Abstract
The objective of this study was to evaluate the antibacterial effects of polymethylmethacrylate (PMMA) bone cements loaded with daptomycin, vancomycin, and teicoplanin against methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-intermediate Staphylococcus aureus (VISA) strains. Standardized cement specimens made from 40 g PMMA loaded with 1 g (low-dose), 4 g (middle-dose) or 8 g (high-dose) antibiotics were tested for elution characteristics and antibacterial activities. The patterns of release of antibiotics from the cement specimens were evaluated using in vitro broth elution assay with high-performance liquid chromatography. The activities of broth elution fluid against different Staphylococcus aureus strains (MSSA, MRSA, and VISA) were then determined. The antibacterial activities of all the tested antibiotics were maintained after being mixed with PMMA. The cements loaded with higher dosages of antibiotics showed longer elution periods. Regardless of the antibiotic loading dose, the teicoplanin-loaded cements showed better elution efficacy and provided longer inhibitory periods against MSSA, MRSA, and VISA than cements loaded with the same dose of vancomycin or daptomycin. Regarding the choice of antibiotics for cement loading in the treatment of Staphylococcus aureus infection, teicoplanin was superior in terms of antibacterial effects.
INTRODUCTION
Most patients with osteomyelitis require adequate drainage, thorough debridement, obliteration of dead space, and long-term antibiotic therapy. Unfortunately, intravenous antibiotic therapy with several antibiotics requires careful monitoring of serum drug concentrations to maintain nontoxic systemic levels. Moreover, the blood supply to the infected tissue may be compromised, which can prevent sufficient local antibiotic levels (16, 21). For these reasons, interest in the local administration of antibiotics has been increasing (16, 21). Antibiotic loaded polymethylmethacrylate (PMMA) bone cements have been used in the treatment of osteomyelitis and in the placement of orthopedic implants in patients at high risk for infection. The ability to deliver high local concentrations of antimicrobial agents with PMMA has made it into one of the standards of treatment for patients with chronic infection of the skeletal system (9).
Staphylococcus aureus is the most common organism implicated in the infection of the skeletal system (6, 8). Approximately 20% of S. aureus isolates from Europe are reported as methicillin-resistant (MRSA), and the prevalence ranges from 33 to 55% in U.S. hospitals (1). The glycopeptides antibiotics vancomycin and teicoplanin remain the standards for treating most MRSA infections. However, reports of vancomycin-intermediate S. aureus (VISA), first isolated in Japan in 1997 (10), and vancomycin-resistant S. aureus in the United States (4) caused widespread alarm. Daptomycin is a cyclic lipopeptide antibiotic which has a rapid bactericidal activity in vitro against a broad spectrum of Gram-positive bacteria (3). Since antibiotic-loaded cement is widely used in treating orthopedic infection, the effective selection of daptomycin, vancomycin, or teicoplanin as the antibiotic loaded into PMMA became an important issue for the treatment of S. aureus infection, especially against resistant strains such as MRSA and VISA.
To the best of our knowledge, the elution characteristics of antibiotic-loaded PMMA which contained daptomycin, vancomycin, and teicoplanin have not been studied. The objective of our study was to measure the elution characteristics of daptomycin, vancomycin, and teicoplanin from antibiotic-loaded cement specimens and to compare their antibacterial activities against methicillin-susceptible S. aureus (MSSA), MRSA, and VISA. We used an in vitro model to measure the antibiotic release characteristics and the bacterial-eradication ability of antibiotic-loaded bone cement.
MATERIALS AND METHODS
Antibiotic-loaded cement specimens.
Surgical Simplex bone cement (Stryker Orthopaedics, Limerick, Ireland) with added daptomycin (Cubist Pharmaceuticals, Inc., Lexington, MA), vancomycin (Gentle Pharmaceutical Co. Yulin, Taiwan), or teicoplanin (Sanofi-Aventis, Paris, France) was tested. The antibiotic-cement mixture consisted of 1 g (low dose), 4 g (middle dose) or 8 g (high dose) of antibiotic mixed with 40 g of bone cement polymer prior to the addition of the liquid counterpart. The cement-antibiotic mixture was hand-mixed in a ceramic container for 2 min to achieve a doughy consistency and then manually pressed into a plastic mold to form uniform cylindrical test specimens. The cement cylinders, 20 mm in height and 15 mm in diameter, were cured at room temperature for 1 h and stored at −80°C for further investigation. The preliminary data showed that the degradation of daptomycin, vancomycin, and teicoplanin was less than 1% after antibiotic-loaded cements were stored at −80°C for 3 months. Hence, −80°C appeared to be the optimal temperature for the storage of antibiotic-loaded cement cylinders.
Antibiotic broth elution assay.
Each cement cylinder was immersed in polypropylene tubes with 5 ml phosphate-buffered saline (PBS; pH 7.3) and shaken in a rotator at 37°C. The study continued for 60 days, with the daily transfer of the cement cylinder into a test tube with PBS after washing with saline. The cumulative and daily antibiotic releases were measured, and 2-ml elution samples of PBS were collected at 14 time points with a gradually expanding interval over a period of 60 days (days 0, 1, 2, 3, 4, 5, 6, 7, 10, 14, 21, 28, 40, and 60) and subsequently stored at −80°C until analysis.
The concentrations of antibiotics were determined using high-performance liquid chromatography (HPLC). The chromatography assay was performed using a model ALC 717 chromatograph (Waters Associates, Milford, MA) with a stainless-steel column (RP18 column, 100 mm by 4.6 mm, 5-μm particle size for vancomycin and daptomycin; C18 column, 250 mm by 4.6 mm, 5-μm particle size for teicoplanin). The mobile phase consisted of water-acetonitrile-100 mM ammonium formate (composite ratio, 78/12/10), acetonitrile-0.5% ammonium phosphate (composite ratio, 34/66) and sodium dihydrogen phosphate-acetonitrile (composite ratio, 90/10) for vancomycin, daptomycin, and teicoplanin, respectively. The HPLC system had sensitivities of 0.5 μg/ml for vancomycin, 0.5 μg/ml for daptomycin, and 0.25 μg/ml for teicoplanin. The concentrations of antibiotics in the cement cylinders were obtained from the HPLC analysis through comparisons made with the prepared daily standard curves where the peak areas were correlated with antibiotic concentrations.
Bioassay of antibiotic activity.
The biological activity of the released antibiotics was studied using the aliquots of the same samples with a modified microtube dilution bioassay. The MSSA strain ATCC 25923, the MRSA strain ATCC 43300, and a laboratory-isolated VISA strain (vancomycin MIC = 4 μg/ml), were selected as the test organisms.
The microtube dilution bioassay was modified from the standardized tube dilution bioassay of the National Committee for Clinical Laboratory Standards, 1993. In brief, the samples were inoculated with tested bacteria of 105 CFU/ml in 96-well culture dishes and incubated at 37°C for 24 h. Since daptomycin required the presence of calcium (at 50 μg/ml for optimal activity), the test was performed in solutions supplemented with calcium to 50 μg/ml (7). The growth was assessed by comparing visibility with that of a positive control (without antibiotic) and standard samples with different concentrations of antibiotics.
Statistical analysis.
The antimicrobial concentration of elution samples from the cements with different preparations at each time point were sampled and tested in triplicate. The results were reported as means ± standard errors of the means. We used analysis of variance to determine the statistical differences in the antibiotic release efficacy between cements loaded with daptomycin, vancomycin, or teicoplanin. A P value of <0.01 was considered significant.
RESULTS
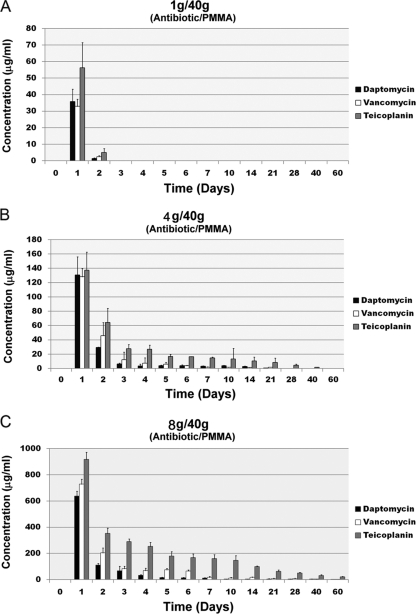
Based on the HPLC results, all tested samples showed a burst release during the first day. The release rates rapidly decreased during the next few days. A higher dose of antibiotic in the cements provided a longer release duration (Fig. 1). In the low-dose group (1 g antibiotic in 40 g PMMA), the specimens released the antibiotics for 2 days, regardless of the antibiotic loaded (Fig. 1A). In the middle-dose group (4 g antibiotic in 40 g PMMA), specimens loaded with daptomycin or vancomycin released the antibiotics for up to 21 days. The specimen loaded with the same amount of teicoplanin released teicoplanin for 40 days (Fig. 1B). In the high-dose group (8 g antibiotic in 40 g PMMA), all of the specimens showed antibiotic release up to 60 days, regardless of the antibiotic loaded (Fig. 1C).
Fig. 1.
Antibiotics released from the cement specimens in broth elution assay for 60 days. (A) Low-dose group; (B) middle-dose group; (C) high-dose group. The data are presented as the absolute release of each antibiotic amount in each cement specimen per day. Values are means ± standard errors of the means for triplicate specimens for each group.
During the 60-day study period, teicoplanin provided a significantly better release efficacy (total amounts of teicoplanin released, 306 ± 17 μg in the low-dose group, 1,716 ± 188 μg in the middle-dose group, and 13,699 ± 621 μg in the high-dose group) compared with vancomycin (total amounts of vancomycin released, 176 ± 5 μg in the low-dose group, 1,052 ± 152 μg in the middle-dose group, and 6,477 ± 648 μg in the high-dose group; P < 0.01) or daptomycin (total amounts of daptomycin released, 186 ± 7 μg in the low-dose group, 944 ± 93 μg in the middle-dose group, and 4,582 ± 420 μg in the high-dose group; P < 0.01). However, there was no significant difference in the release efficacy between cement samples loaded with vancomycin and daptomycin (P = 0.2).
With regard to the bioactivity against test organisms, all tested organisms were susceptible (by MIC) to the three drugs. The MICs of teicoplanin for MSSA was 0.125 μg/ml, followed by vancomycin (0.5 μg/ml) and daptomycin (1 μg/ml). The MICs for MRSA were 1 μg/ml for daptomycin and 2 μg/ml for vancomycin and teicoplanin. The MICs for VISA were 2 μg/ml for daptomycin and 4 μg/ml for vancomycin and teicoplanin.
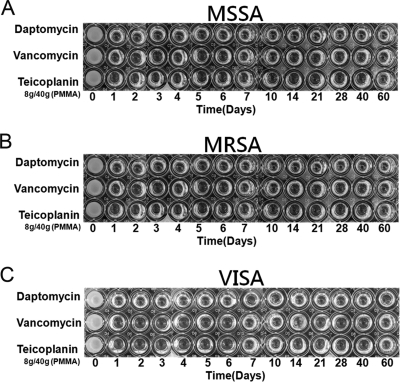
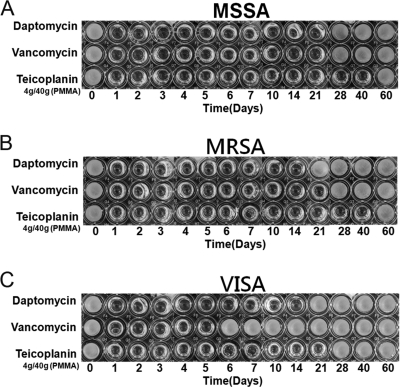
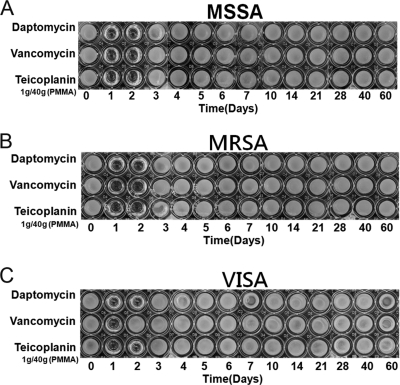
Daptomycin, vancomycin, and teicoplanin retained their antibacterial effects after incorporation into PMMA. All test specimens in the high-dose group exhibited antibacterial activities against MSSA, MRSA, and VISA throughout the 60-day elution period, as determined by the microtube dilution assay (Fig. 2). In the middle-dose group, the cements loaded with daptomycin or vancomycin showed antibacterial effects against MSSA for 21 days. At the same time, the cement loaded with the same dose of teicoplanin provided a longer duration (40 days) of antibacterial effect against MSSA (Fig. 3A). For bioactivities against MRSA, the antibacterial effects lasted for 14, 21, and 40 days in the cements loaded with daptomycin, vancomycin, and teicoplanin, respectively (Fig. 3B). For antibacterial activities against VISA, the cements loaded with middle-dose daptomycin or teicoplanin showed similar antibacterial effects against MRSA. However, the bioactivity against VISA lasted for only 5 days in the cement loaded with middle-dose vancomycin (Fig. 3C). In the low-dose group, the antibacterial effects did not last more than 2 days, regardless of the test organism or the antibiotic loaded (Fig. 4). In addition, the cement loaded with low-dose vancomycin provided only 1 day of antibacterial activity against VISA (Fig. 4C).
Fig. 2.
Antibacterial activities of broth elution samples from cements loaded with high-dose antibiotic (8 g antibiotic in 40 g PMMA) in a 60-day elution period. The figure shows the inhibition of MSSA (A), MRSA (B), and VISA (C) in a microtube dilution bioassay.
Fig. 3.
Antibacterial activities of broth elution samples from cements loaded with middle-dose antibiotic (4 g antibiotic in 40 g PMMA) in a 60-day elution period. The figure shows the inhibition of MSSA (A), MRSA (B), and VISA (C) in a microtube dilution bioassay.
Fig. 4.
Antibacterial activities of broth elution samples from cements loaded with low-dose antibiotic (1 g antibiotic in 40 g PMMA) in a 60-day elution period. The figure shows the inhibition of MSSA (A), MRSA (B), and VISA (C) in a microtube dilution bioassay.
DISCUSSION
Staphylococcus aureus is a Gram-positive pathogen with importance in orthopedic infection. The increasing resistance of S. aureus to antibiotics is a major concern in the treatment of infections. There have been increasing warnings regarding the apparent shift in vancomycin MICs, along with reported cases of clinical failure with VISA strains (11, 19). It has been shown that the time for MRSA bacteremia clearance is longer for strains with vancomycin MICs of 2 μg/ml than for strains with MICs of ≤1 μg/ml (15). Since there is uncertainty about the likelihood of successful outcomes with vancomycin, newer agents such as daptomycin, a bactericidal, broad-spectrum anti-Gram-positive cyclic lipopeptide antibiotic, have been utilized. Daptomycin provided better antibacterial activities against S. aureus, especially against resistant types such as MRSA or VISA, than vancomycin or teicoplanin (2). In theory, daptomycin should provide antibacterial activity against S. aureus superior to that of vancomycin or teicoplanin when it is loaded into PMMA for local implantation. However, there is no report to compare the antibacterial activities of daptomycin, vancomycin, and teicoplanin after they are loaded into PMMA.
Based on the results of this study, low-dose daptomycin added to cement (1 g antibiotic in 40 g PMMA), which is the dose frequently used as an infection prophylaxis in total joint arthroplasty (5), did not show effects against either MSSA or MRSA superior to those obtained with the same doses of vancomycin or teicoplanin in cement. The cement loaded with low-dose vancomycin provided a shorter period (1 day) of antibacterial effect than cement loaded with teicoplanin or daptomycin (2 days) against VISA. Low-dose teicoplanin and daptomycin in cement used as prophylaxis for infection showed similar antibacterial effects against S. aureus, regardless of whether the strains were resistant.
It has been shown that at least 3.6 g of antibiotic per 40 g of PMMA is desirable for effective elution kinetics and sustained therapeutic levels of antibiotic for local implantations (17). We selected the 4-g antibiotic dose (middle dose) as another test dose in this study. In the middle-dose group, teicoplanin provided a 40-day antibacterial effect against MSSA and MRSA, compared with 21-day antibacterial effects of cement loaded with the same dose of vancomycin or daptomycin. Against VISA, the cement loaded with middle-dose teicoplanin and daptomycin provided 21-day and 14-day antibacterial activities, respectively. The cement loaded with middle-dose vancomycin provided only a 5-day antibacterial effect. Hence, it can be speculated that 4 g of vancomycin in 40 g PMMA may not be sufficient for the treatment of osteomyelitis with VISA infection. In the high-dose group (8 g of antibiotic, the highest dose which can be loaded into 40 g of PMMA without disrupting the polymerization of cement), all of the cement specimens showed 60-day antibacterial activities against MSSA, MRSA, or VISA, regardless of the antibiotic.
Our results did not show that daptomycin had antibacterial effects against MSSA or MRSA superior to those of vancomycin or teicoplanin in PMMA. In contrast, teicoplanin-loaded cement provided equal (low-dose and high-dose groups) or even better (middle-dose group) antibacterial effects against S. aureus, regardless of whether the strains were resistant strains, compared with daptomycin-loaded cement.
The superior antibacterial effects of teicoplanin-loaded cement could be caused by a antibiotic release efficacy better that that of cement loaded with daptomycin or vancomycin. The antibiotic-loaded cement can achieve extremely high local concentrations of antibiotics, compared with the parenteral route (12). The superior antibacterial effects of daptomycin could be counteracted by a better antibiotic release efficacy of teicoplanin-loaded cement, which can achieve an extremely high local concentration. Daptomycin has superior antibacterial effects similar to those of vancomycin or teicoplanin after systemic administration in most situations (2). However, for antibiotic in cement, the superiority of daptomycin above vancomycin or teicoplanin was not observed in this study. When these antibiotics are used in cement, the release efficacy is the critical point which should be used to determine antibacterial activities.
To date, the exact mechanism by which antibiotics are released from PMMA is unclear. Many factors are known to affect the elution profiles, including the type of cement used, the choice and amount of antibiotics added, and the method of preparing the PMMA beads or spacers (13). These factors contribute to the porosity of cements. The porosity of antibiotic-loaded cements has been reported to play an important role in the release of antibiotics from the cement (17). We tried to compare the difference of porosities between the cement specimens loaded with different antibiotics in this study. After the specimens were polished with a grinder polisher, we inspected the porosity of the cements loaded with different antibiotics with a reflected-light microscope. We could not find any difference in size or the number of pores between the different samples (data not shown). Therefore, we were not able to determine the exact mechanism which contributed to the differences in release efficacy between the cements loaded with daptomycin, vancomycin, and teicoplanin.
Daptomycin (18), vancomycin (14), and teicoplanin (20) have been shown to be useful for the local treatment of drug-resistant S. aureus strains which cause osteomyelitis. In the literature to date, there is no comparative study determining the superiority of daptomycin, vancomycin, or teicoplanin incorporated into PMMA. This is the first study to compare the efficacy of these three antibiotics in PMMA, and the results showed that teicoplanin was the most effective. At the same time, in the high-dose group (8 g antibiotic in 40 g PMMA), all of the cement specimens retained antibacterial activities for up to 60 days at 37°C. All the tested antibiotics not only were stable under high temperatures of PMMA polymerization but also retained bioactivity after exposure to body temperature (37°C) for up to 60 days.
Our study has certain limitations. First, this is an in vitro study of specimens prepared in a laboratory environment, which does not necessarily reflect actual clinical circumstances. The quantity and flow of body fluid, limb mobility, host response, and antibiotic stability in vivo were not taken into consideration. Second, only one type of bone cement and method of preparation was used. Third, only resistant or nonresistant strains of S. aureus were selected as test organisms in the experiment. We do not know the bioactivities of specimens against other organisms, especially biofilm-forming organisms. Even though teicoplanin has been widely distributed in the majority of European and Asian countries, it is still not available in North America, including the U.S. and Canada. Since the specimens in this study were all prepared and tested in a uniform and reproducible manner, these results should provide useful information.
In conclusion, teicoplanin, daptomycin, and vancomycin all retained antibacterial activities after they were loaded into PMMA. The teicoplanin-loaded cement provided equal or even better antibacterial activity against MSSA, MRSA, and VISA strains compared with cements loaded with daptomycin or vancomycin in an in vitro assay. Teicoplanin was the better choice for the treatment of S. aureus infection with antibiotic-loaded PMMA cements, compared with daptomycin or vancomycin.
ACKNOWLEDGMENTS
This work was supported in part by the Chang Gung Memorial Hospital (grant no. BMRP 982) and Taiwan National Science Council (grant no. 98-2314-B-182A-020-MY3 to Y.C.).
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Appelbaum P. C. 2006. MRSA—the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl. 2): 3–10 [DOI] [PubMed] [Google Scholar]
- 2. Bowker K. E., Noel A. R., MacGowan A. P. 2009. Comparative antibacterial effects of daptomycin, vancomycin and teicoplanin studied in an in vitro pharmacokinetic model of infection. J. Antimicrob. Chemother. 64: 1044–1051 [DOI] [PubMed] [Google Scholar]
- 3. Carpenter C. F., Chambers H. F. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38: 994–1000 [DOI] [PubMed] [Google Scholar]
- 4. Chang S., et al. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348: 1342–1347 [DOI] [PubMed] [Google Scholar]
- 5. Chiu F. Y., Chen C. M., Lin C. F., Lo W. H. 2002. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J. Bone Joint Surg. Am. 84-A: 759–762 [PubMed] [Google Scholar]
- 6. Davis J. S. 2005. Management of bone and joint infections due to Staphylococcus aureus. Intern. Med. J. 35 s. 2: S79–96 [DOI] [PubMed] [Google Scholar]
- 7. Fuchs P. C., Barry A. L., Brown S. D. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 38: 51–58 [DOI] [PubMed] [Google Scholar]
- 8. Goldenberg D. L. 1998. Septic arthritis. Lancet 351: 197–202 [DOI] [PubMed] [Google Scholar]
- 9. Hanssen A. D. 2005. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 437: 91–96 [DOI] [PubMed] [Google Scholar]
- 10. Hiramatsu K., et al. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40: 135–136 [DOI] [PubMed] [Google Scholar]
- 11. Howden B. P., Johnson P. D., Charles P. G., Grayson M. L. 2004. Failure of vancomycin for treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 39: 1544. [DOI] [PubMed] [Google Scholar]
- 12. Hsieh P. H., Chang Y. H., Chen S. H., Ueng S. W., Shih C. H. 2006. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J. Orthop. Res. 24: 1615–1621 [DOI] [PubMed] [Google Scholar]
- 13. Jiranek W. A., Hanssen A. D., Greenwald A. S. 2006. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Joint Surg. Am. 88: 2487–2500 [DOI] [PubMed] [Google Scholar]
- 14. Kuechle D. K., Landon G. C., Musher D. M., Noble P. C. 1991. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin. Orthop. Relat. Res. 264: 302–308 [PubMed] [Google Scholar]
- 15. Moise-Broder P. A., Forrest A., Birmingham M. C., Schentag J. J. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43: 925–942 [DOI] [PubMed] [Google Scholar]
- 16. Murray W. R. 1984. Use of antibiotic-containing bone cement. Clin. Orthop. Relat. Res. 190: 89–95 [PubMed] [Google Scholar]
- 17. Penner M. J., Masri B. A., Duncan C. P. 1996. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J. Arthroplasty. 11: 939–944 [DOI] [PubMed] [Google Scholar]
- 18. Rouse M. S., et al. 2006. Daptomycin treatment of Staphylococcus aureus experimental chronic osteomyelitis. J. Antimicrob. Chemother. 57: 301–305 [DOI] [PubMed] [Google Scholar]
- 19. Sakoulas G., et al. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42: 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuzuner T., et al. 2006. In vivo evaluation of teicoplanin- and calcium sulfate-loaded PMMA bone cement in preventing implant-related osteomyelitis in rats. J. Chemother. 18: 628–633 [DOI] [PubMed] [Google Scholar]
- 21. Zalavras C. G., Patzakis M. J., Holtom P. 2004. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin. Orthop. Relat. Res. 427: 86–93 [DOI] [PubMed] [Google Scholar]