs based on a systematic review of the literature. The WHO now advises giving INH at 10 mg/kg (range, 10 to 15 mg/kg), RMP at 15 mg/kg (range, 10 to 20 mg/kg), and PZA at 35 mg/kg (range, 30 to 40 mg/kg). This compares to the previous recommendation of INH at 5 mg/kg (range, 4 to 6 mg/kg), RMP at 10 mg/kg (range, 8 to 12 mg/kg), and PZA at 25 mg/kg (range, 20 to 30 mg/kg). All doses are advised for once-daily administration.
The serum INH, RMP, and PZA concentrations achieved following previous and revised WHO dosing guidelines for young children have not been examined. The aim of the present study was to investigate the pharmacokinetics of the first-line anti-TB agents INH, RMP, and PZA at previous and revised WHO-recommended doses for children younger than 2 years of age. We also investigated the effects of important clinical covariates, including HIV coinfection, nutritional status, age, gender, and type of TB, and the effect of NAT2 acetylator status.
MATERIALS AND METHODS
Study population and setting.
This was a prospective, single-center hospital-based, observational intensive sampling pharmacokinetic study. The study was conducted at the Brooklyn Hospital for Chest Diseases (BHCD), Cape Town, South Africa, from May to October 2010. Eligible children were <2 years of age and on anti-TB treatment with a regimen that included INH and PZA, with or without RMP (all licensed for use in South Africa). Children had to be medically stable, and written informed consent was obtained from a parent or legal guardian, including permission for HIV testing. The study was approved by the Health Research Ethics Committee of the Faculty of Health Sciences, Stellenbosch University, Stellenbosch, South Africa.
Diagnosis of TB.
The diagnosis of TB was based on a history of TB contact, a positive tuberculin skin test (TST), a chest radiograph (CR) compatible with the diagnosis of pulmonary TB, and/or clinical signs of extrapulmonary TB. Whenever possible, the diagnosis was confirmed by culture of Mycobacterium tuberculosis from sputum or another clinical specimen. Children were treated according to the mycobacterial drug susceptibility test pattern from the child or that obtained from the most likely adult source case.
Drug administration.
Fixed dosage combinations formulated for pediatric use are routinely prescribed for anti-TB treatment, as recommended by the South African National Tuberculosis Programme. Rimcure (INH at 30 mg, RMP at 60 mg and PZA at 150 mg) was manufactured by Sandoz SA Pty. Ltd., Spartan, South Africa. The doses given were calculated according to body weight at both WHO recommendations. For an adequate dose of INH, tablets (or fractions of tablets, respectively) containing INH at 100 mg (Be-Tabs Isoniazid; Be-Tabs Pharmaceuticals Pty. Ltd., Roodepoort, South Africa) were added. Fractions of 500-mg PZA (Pyrazide; Sanofi Aventis, Johannesburg, South Africa) and 100-mg INH tablets were given to children not receiving RMP. All formulations used were approved by the South African Medicines Control Council. Tablets were routinely crushed and dissolved in 2 to 5 ml of water and orally administered. All children were supplemented with pyridoxine and multivitamin syrup. Antiretroviral therapy (ART) consisted of two nucleoside reverse transcriptase inhibitors (lamivudine and stavudine) and a protease inhibitor (lopinavir/ritonavir). This was boosted with extra ritonavir if the child was prescribed RMP. Trimethoprim-sulfamethoxazole was given to all HIV-infected children.
Pharmacokinetic sampling.
The first pharmacokinetic assessment was performed at 2 weeks or more following the initiation of anti-TB treatment. Two days prior to the assessment, the child was prescribed PZA at the previous WHO dosage of 25 mg/kg, due to the long half-life of the drug. On the morning of the assessment, the child was fasted for 4 h prior to receiving medications. An intravenous catheter was inserted, and a baseline blood sample was taken. The child was given INH, PZA, and, if appropriate, RMP at the previous WHO dosages. Further sampling took place at 0.5, 1.5, 3, and 5 h after dosing. Blood samples were collected in EDTA-coated tubes and placed on ice immediately. Plasma was separated by centrifugation within 15 min and then deep-frozen until analysis. Children received breakfast only after the 1.5-h blood sample was taken. If the child was HIV infected, combination ART (cART) was given with breakfast, while for all children other treatment was given after the last blood sample was taken. Following assessment, children were represcribed TB medications at the revised WHO dosages. One week later, a second assessment of each child took place, using identical methods but using the revised WHO dosages.
Laboratory sampling.
The serum INH and PZA concentrations of plasma extract was determined by high-performance liquid chromatography (HPLC) and UV detection. INH had to be derivatized with cinnamaldehyde to be detectable at 340 nm, while PZA could be measured directly at 269 nm. For both compounds, a solvent gradient of 50 mM phosphate buffer (solvent A) and a 1:4 acetonitrile-isopropanol mixture (solvent B) was used. The RMP concentration was determined by HPLC-tandem mass spectrometry technology with an m/z ratio of 823.3/95.2, a gradient of water and acetonitrile, both containing 0.1% formic acid, was used in this instance as previously described (36, 37). The lower limit of detection was 0.5 μg/ml for both INH and PZA and 0.1 μg/ml for RMP; the lower limit of quantification was determined at a concentration where the inter- and intraday variations were less than 5.0% (n = 10) for each compound. For all three compounds, we found a linear calibration range of 8 spiked samples (R2, >0.9950).
Following centrifugation, the remaining blood cells were pooled and used for the extraction of DNA for NAT2 genotyping. After preparation of genomic DNA by a salting-out procedure, the NAT2 gene was analyzed by PCR and restriction fragment length polymorphism as previously described (22, 34). The NAT2 gene was analyzed for single nucleotide polymorphisms defining the NAT2*5, NAT2*6, NAT2*7, NAT2*12, NAT2*13, and NAT2*14 alleles. According to the current NAT nomenclature, the wild-type allele is designated NAT2*4, which together with the NAT2*12 and NAT2*13 alleles, defines the rapid-acetylator (F) status. Decreased or impaired NAT2 enzyme activity is encoded by the mutant alleles NAT2*5, NAT2*6, NAT2*7, and NAT2*14, which define the slow-acetylator (S) status. Accordingly, individuals were classified as homozygous fast (FF), heterozygous intermediate (FS), or homozygous slow (SS) acetylators, depending upon the allele combinations observed.
Pharmacokinetic parameters.
Study endpoints were the following standard parameters for each patient: Cmax (the highest drug concentration measured), tmax (the time after drug administration taken to reach Cmax), and the area under the concentration-time curve (AUC) from 0 to 5 h of INH, PZA, and RMP. AUC was calculated according to the linear trapezoidal rule.
Statistical methods.
Data were graphically checked for distribution and considered adequate to be analyzed using original (untransformed) values for all subsequent analyses. Data were summarized as means and 95% confidence intervals (95% CIs). Paired t tests were used to assess differences in pharmacokinetic parameters between the two doses. Independent t tests were used to assess the effect of HIV, type of TB, RMP coadministration, and gender on pharmacokinetic parameters. The effect of the NAT2 genotype was evaluated using one-way analysis of variance (ANOVA). The effects of age and nutritional status (Z score for weight) was assessed by linear regression. The Z score for weight was calculated according to WHO growth charts (43). All tests were two sided, and the significance level was set at 5% without adjustment for multiple testing. Data were analyzed using SAS for Windows, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics.
Twenty children were studied (mean age, 1.09 years; standard deviation [SD], 0.49 years); 9 (45%) were female. Demographic, diagnostic, and clinical features are displayed in Table 1. At enrolment, all HIV-infected children (n = 5) were established on cART. Eleven children received a multidrug regimen including RMP, and nine children received treatment excluding RMP due to the presence of resistance. Eight children were treated for multidrug-resistant TB (i.e., resistance to INH and RMP with or without other drugs). INH at a dose of 20 mg/kg was continued in these children to overcome possible low-dose INH resistance.
Table 1.
Demographic, diagnostic, and clinical features of 20 children with TB
| Demographic, diagnostic, or clinical feature | Value |
|---|---|
| Demographic features | |
| Mean age, yr (SD) | 1.09 (0.49) |
| No. (%) of females | 9 (45) |
| No. (%) M. tuberculosis or AFBa | |
| culture positive | 9 (45) |
| No. (%) with household TB contact | 16 (80) |
| No. (%) TSTb positive | 10/19 (53) |
| Mean no. of days on anti-TB treatment (SD) | 106.0 (62.7) |
| No. (%) HIV infected | 5 (25) |
| Mean no. of days on cARTc (SD) | 168.6 (97.7) |
| Clinical features | |
| No. (%) with pulmonary TB | 11 (45) |
| No. (%) with extrapulmonary TB | 9 (45) |
| No. (%) with TB meningitis | 8 (40) |
| No. (%) with abdominal TB | 1 (5) |
| No. (%) with hepatomegaly at inclusion | 11 (55) |
| Nutritional status | |
| No. (%) with mass <3rd percentile for age | 10 (50) |
| Mean Z score for wt (SD) | −1.74 (1.84) |
| Mean BMId (SD) | 16.02 (2.62) |
AFB, acid-fast bacilli.
TST, tuberculin skin test.
cART, combination antiretroviral therapy.
BMI, body mass index.
Differences between previous and revised WHO dosages.
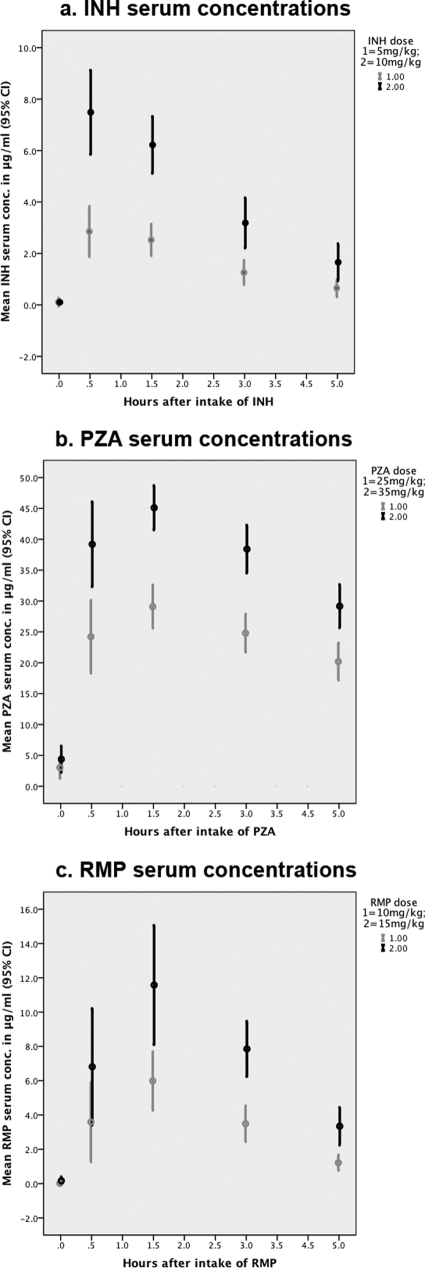
Pharmacokinetic parameters for the two different dosages of INH, PZA, and RMP are shown in Table 2. Giving INH at a dose of 10 mg/kg resulted in a higher Cmax and a correspondingly higher AUC than giving INH at 5 mg/kg. No difference in tmax was seen between the two INH doses. The same was true for the two doses of PZA and RMP: Cmax and AUC were significantly higher following the revised dose, while there was no difference regarding tmax (Fig. 1). A high intraindividual variability was found: children with high serum drug concentrations at the lower INH, PZA, and RMP doses did not necessarily have comparatively high serum drug concentrations following the higher dose.
Table 2.
Mean maximum serum concentrations (Cmax), area under the curve (AUC), and mean time until maximum serum concentration is reached (tmax) with 95% confidence interval for INH, PZA, and RMP in children <2 years of age
| Parameter | INH (n = 20) |
PZA (n = 20) |
RMP (n = 11) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg | 10 mg/kg | P valuea | 25 mg/kg | 35 mg/kg | P valuea | 10 mg/kg | 15 mg/kg | P valuea | |
| Cmax(μg/ml) | 3.19 (2.39-3.99) | 8.11 (6.68-9.53) | <0.001b | 29.95 (26.16-33.73) | 47.11 (42.64-51.58) | <0.001b | 6.36 (4.45-8.27) | 11.69 (8.71-14.67) | 0.005b |
| tmax (h) | 0.70 (0.72-1.17) | 0.52(0.64-1.12) | 0.640 | 1.44 (0.98-1.41) | 1.45 (0.96-1.41) | 0.961 | 1.49 (1.07-1.91) | 1.54 (1.16-1.93) | 0.865 |
| AUC (μg·h/ml) | 8.09 (5.76-10.42) | 20.36 (15.76-24.97) | <0.001b | 118.01 (101.33-134.70) | 175.23 (155.50-194.96) | <0.001b | 17.78 (12.81-22.76) | 36.95 (27.64-46.26) | 0.006b |
From paired t test comparing previous and revised doses.
Significant on the 0.05 level.
Fig. 1.
Serum INH, PZA, and RMP concentrations following old and revised WHO dosage recommendations. Shown are means with 95% confidence intervals. Panels: a, INH; b, PZA; c, RMP.
A low Cmax of INH (<3 μg/m) was seen in half of the children (10 of 20) following an INH dose of 5 mg/kg, but in none after an INH dose of 10 mg/kg. For PZA, 15 of 20 children had a low Cmax (<35 μg/ml) following a dose of 25 mg/kg (2 children <20 μg/ml), while only one child had a concentration below this threshold following PZA at 35 mg/kg. Low (<8 μg/ml) RMP Cmaxs were found in 6 of 11 children following a 10-mg/kg dose of RMP compared with three following a dose of 15 mg/kg. Very low (<4 μg/ml) RMP Cmax s were found in 3 of the 11 children following 10 mg/kg, whereas none had such low concentrations following dosing at 15 mg/kg.
Influence of RMP on pharmacokinetics.
There were no differences in INH pharmacokinetics (with either 5 or 10 mg/kg) between children receiving RMP and those not receiving RMP (Table 3). Children receiving RMP achieved a significantly lower PZA Cmax and AUC following a PZA dose of 25 mg/kg than children not receiving RMP (Table 4). Following the higher PZA dose (35 mg/kg), the differences between children with and without RMP were diminished and not statistically significantly different. The tmax of PZA was not influenced by RMP at either the previous or the revised dosage.
Table 3.
Influence of HIV, type of TB, RMP, gender, age and nutritional status (weight for age Z score) on pharmacokinetic parameters of INH
| Parameter | No. |
Cmax(μg/ml) |
tmax (h) |
AUC0-5(μg·h/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg |
10 mg/kg |
5 mg/kg |
10 mg/kg |
5 mg/kg |
10 mg/kg |
||||||||
| Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | ||
| HIV status | |||||||||||||
| Negative | 15 | 3.19 (2.18-4.19) | 0.990 | 8.50 (6.69-10.32) | 0.229 | 0.98 (0.71-1.24) | 0.688 | 0.76 (0.49-1.02) | 0.078 | 7.39 (4.98-9.80) | 0.413 | 19.54 (14.04-25.06) | 0.552 |
| Positive | 5 | 3.20 (1.25-5.15) | 6.91 (4.37-9.46) | 0.86 (0.21-1.52) | 1.26 (0.67-1.85) | 10.21 (1.98-18.44) | 22.81 (10.19-35.42) | ||||||
| Type of TB | |||||||||||||
| Extrapulmonary | 9 | 2.93 (1.89-3.97) | 0.539 | 7.07 (5.17-8.98) | 0.166 | 0.87 (0.50-1.23) | 0.515 | 1.04 (0.64-1.44) | 0.224 | 8.15 (3.86-12.44) | 0.966 | 18.91 (11.10-26.73) | 0.567 |
| Pulmonary | 11 | 3.40 (2.06-4.75) | 8.95 (6.72-11.18) | 1.01 (0.68-1.34) | 0.75 (0.42-1.08) | 8.05 (4.88-11.22) | 21.55 (14.90-28-19) | ||||||
| RMP | |||||||||||||
| No RMP | 9 | 3.31 (1.43-4.07) | 0.791 | 8.89 (6.03-11.75) | 0.337 | 0.91 (0.52-1.30) | 0.758 | 0.70 (0.36-1.05) | 0.169 | 7.24 (3.97-10.51) | 0.491 | 19.26 (11.05-27.47) | 0.667 |
| RMP included | 11 | 3.09 (0.98-2.45) | 7.46 (5.88-9.05) | 0.98 (0.66-1.30) | 1.02 (0.67-1.39) | 8.79 (5.03-12.56) | 21.26 (9.52-2.87) | ||||||
| Gender | |||||||||||||
| Male | 11 | 3.31 (2.05-4.56) | 0.742 | 8.73 (6.87-10.60) | 0.332 | 1.11 (0.79-1.44) | 0.083 | 0.93 (0.57-1.28) | 0.670 | 8.82 (5.13-12.51) | 0.476 | 23.02 (16.58-29.46) | 0.19 |
| Female | 9 | 3.05 (1.82-4.28) | 7.34 (4.76-9.92) | 0.74 (0.42-1.07) | 0.83 (0.42-1.23) | 7.21 (3.81-10.60) | 17.11 (9.67-24) | ||||||
| Age | 20 | 0.044b | 0.083 | 0.764 | 0.787 | <0.001b | 0.007b | ||||||
| Nutritional status (Z score wt for age) | 20 | 0.237 | 0.228 | 0.909 | 0.207 | 0.777 | 0.582 | ||||||
P values are from independent t test (HIV, TB, RMP, gender) or linear regression analysis (age, nutrition).
Significant at the 0.05 level.
Table 4.
Influence of HIV, type of TB, RMP, gender, age and nutritional status (weight for age Z score) on pharmacokinetic parameters of PZA
| Parameter | N |
Cmax(μg/ml) |
tmax (h) |
AUC0-5(μg·h/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 mg/kg |
35 mg/kg |
25 mg/kg |
35 mg/kg |
25 mg/kg |
35 mg/kg |
||||||||
| Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | ||
| HIV status | |||||||||||||
| Negative | 15 | 30.91 (26.15-25.67) | 0.304 | 49.20 (43.62-54.77) | 0.015b | 1.16 (0.91-1.42) | 0.654 | 1.08 (0.79-1.37) | 0.009b | 122.9 (101.6-144.3) | 0.183 | 180.1 (154.0-206.3) | 0.186 |
| Positive | 5 | 27.05 (19.34-34.76) | 40.84 (36.22-45.46) | 1.28 (0.69-1.86) | 1.50 (1.39-1.61) | 103.2 (75.80-130.6) | 160.5 (139.9-181.1) | ||||||
| Type of TB | |||||||||||||
| Extrapulmonary | 9 | 29.06 (22.29-35.83 | 0.675 | 44.65 (35.77-53.53) | 0.335 | 1.20 (0.84-1.57) | 0.911 | 1.43 (0.75-1.53) | 0.737 | 114.3 (84.97-143.6) | 0.686 | 173.8 (140.6-207.0 | 0.895 |
| Pulmonary | 11 | 30.67 (25.43-35.91) | 49.12 (44.06-54.19) | 1.18 (0.87-1.49) | 1.22 (0.89-1.55) | 121.1 (97.53-144.6) | 176.4 (147.2-205.6) | ||||||
| RMP | |||||||||||||
| No RMP | 9 | 34.07 (27.17-40.97) | 0.049 | 50.70 (45.50-55.90) | 0.117 | 1.12 (0.74-1.50) | 0.541 | 1.25 (0.89-1.60) | 0.616 | 138.2 (107.7-168.7) | 0.029b | 182.7 (146.2-219.3) | 0.498 |
| RMP included | 11 | 26.58 (22.77-30.38) | 44.17 (36.94-51.40) | 1.25 (0.96-1.55) | 1.13 (0.78-1.49) | 101.5 (86.63-116.3) | 169.1 (143.3-194.9) | ||||||
| Gender | |||||||||||||
| Male | 11 | 28.03 (24.31-31.75) | 0.287 | 48.89 (42.84-54.95) | 0.378 | 1.30 (1.03-1.57) | 0.270 | 1.20 (0.87-1.52) | 0.895 | 108.2 (92.19-124.1) | 0.212 | 188.4 (164.8-212.1) | 0.138 |
| Female | 9 | 32.29 (24.38-40.19) | 44.93 (37.03-52.83) | 1.06 (0.67-1.46) | 1.17 (0.76-1.57) | 130.1 (95.55-164.6) | 159.1 (123.6-194.6) | ||||||
| Age | 20 | 0.972 | 0.373 | 0.998 | 0.258 | 0.550 | 0.198 | ||||||
| Nutritional status (Z score wt for age) | 20 | 0.529 | 0.676 | 0.787 | 0.693 | 0.679 | 0.416 | ||||||
P values are from independent t test(HIV, TB, RMP, gender) or linear regression analysis(age, nutrition).
Significant at the 0.05 level.
Influence of patient characteristics on pharmacokinetics.
Nutritional status, assessed by weight for age, body mass index, and weight-for-age Z score according to WHO growth charts, did not influence the pharmacokinetics of any of the drugs, irrespective of the dose (only data for Z scores are shown in Tables 3 to 5).
Table 5.
Influence of HIV, type of TB, RMP, gender, age and nutritional status (weight for age Z score) on pharmacokinetic parameters of RMP
| Parameter | N |
Cmax(μg/ml) |
tmax (h) |
AUC0-5(μg·h/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 mg/kg |
15 mg/kg |
10 mg/kg |
15 mg/kg |
10 mg/kg |
15 mg/kg |
||||||||
| Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | ||
| HIV status | |||||||||||||
| Negative | 7 | 6.85 (4.43-9.27) | 0.520 | 12.52 (7.65-27.39) | 0.350 | 1.612 (0.97-2.25) | 0.390 | 1.56 (0.88-1.38) | 0.901 | 18.41 (12.80-24.02) | 0.774 | 36.12 (22.67-49.57) | 0.810 |
| Positive | 4 | 5.49 (0.06-10.92) | 10.24 (6.46-14.01) | 1.283 (0.48-2.08) | 1.52 (1.38-1.66) | 16.68 (0.24-33.12) | 38.40 (15.21-61.59) | ||||||
| Type of TB | |||||||||||||
| Extrapulmonary | 8 | 5.77 (3.23-8.31) | 0.185 | 11.49 (7.19-15.80) | 0.753 | 1.62 (1.09-2.15) | 0.271 | 1.54 (0.97-2.11) | 0.998 | 17.16 (10.10-24.23 | 0.576 | 37.29 (23.97-50.62 | 0.870 |
| Pulmonary | 3 | 7.93 (3.66-12.20) | 12.21 (6.77-17.66) | 1.14 (0.17-2.46) | 1.54 (1.32-1.77) | 19.44 (8.70-30.19) | 36.03 (15.40-56.67) | ||||||
| Gender | |||||||||||||
| Male | 6 | 6.20 (3.81-8.60) | 0.866 | 12.20 (9.09-15.31) | 0.724 | 1.50 (1.43-1.57) | 0.961 | 1.31 (0.87-1.75) | 0.176 | 18.19 (11.28-25.09) | 0.860 | 37.51 (28.27-46.75) | 0.901 |
| Female | 5 | 6.53 (1.96-11.12) | 11.09 (3.50-18.67) | 1.48 (0.26-2.70) | 1.82 (1.01-2.63) | 17.30 (6.03-28.57) | 36.28 (12.01-60.55) | ||||||
| Age | 11 | 0.240 | 0.232 | 0.351 | 0.153 | 0.415 | 0.527 | ||||||
| Nutritional status (Z score wt for age) | 11 | 0.344 | 0.774 | 0.437 | 0.217 | 0.832 | 0.270 | ||||||
P values are from independent t test (HIV, TB, RMP, gender) or linear regression analysis (age, nutrition).
There were no differences in the pharmacokinetics of INH and RMP between HIV-infected and HIV-uninfected children (Tables 3 and 5). Following a PZA dose of 35 mg/kg, HIV-uninfected children achieved higher maximum serum PZA concentrations than did HIV-infected children after a shorter time (tmax, 1.08 versus 1.50 h) (Table 4). The AUC for HIV-uninfected children was also greater than that for HIV-infected children, but this difference was not significant. No difference in Cmax, tmax, or AUC was found in HIV-infected versus uninfected children following a PZA dose of 25 mg/kg.
Neither the type of TB (pulmonary versus extrapulmonary disease) nor gender affected the pharmacokinetics of INH, PZA, or RMP (Tables 3 to 5). Age had an impact on the pharmacokinetic parameters of INH, but not on those of PZA and RMP (Tables 3 to 5). At both INH dosages, younger age was associated with a higher maximum serum INH concentration (INH at 5 mg/kg, P = 0.04; INH at 10 mg/kg, P = 0.22) and a greater AUC (INH at 5 mg/kg, P = 0.004; INH at 10 mg/kg, P = 0.007). No difference was detected for tmax.
The influence of acetylator status.
The NAT2 genotype was distributed as follows: 8 children were FF acetylators (5 of 8 children <1 year old), 4 were FS acetylators (3 of 4 children <1 year old), and 8 were SS acetylators (4 of 8 children <1 year old). The maximum serum INH concentration and the AUC were greater for the SS acetylators than for the FF acetylators following INH dosing at both 5 mg/kg and 10 mg/kg (Table 6).
Table 6.
Mean maximum serum INH concentrations and AUCs after administration of 5 and 10 mg/kg according to acetylator status
| NAT2 status | No. |
Cmax |
AUC0-5 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg |
10 mg/kg |
5 mg/kg |
10 mg/kg |
||||||
| Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | Mean (95% CI) | P valuea | ||
| Fast | 8 | 2.16 (0.10-3.32) | 0.139 | 6.69 (4.58-8.79) | 0.650 | 4.28 (1.70-6.87) | 0.196 | 12.89 (8.66-17.12) | 0.416 |
| Intermediate | 4 | 3.64 (2.00-5.29) | 0.031b | 7.48 (4.51-10.53) | 0.039b | 7.14 (3.48-10.80) | <0.001b | 15.79 (9.80-21.77) | <0.001b |
| Slow | 8 | 4.00 (2.83-5.16) | 0.714 | 9.84 (7.74-11.94) | 0.189 | 12.38 (9.80-14.97) | 0.024 | 30.12 (25.89-34.35) | <0.001b |
From one-way ANOVA.
Significant at the 0.05 level.
The impact of acetylator status is shown in Table 6. At both doses, Cmax and AUC differ significantly between SS and FF acetylators. There were no differences found regarding tmax based on acetylator status.
At an INH dose of 5 mg/kg, 1 of 8 children with FF acetylator status, 1 of 4 with FS acetylator status, and 7 of 8 with SS acetylator status achieved Cmaxs above the recommended minimal therapeutic concentration for INH (3 μg/ml), while with INH at 10 mg/kg, all children achieved INH Cmaxs above the therapeutic target concentration.
DISCUSSION
We show that following administration of INH, PZA, and RMP at the revised WHO dosage recommendations, most children under 2 years of age had serum drug concentrations within the recommended therapeutic range. In contrast, at the previous WHO dosages, serum INH, PZA, and RMP concentrations were significantly lower and often below the recommended concentrations for anti-TB therapy. Serum INH concentrations in children are generally reported to be lower than in adults following the same mg/kg dose (3, 21, 34, 41). Although INH is the most extensively studied anti-TB drug, we are aware of only two reports of INH pharmacokinetics that included only children younger than 3 years (19, 30). In these studies, INH was given at doses (15 mg/kg and 10 mg/kg, respectively) similar to those currently recommended by the WHO (10 to 15 mg/kg). The peak serum INH concentrations were comparable to those in our study and above the therapeutic minimum concentration of INH. In a previous study by our group, serum INH concentrations in children younger than 6 years of age receiving a dose of 4 to 6 mg/kg were significantly lower than in children prescribed a dose of 8 to 10 mg/kg (2.39 μg/ml versus 5.71 μg/ml) (21). The proportion of children with peak serum INH concentrations of <3 μg/ml following an INH dose of 5 mg/kg was even higher than in the present study (70% versus 50%).
In our study, coadministration with RMP had no influence on INH pharmacokinetics, while some inconsistent influence on PZA pharmacokinetics was found. Serum INH, PZA, and RMP concentrations were not associated with nutritional status, type of TB, or gender. The HIV status of the child did not affect INH and RMP pharmacokinetics, while the findings for PZA differed, dependent on the dosage given. Younger children achieved higher serum INH concentrations than older children, but for PZA and RMP, age did not influence the pharmacokinetic profile (within this 2-year age range). Children with a NAT2 fast-acetylator status had significantly lower serum INH concentrations than children with slow-acetylator status.
Rey et al. demonstrated a marked difference between slow and fast INH acetylators, with slow acetylators achieving higher serum INH levels. In further studies, a trimodal INH elimination was demonstrated (18, 26). Several studies have confirmed that fast- or intermediate-acetylator status is associated with lower serum INH concentrations than slow-acetylator status (21, 34). The distribution of acetylator phenotypes is population specific. This might explain the comparatively higher serum INH concentrations among Indian children, with mean serum INH concentrations of 4.75 μg/ml following an INH dose of 5 mg/kg and 10.1 μg/ml following a dose of 10 mg/kg (31). Routine determination of the acetylator status for clinical care is not feasible in resource-limited settings; however, with an INH dosage of 10 mg/kg, appropriate serum INH concentrations can be ensured in most children, irrespective of their acetylator status. Higher dosages might not be required in pediatric populations with predominantly slow acetylators and would unnecessarily expose patients to a higher risk of side effects.
Age-dependent elimination of INH has been demonstrated, with younger children eliminating INH more rapidly than older children and adults (21, 34, 46). This has been attributed mainly to a relatively larger liver size in proportion to total body weight in young children (3, 34). In our study, however, where all children were less than 2 years old, serum INH levels were higher in the younger children despite more children <1 year of age with the fast- and intermediate-acetylator phenotypes. In this very young age group, maturation of the NAT2 enzyme system might play an important role in INH metabolism. There is some evidence that maturation of acetylation occurs within the first 2 years of life and the cumulative percentage of fast acetylators increases with age, although the final acetylator status is genetically determined (25, 46). The effect of age in our study population might therefore be confounded by maturation of the NAT2 phenotype.
There are no published studies of the pharmacokinetics of PZA specifically investigating children younger than 2 years of age. In two studies of older children, PZA was used at 35 mg/kg, the same dosage currently recommended by the WHO; in one study (n = 10), the ages of the children studied ranged from 6 to 12 years and in the second (n = 27), the median age was 5.7 years (12, 31, 44). The mean Cmax in these studies was 40 to 50 μg/ml, similar to that observed in our study. In a study of serum PZA concentrations across different age groups, PZA pharmacokinetics did not differ significantly with age (39). In our study, no influence of age on PZA pharmacokinetics was found. There is evidence that serum PZA concentrations increase linearly with increasing dose (45). In our study, serum PZA concentrations following a dose of 25 mg/kg were significantly lower than those following a 35-mg/kg dose. This is in contrast to a small study of PZA pharmacokinetics in children (>5 years of age) where serum PZA concentrations of 42.4 (SD, 3.3) μg/ml were reported following a dose of 25 mg/kg (13).
Of note is that the lower recommended limit of serum PZA concentrations (20 μg/ml) was achieved in 18 children following the previous WHO dosage recommendations and in all 20 following the higher dosages. However, mean serum PZA concentrations above 35 μg/ml were only achieved following higher doses.
Most of the published pharmacokinetic studies of RMP in children were performed after a single dose of RMP and not after anti-TB therapy with RMP had been established. Due to self-induction of RMP metabolism, these findings might show higher serum drug concentrations than actually present during established anti-TB therapy. In a study with children 2 months to 5 years of age, low maximum serum drug levels (5.2 μg/ml) were found after a dose of 15 mg/kg (9). A more recent study of children (mean age, 3.8 years) established on RMP therapy assessed RMP parameters following a dose of 10 mg/kg (35). Serum RMP concentrations and AUCs were comparable to those in our younger study population. In both studies, the mean peak serum drug concentrations were low (<8 μg/ml). Again, our data confirm that the revised dosages recommended by WHO are necessary to achieve satisfactory serum RMP concentrations in young children. Although children have been documented to achieve lower serum RMP concentrations than adults in several studies, we could not find any influence of age within our young study population (1, 16, 35, 40).
Because RMP is a potent inducer of the cytochrome P450 system, concomitant treatment can result in a decreased half-life of a number of compounds (20, 42). Serum INH concentrations in our study were not affected by RMP, as has been previously described (24, 33). Our findings indicate that when PZA is given at a dose of 25 mg/kg, the concomitant use of RMP reduces the Cmax of PZA. However, following a PZA dose of 35 mg/kg, RMP did not influence the PZA concentration. The clinical significance and mechanism of this finding are not clear. In previous studies, concurrent RMP did not influence the pharmacokinetics of PZA (2, 32). However, Bouhlabal et al. reported significantly lower serum PZA concentrations in adults when the drug was given in combination with INH and RMP rather than as monotherapy (7).
HIV-infected patients have been reported to achieve lower serum anti-TB drug concentrations (8, 12, 14). This has been attributed to malabsorption caused by drug-drug interactions, gastrointestinal infections, or wasting, rather than the HIV infection itself. The different anti-TB drugs seem to be affected in various ways. Graham et al. demonstrated decreased serum PZA levels in Malawian HIV-infected children, while serum ethambutol concentrations were not lower in HIV-infected children than in non-HIV-infected children (12). In our study, HIV-infected children had lower serum concentrations of all of the first-line drugs tested, but only for PZA did this difference reach statistical significance. As only five children in our study were HIV infected, we may, however, not have been sufficiently powered to demonstrate such differences. The effect of HIV coinfection on the pharmacokinetics of anti-TB drugs in children requires further investigation.
Conclusions.
Our data document low serum INH, PZA, and RMP concentrations following the previous WHO dose recommendations for children younger than 2 years of age. In contrast, administration of the revised WHO dose recommendations of INH at 10 mg/kg, RMP at 15 mg/kg, and PZA at 35 mg/kg results in satisfactory serum drug concentrations. Our data provide supportive evidence for the implementation of the revised WHO guidelines for first-line anti-TB therapy in young children.
Footnotes
Published ahead of print on 3 October 2011.
REFERENCES
- 1. Acocella G., Buniva G., Flauto U., Nicolis F. 1970. Absorption and elimination of the antibiotic rifampicin in newborns and children, p. 755–760 In Proceedings of the Sixth International Congress of Chemotherapy 1969 University of Tokyo Press, Tokyo, Japan [Google Scholar]
- 2. Acocella G., Nonis A., Gialdroni-Grassi G., Grassi C. 1988. Comparative bioavailability of isoniazid, rifampin, and pyrazinamide administered in free combination and in a fixed triple formulation designed for daily use in antituberculosis chemotherapy. I. Single-dose study. Am. Rev. Respir. Dis. 138: 882–885 [DOI] [PubMed] [Google Scholar]
- 3. Advenier C., Saint-Aubin A., Scheinmann P., Paupe J. 1981. The pharmacokinetics of isoniazid in children (author's transl). Rev. Fr. Mal. Respir. 9: 365–374 [PubMed] [Google Scholar]
- 4. Alcorn J., McNamara P. J. 2002. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin. Pharmacokinet. 41: 959–998 [DOI] [PubMed] [Google Scholar]
- 5. Bartelink I. H., Rademaker C. M., Schobben A. F., N. van den Anker J. 2006. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin. Pharmacokinet. 45: 1077–1097 [DOI] [PubMed] [Google Scholar]
- 6. Blake M. J., et al. 2006. Pharmacokinetics of rifapentine in children. Pediatr. Infect. Dis. J. 25: 405–409 [DOI] [PubMed] [Google Scholar]
- 7. Boulahbal F., Khaled S., Bouhassen H., Larbaoui D. 1978. Absorption and urinary elimination of pyrazinamid administered alone or in combination with isoniazid and rifampicin. Arch. Inst. Pasteur Alger. 53: 165–185 [PubMed] [Google Scholar]
- 8. Chideya S., et al. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 48: 1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Rautlin de la Roy Y., Hoppeler A., Creusot G., Brault A. M. 1974. Rifampicin levels in the serum and cerebrospinal fluid in children. Arch. Fr. Pediatr. 31: 477–488 [PubMed] [Google Scholar]
- 10. Donald P. R., Maher D., Maritz J. S., Qazi S. 2006. Ethambutol dosage for the treatment of children: literature review and recommendations. Int. J. Tuberc. Lung Dis. 10: 1318–1330 [PubMed] [Google Scholar]
- 11. Ellard G. A. 1969. Absorption, metabolism and excretion of pyrazinamide in man. Tubercle 50: 144–158 [DOI] [PubMed] [Google Scholar]
- 12. Graham S. M., et al. 2006. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob. Agents Chemother. 50: 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta P., Roy V., Sethi G. R., Mishra T. K. 2008. Pyrazinamide blood concentrations in children suffering from tuberculosis: a comparative study at two doses. Br. J. Clin. Pharmacol. 65: 423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurumurthy P., et al. 2004. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin. Infect. Dis. 38: 280–283 [DOI] [PubMed] [Google Scholar]
- 15. Heifets L. B., Lindholm-Levy P. J., Flory M. 1991. Comparison of bacteriostatic and bactericidal activity of isoniazid and ethionamide against Mycobacterium avium and Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 143: 268–270 [DOI] [PubMed] [Google Scholar]
- 16. Hussels H., Kroening U., Magdorf K. 1973. Ethambutol and rifampicin serum levels in children: second report on the combined administration of ethambutol and rifampicin. Pneumonologie 149: 31–38 [DOI] [PubMed] [Google Scholar]
- 17. Kearns G. L., et al. 2003. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 349: 1157–1167 [DOI] [PubMed] [Google Scholar]
- 18. Kinzig-Schippers M., et al. 2005. Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob. Agents Chemother. 49: 1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llorens J., Serrano R. J., Sanchez R. 1978. Pharmacodynamic interference between rifampicin and isoniazid. Chemotherapy 24: 97–103 [DOI] [PubMed] [Google Scholar]
- 20. McIlleron H., et al. 2007. Elevated gatifloxacin and reduced rifampicin concentrations in a single-dose interaction study amongst healthy volunteers. J. Antimicrob. Chemother. 60: 1398–1401 [DOI] [PubMed] [Google Scholar]
- 21. McIlleron H., et al. 2009. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin. Infect. Dis. 48: 1547–1553 [DOI] [PubMed] [Google Scholar]
- 22. Miller S. A., Dykes D. D., Polesky H. F. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore D. P., Schaaf H. S., Nuttall J., Marais B. J. 2009. Childhood tuberculosis guidelines of the Southern African Society for Paediatric Infectious Diseases. South. Afr. J. Epidemiol. Infect. 29: 57–68 [Google Scholar]
- 24. Mouton R. P., Mattie H., Swart K., Kreukniet J., de Wael J. 1979. Blood levels of rifampicin, desacetylrifampicin and isoniazid during combined therapy. J. Antimicrob. Chemother. 5: 447–454 [DOI] [PubMed] [Google Scholar]
- 25. Pariente-Khayat A., et al. 1997. Isoniazid acetylation metabolic ratio during maturation in children. Clin. Pharmacol. Ther. 62: 377–383 [DOI] [PubMed] [Google Scholar]
- 26. Parkin D. P., et al. 1997. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 155: 1717–1722 [DOI] [PubMed] [Google Scholar]
- 27. Peloquin C. 1992. Therapeutic drug monitoring: principles and applications in mycobacterial infections. Drug Ther. 22: 31–36 [Google Scholar]
- 28. Peloquin C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62: 2169–2183 [DOI] [PubMed] [Google Scholar]
- 29. Pinheiro V. G., et al. 2006. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz. J. Infect. Dis. 10: 374–379 [DOI] [PubMed] [Google Scholar]
- 30. Rey E., et al. 2001. Isoniazid pharmacokinetics in children according to acetylator phenotype. Fundam. Clin. Pharmacol. 15: 355–359 [DOI] [PubMed] [Google Scholar]
- 31. Roy V., Tekur U., Chopra K. 1999. Pharmacokinetics of pyrazinamide in children suffering from pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 3: 133–137 [PubMed] [Google Scholar]
- 32. Ruslami R., et al. 2007. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob. Agents Chemother. 51: 2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarma G. R., Kailasam S., Nair N. G., Narayana A. S., Tripathy S. P. 1980. Effect of prednisolone and rifampin on isoniazid metabolism in slow and rapid inactivators of isoniazid. Antimicrob. Agents Chemother. 18: 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaaf H. S., et al. 2005. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch. Dis. Child. 90: 614–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schaaf H. S., et al. 2009. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med. 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seifart H. I., Gent W. L., Parkin D. P., van Jaarsveld P. P., Donald P. R. 1995. High-performance liquid chromatographic determination of isoniazid, acetylisoniazid and hydrazine in biological fluids. J. Chromatogr. B Biomed. Appl. 674: 269–275 [DOI] [PubMed] [Google Scholar]
- 37. Smith P. J., van Dyk J., Fredericks A. 1999. Determination of rifampicin, isoniazid and pyrazinamide by high performance liquid chromatography after their simultaneous extraction from plasma. Int. J. Tuberc. Lung Dis. 3: S325–S328 (Discussion,S351-S352). [PubMed] [Google Scholar]
- 38. Thee S., Detjen A., Quarcoo D., Wahn U., Magdorf K. 2007. Ethambutol in paediatric tuberculosis: aspects of ethambutol serum concentration, efficacy and toxicity in children. Int. J. Tuberc. Lung Dis. 11: 965–971 [PubMed] [Google Scholar]
- 39. Thee S., Detjen A., Wahn U., Magdorf K. 2008. Pyrazinamide serum levels in childhood tuberculosis. Int. J. Tuberc. Lung Dis. 12: 1099–1101 [PubMed] [Google Scholar]
- 40. Thee S., Detjen A., Wahn U., Magdorf K. 2009. Rifampicin serum levels in childhood tuberculosis. Int. J. Tuberc. Lung Dis. 13: 1106–1111 [PubMed] [Google Scholar]
- 41. Thee S., Detjen A. A., Wahn U., Magdorf K. 2010. Isoniazid pharmacokinetic studies of the 1960s: considering a higher isoniazid dose in childhood tuberculosis. Scand. J. Infect. Dis. 42: 294–298 [DOI] [PubMed] [Google Scholar]
- 42. Venkatesan K. 1992. Pharmacokinetic drug interactions with rifampicin. Clin. Pharmacokinet. 22: 47–65 [DOI] [PubMed] [Google Scholar]
- 43. WHO 2011. Child growth standards. WHO, Geneva, Switzerland: http://www.who.int/childgrowth/standards/weight_for_age/en/index.html [Google Scholar]
- 44. WHO September 2009, posting date. Dosing instructions for the use of currently available fixed-dose combination TB medicines for children. WHO, Geneva, Switzerland: www.who.int/entity/tb/challenges/interim_paediatric_fdc_dosing_instructions_sept09.pdf [Google Scholar]
- 45. Zhu M., et al. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22: 686–695 [DOI] [PubMed] [Google Scholar]
- 46. Zhu R., et al. 10 May 2011, posting date. The pharmacogenetics of NAT2 enzyme maturation in perinatally HIV exposed infants receiving isoniazid. J. Clin. Pharmacol. [Epub ahead of print.] doi:10.1177/0091270011402826 [DOI] [PMC free article] [PubMed] [Google Scholar]