Abstract
Antibiotic resistance of biofilm-grown bacteria contributes to chronic infections, such as marginal and periapical periodontitis, which are strongly associated with Porphyromonas gingivalis. Concurrent azithromycin (AZM) administration and mechanical debridement improve the clinical parameters of periodontal tissue in situ. We examined the in vitro efficacy of AZM against P. gingivalis biofilms. The susceptibilities of adherent P. gingivalis strains 381, HW24D1, 6/26, and W83 to AZM, erythromycin (ERY), ampicillin (AMP), ofloxacin (OFX), and gentamicin (GEN) were investigated using a static model. The optical densities of adherent P. gingivalis cells were significantly decreased by using AZM and ERY at sub-MIC levels compared with those of the controls in all the strains tested, except for the effect of ERY on strain W83. AMP and OFX inhibited P. gingivalis adherent cells at levels over their MICs, and GEN showed no inhibition in the static model. The effects of AZM and ERY against biofilm cells were investigated using a flow cell model. The ATP levels of P. gingivalis biofilms were significantly decreased by AZM at concentrations below the sub-MICs; however, ERY was not effective for inhibition of P. gingivalis biofilm cells at their sub-MICs. Furthermore, decreased density of P. gingivalis biofilms was observed three-dimensionally with sub-MIC AZM, using confocal laser scanning microscopy. These findings suggest that AZM is effective against P. gingivalis biofilms at sub-MIC levels and could have future clinical application for oral biofilm infections, such as chronic marginal and periapical periodontitis.
INTRODUCTION
Biofilms are matrix-enclosed bacterial populations and are formed on inactive or bioactive surfaces (7). In medicine and many other fields, biofilms cause many problems, such as metallic corrosion and food contamination in processing. The characteristics of biofilms, such as the structure and composition of the extracellular matrix, have been reported (7). In medicine, bacterial biofilms cause chronic or refractory infections, so-called “biofilm infections,” by forming films on medical devices, such as catheters or artificial joints. Biofilms formed on teeth or oral soft tissues are the main causes of oral biofilm infections, including caries, periodontitis, and mucosal disease.
Porphyromonas gingivalis is a Gram-negative obligate anaerobic bacterium, and it is detected throughout periodontal pockets in patients with severe chronic marginal periodontitis (34). P. gingivalis is also frequently detected from extraradicular biofilms, which are refractory infectious lesions that are located outside the root apex (31). The virulence factors of P. gingivalis, such as proteolytic activity, fimbriae, capsules, and hemagglutinating activity, have been described (29). P. gingivalis biofilms are resistant to chemotherapeutic agents, such as minocycline and metronidazole, although these agents are effective against planktonic P. gingivalis cells (33).
Generally, biofilms are resistant to antibiotics, and this plays an important role in the failure of chemotherapy for biofilm infections. It has been reported that azithromycin (AZM) has antibiofilm effects (14, 37). Pseudomonas aeruginosa biofilm is inhibited by sub-MIC AZM (14). Also, in Haemophilus influenzae, AZM suppresses monospecies biofilm formation and detachment of formed biofilms (37). More than 500 different bacterial species are known to form human oral biofilms (19). Most oral biofilm-forming species are opportunistic pathogens, and they seldom cause monospecies infections. It is unknown how AZM exerts its effect against oral biofilms.
AZM is a 15-membered macrolide antibiotic that is obtained from erythromycin (ERY) by inserting a methyl-substituted nitrogen into a 14-membered ring. AZM has two amine bases, which have been reported to give AZM excellent tissue transition and an exceedingly long half-life compared with those of other macrolide antibiotics (9). Oral administration of 500 mg AZM can maintain a high concentration in the target tissue after the blood level has decreased to an undetectable level (9, 11). AZM administration is once daily, which is different from other antibiotics, because of its unique characteristics, such as long half-life and tissue accumulation (9). AZM is effective mainly against Gram-positive bacteria; however, it has a wide spectrum and also shows efficacy against Gram-negative bacteria (18).
In periodontal treatment, a combination of AZM and mechanical debridement significantly inhibits regrowth of periodontopathic bacteria, such as P. gingivalis and Tannerella forsythia, and improves clinical features, such as periodontal pockets and clinical attachment levels (10). In respiratory medicine, low-dose, long-term ERY is an established treatment for diffuse panbronchiolitis (DPB) (21). The characteristics of this treatment show that ERY also has other effects apart from antimicrobial activity.
Sub-MICs of antibiotics are effective for the treatment of DPB or cystic fibrosis (8, 21, 42). Research on the efficacy of sub-MICs of antibiotics against bacteria or biofilms of various strains in vitro is progressing in many fields, such as pathogenicity, antibiotic resistance, and quorum sensing (39). It has been reported that sub-MICs of AZM inhibit expression of fimbriae of P. gingivalis (23). Also, sub-MICs of AZM and ERY inhibit P. gingivalis biofilm formation in static models (38). However, macrolides with 16-membered rings, such as josamycin or midecamycin, cannot inhibit P. gingivalis biofilm formation or mucoid alginate biosynthetic enzyme activity of P. aeruginosa (27, 38).
Therefore, in this study, we focused on the effects of macrolides with 15- and 14-membered rings, including AZM and ERY, on biofilms of four strains of P. gingivalis, at several concentrations, including sub-MICs, using two biofilm models.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
We used four strains of P. gingivalis with different fimA genes, which encode fimbrial proteins (2): strain 381 with type I fimbriae, strain HW24D1 with type II, strain 6/26 with type III, and strain W83 with type IV. They were incubated at 37°C under anaerobic conditions (90% N2, 5% CO2, 5% H2) using Gifu anaerobic medium (GAM) bouillon (Nissui, Tokyo, Japan), supplemented with 5 μg/ml hemin (Sigma-Aldrich, St. Louis, MO) and 1 μg/ml menadione (Wako Pure Chemical Industries, Osaka, Japan), as previously described (28, 32, 34).
Antibiotics.
We used the 15-membered macrolide AZM (Wako), 14-membered macrolide ERY (Wako), ampicillin (AMP; Sigma-Aldrich), new quinolone ofloxacin (OFX; Wako), and aminoglycoside gentamicin (GEN; Nacalai Tesque, Kyoto, Japan).
Antimicrobial susceptibility test.
MICs of AZM, ERY, AMP, OFX, and GEN for planktonic P. gingivalis were determined in the range 0 to 640 μg/ml by using 96-well U-bottom microplates (Becton Dickinson, Sparks, MD) according to the standard method of the Japan Society of Chemotherapy (20). All assays were performed in triplicate.
P. gingivalis adherent cells by static model.
Adherent cells using a static model were formed on 96-well, flat-bottom plates as previously described by Azakami et al. (4). Each of four strains of cultured P. gingivalis was dispensed in 200 μl per well and incubated for 3 days at 37°C under anaerobic conditions. Antibiotics were added at the final concentrations of 3.9 × 10−3 to 640 μg/ml using a serial dilution method and incubated for a further 3 days. In the controls, hemin-and-menadione-supplemented GAM broth was added. Quantitative analysis of adherent cells was also determined by a previously described assay (4). After 3 days of cultivation, supernatants were removed and stained with 1% crystal violet (Sigma-Aldrich). Plates were washed three times with distilled water and decolorized with ethanol. Optical density (OD) was measured at 595 nm using a spectrophotometer (model 680; Bio-Rad, Hercules, CA), and the minimum biofilm inhibitory concentration (MBIC) was determined.
Biofilm formation and biological assay of the P. gingivalis flow cell biofilm model.
Details of the P. gingivalis biofilm formation model using a modified Robbins device (MRD) and hydroxyapatite (HA) disks have been described previously (33). Four separate MRDs and 40 HA disks (10 disks/MRD), which were processed with saliva for 8 h, were prepared for each strain. P. gingivalis biofilms were formed by anaerobic perfusion of culture medium containing bacterial cells for 14 days. Culture medium that contained antibiotics at various concentrations (10, 1, and 0.125 μg/ml) was perfused through three separate MRDs for a 7-day period. In the control group, only culture medium was circulated. HA disks were removed aseptically from each MRD after exposure to antibiotics and examined using an ATP bioluminescence assay. The latter was performed using an ATP kit (AF-3X2; DKK-TOA, Tokyo, Japan) as described previously (3, 13, 33). After being aseptically sampled from each MRD, the specimens were washed and ultrasonicated in 1.5 ml distilled water at 4°C for 30 min to remove the P. gingivalis biofilms from the HA disks. After sample processing according to the kit manufacturer's instructions, bioluminescence was measured using an ATP analyzer (AF-100 or DF-10; DKK-TOA). Antibiotics were selected that inhibited P. gingivalis adherent cells at their sub-MICs in the static model.
Three-dimensional observations of P. gingivalis flow cell biofilms.
To examine the effect of AZM on P. gingivalis biofilms three-dimensionally, flow cell biofilm samples on celluloid disks (Celltight; Sumitomo Bakelite Co., Tokyo, Japan) were prepared using an MRD as described above. Biofilm samples were stained with the Live/Dead BacLight bacterial viability kit L7007 (Invitrogen, Carlsbad, CA) for 15 min at room temperature. The samples were observed by confocal laser scanning microscopy (CLSM; LSM 510; Carl Zeiss, Oberkochen, Germany). Images were processed by image analysis software (Imaris; Bitplane AG, Zurich, Switzerland).
Changes in drug susceptibility by exposure to sub-MIC AZM on P. gingivalis biofilm-detached cells from the flow cell model.
Culture fluid of P. gingivalis 381, HW24D1, 6/26, and W83 was perfused for 14 days using an MRD as described above and formed biofilms. Medium that contained 0.125 μg/ml AZM, which is sub-MIC, was perfused for 3 days, and biofilm-detached cells were collected from perfused cultures. Control samples were obtained from antibiotic-unexposed P. gingivalis biofilm-detached cells. P. gingivalis biofilm-detached cells exposed/unexposed to sub-MIC AZM were adjusted to 106 CFU/ml using GAM broth. After that, MICs for P. gingivalis biofilm-detached cells exposed/unexposed to sub-MIC AZM were determined using 96-well microtiter plates, according to the standard method of the Japan Society of Chemotherapy (20).
Statistical analysis.
The significances of intergroup differences in the static model and flow cell biofilm model were analyzed using Student's t tests. P values of <0.01 and <0.005 were considered to indicate statistical significance for static and flow cell models, respectively. The minimum concentration that inhibited P. gingivalis biofilms significantly was designated MBIC in the static model.
RESULTS
MICs for P. gingivalis planktonic cells.
MICs of AZM, ERY, AMP, OFX, and GEN for P. gingivalis strain 381, HW24D1, 6/26, and W83 are shown in Table 1. MICs for AZM, ERY, and OFX were similar for each strain examined; however, AMP MICs showed large differences among the strains. GEN was ineffective against the biofilms of the four strains examined.
Table 1.
MICs of AZM, ERY, AMP, OFX, and GEN for planktonic P. gingivalis strains 381, HW24D1, 6/26, and W83
| Bacterial strain | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
| AZM | ERY | AMP | OFX | GEN | |
| P. gingivalis 381 | 0.5 | 0.5 | 0.08 | 0.5 | >640 |
| P. gingivalis HW24D1 | 0.3 | 0.16 | 2.5 | 0.6 | 320 |
| P. gingivalis 6/26 | 5 | 1.25 | 0.08 | 1.25 | 10 |
| P. gingivalis W83 | 0.6 | 0.16 | 640 | 0.3 | 640 |
Susceptibilities for P. gingivalis adherent cells.
We measured susceptibilities of antibiotics using a static model and established the MBICs. The MBICs of AZM, ERY, AMP, OFX, and GEN against four strains of P. gingivalis are summarized in Table 2. In P. gingivalis strain 381, the OD of the adherent cells was significantly decreased at AZM concentrations of ≥0.125 μg/ml compared with that of the control group. In P. gingivalis HW24D1, 6/26, and W83, the OD of the adherent cells was significantly decreased at AZM concentrations of ≥0.00061, ≥1.25, and ≥0.3 μg/ml, respectively. AZM inhibited P. gingivalis adherent cells of all the strains at sub-MIC. ERY decreased adherent cells of P. gingivalis 381, HW24D1, 6/26, and W83 at concentrations of ≥0.25, ≥0.00061, ≥0.6, and ≥ 0.16, respectively. This meant that adherent cells of three strains except for W83 were significantly inhibited at their sub-MIC levels compared with the corresponding control groups. In the AMP-treated group, the MBICs of strains 381, HW24D1, and 6/26 were higher than their MICs. In strain W83, MBIC of AMP was below its MIC; however, the MIC was much higher than the breakpoint of AMP for anaerobic bacteria (0.25 μg/ml) (6). In the OFX-treated group, the MBICs for all the strains tested were above their MICs. The MBICs of GEN were ≥640 μg/ml in tested strains, and they showed no inhibition of biofilm elimination.
Table 2.
MBICs of AZM, ERY, AMP, OFX, and GEN for adherent cells of P. gingivalis strains 381, HW24D1, 6/26, and W83 using a static model
| Bacterial strain | MBIC (μg/ml)a | ||||
|---|---|---|---|---|---|
| AZM | ERY | AMP | OFX | GEN | |
| P. gingivalis 381 | 0.125 | 0.25 | 20 | 1.25 | >640 |
| P. gingivalis HW24D1 | 0.00061 | 0.00061 | >640 | 2.5 | >640 |
| P. gingivalis 6/26 | 1.25 | 0.6 | 160 | 640 | 640 |
| P. gingivalis W83 | 0.3 | 0.16 | 160 | 1.25 | >640 |
Antibiotics could inhibit biofilm formation in a static model at sub-MICs.
Susceptibilities for P. gingivalis biofilms.
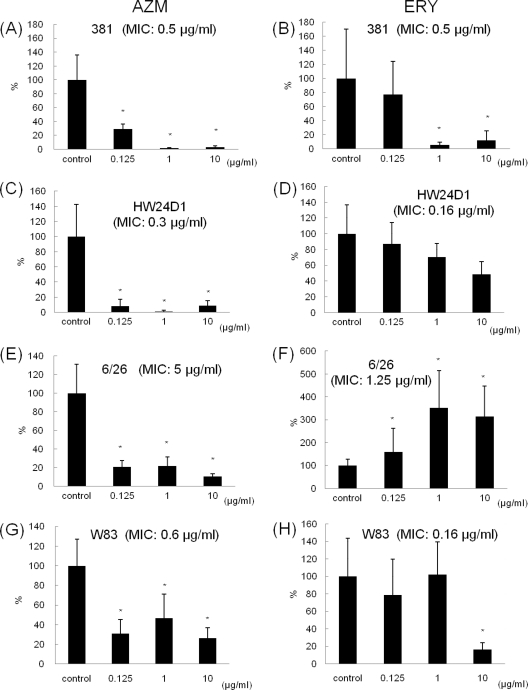
In P. gingivalis 381, HW24D1, 6/26, and W83, the ATP values of biofilm cells from the flow cell biofilm model were significantly decreased by AZM at all concentrations (0.125, 1, and 10 μg/ml) compared with the control group (Fig. 1A, C, E, and G). In the P. gingivalis 381 and W83 groups, the ATP values were significantly decreased at ERY concentrations above their MICs (Fig. 1B and H), whereas strains HW24D1 and 6/26 did not show an inhibitory effect at their sub-MICs (Fig. 1D and F).
Fig. 1.
Effects of antibiotics on biofilms of P. gingivalis strains 381, HW24D1, 6/26, and W83, determined by using the flow cell model. Biofilms of P. gingivalis strain 381 were prepared by using perfused culture medium and an MRD for 14 days, and then each antibiotic was added for 3 days and ATP bioluminescence assay was performed. The ATP volume of the control group was set to 100%, and these figures indicate the percentage amounts of ATP when antibiotics were added. AZM inhibited biofilms at sub-MIC levels. ERY decreased ATP levels of the biofilm cells at levels over its MIC in strains 381 and W83. (A) AZM for strain 381; (B) ERY for strain 381; (C) AZM for strain HW24D1; (D) ERY for strain HW24D1; (E) AZM for strain 6/26; (F) ERY for strain 6/26; (G) AZM for strain W83; (H) ERY for strain W83. Ten biofilm samples were examined at each concentration. *, P < 0.005 (Student's t test).
CLSM observations.
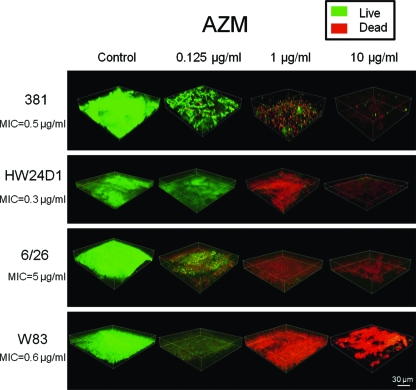
In the control group of every strain, biofilms observed consisted mostly of live cells, and their thickness was 20 to 60 μm. However, biofilms treated with sub-MIC AZM (0.125 μg/ml) were decreased in thickness and density. In groups treated with 1 and 10 μg/ml AZM, live cells in the biofilms almost disappeared (Fig. 2). In P. gingivalis strain 381, ERY treatment decreased the density of the live cells in the biofilm at a concentration of 1 and 10 μg/ml, which was above the MIC (data not shown).
Fig. 2.
CLSM images of AZM-treated biofilms of P. gingivalis strains 381, HW24D1, 6/26, and W83. P. gingivalis biofilms were formed on celluloid disks, and after adding AZM, biofilms were stained by LIVE/DEAD staining. Biofilms were observed using CLSM. Images were processed using Imaris software. Green shows live cells and red dead cells. In all the strains, at the higher concentrations of AZM, fewer residual cells were observed.
Drug susceptibility for biofilm-detached cells exposed to sub-MIC levels of AZM.
MICs for AZM and ERY for biofilm-detached cells exposed/unexposed to sub-MICs of AZM are shown in Table 3. MICs for P. gingivalis exposed to AZM were not increased compared with those for unexposed cells, and exposure to sub-MICs of AZM did not cause resistance to AZM. Similarly, MICs for ERY for biofilm-detached cells exposed to sub-MICs of AZM were not above the MICs for unexposed cells, and resistance to ERY was not observed.
Table 3.
Susceptibility of detached cells from biofilms of P. gingivalis strains 381, HW24D1, 6/26, and W83 exposed to sub-MICs of AZMa
| Bacterial strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| AZM |
ERY |
|||
| Unexposed | Exposed | Unexposed | Exposed | |
| 381 | 1.56 | 0.39 | 0.19 | 0.09 |
| HW24D1 | 0.39 | 0.09 | 0.09 | <0.02 |
| 6/26 | 6.25 | 6.25 | 0.78 | 0.78 |
| W83 | 6.25 | 6.25 | 0.78 | 0.78 |
Culture fluid of P. gingivalis was perfused for 14 days using a flow cell model, and biofilms were formed. Medium that contained a sub-MIC of AZM was perfused for 3 days, and biofilm-detached cells were collected. Controls were perfused with hemin-and-menadione-supplemented GAM broth only. MICs were determined for P. gingivalis biofilm-detached cells exposed/unexposed to sub-MICs of AZM, according to the standard method of the Japan Society of Chemotherapy.
DISCUSSION
The macrolide antibiotic AZM is effective against chronic infections caused by P. aeruginosa biofilms, such as cystic fibrosis or DPB (27, 42); however, it has not been shown to have efficacy against oral biofilms of many bacterial species. AZM is already in clinical use, and there are many reports about its efficacy both in vitro and in vivo (9–11, 18, 38). However, the mechanism of its effects has yet to be determined. Oral biofilms consist of many bacterial species (19), and their structure and biological properties differ in each microenvironment. It is difficult to assess the mechanism of action of antibiotics and their effects against oral biofilms that comprise mixed species in vitro. P. gingivalis, which is one of the key pathogens in oral biofilm infections, was selected for the present study to clarify the effect of AZM on P. gingivalis biofilms using ATP measurement and CLSM in vitro. Mono- and multispecies biofilms differ with regard to structure, and therefore, it might change the permeability to antibiotics and exert an influence on their effects. The extracellular polymeric substances and biofilm-forming bacteria differ from those in actual oral biofilms; thus, it will be necessary to verify the results in vivo.
The genotype of fimA, which encodes an important fimbrial protein, FimA, is classified into six groups, from I to V and Ib, because of slight differences in nucleotide sequence and is related to expression of pathogenicity of P. gingivalis (28). A lot of research has been carried out to study the relationship between the stage of progression of periodontitis and differences in genotypes (2, 24, 35, 41). These studies have shown that, in severe marginal periodontitis, type II is most frequently detected, followed by type IV (2, 24). Similarly, in apical periodontitis, type II P. gingivalis shows the highest appearance ratio in symptomatic focus and type I is isolated from asymptomatic focus (41). The present study was designed to investigate the effect of antibiotics on adherent cells and multilayer biofilms. AZM inhibited biofilms of all the P. gingivalis strains tested in the static and flow cell models at sub-MICs (Table 2, Fig. 1). P. gingivalis biofilms decreased in thickness after the addition of AZM, and in the presence of 1 and 10 μg/ml AZM, bacterial cells in the biofilm almost disappeared (Fig. 2). Promotion of biofilm detachment or some impact, such as water flow, on the biofilm matrix is considered a possible reason. It has been reported previously that AZM and ERY are effective biofilm inhibitors in a static model (38), and this is similar to our results with biofilm adherent cells in the static model. However, in the flow cell biofilm model, ERY was ineffective for biofilm inhibition. This differs from the static model and previous studies (38). The flow cell biofilm model resulted in formation of a P. gingivalis biofilm with a >20-μm thickness. With regard to actual dental biofilms, the flow cell biofilm model is preferable for investigation of the antibiofilm effect.
AMP and OFX were examined for activity against all the strains in a static model, and they inhibited P. gingivalis biofilms at concentrations over their MICs, except for AMP inhibition of strain W83. Previous studies have shown that inhibitory concentrations against biofilms are often 10 to 1,000 times higher than MICs for planktonic cells (15, 22, 25, 30). In the present study, a similar tendency was seen for AMP and OFX. GEN showed no inhibitory effect for P. gingivalis in planktonic and adherent cells. We found that AMP, OFX, and GEN were ineffective against adherent P. gingivalis cells; therefore, we predicted that these antibiotics would be ineffective against P. gingivalis biofilm cells in the flow cell model and did not use that model. These results with AMP, OFX, and GEN led us to suggest that AMP and OFX are effective in the acute stage of biofilm infection, but they might be useless in the chronic stage. Even when single antibiotics are not effective to inhibit biofilms significantly, we can strengthen the antibiofilm effect by combination with other agents (5, 36). When adjunctive antibiotics are administered, it would appear that they can exhibit wider antimicrobial activity.
The acquisition of drug resistance because of sub-MICs of antibiotics has been a problem. Among children, AZM-resistant bacteria are detected in 85% of patients after 6 weeks of oral administration, and AZM shows more resistance than other macrolide antibiotics do (17). Several other reports about increasing AZM resistance have been published recently (1, 12, 26). However, we did not find any evidence of acquisition of resistance after exposure to sub-MICs of AZM or ERY (Table 3). Moreover, clinical isolates of P. gingivalis have been reported not to show resistance to AZM (16, 40), and these data support the future possibility of clinical application of AZM as a new therapy for chronic infections caused by biofilms.
In conclusion, the present study demonstrated the efficacy of AZM at subinhibitory concentrations, and it was capable of inhibiting P. gingivalis biofilm. We suggest that AZM is likely to be useful for the treatment of diseases caused by P. gingivalis biofilms. However, the mechanism of action of AZM against P. gingivalis biofilms has not yet been clarified and remains a subject for future studies.
ACKNOWLEDGMENTS
This study was supported by Grants-in-Aid for Scientific Research (no. 17209061, 18390505, 19592198, and 23890103) and the 21st Century COE program of the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 12 September 2011.
REFERENCES
- 1. Ahmed M. U., et al. 2010. Monitoring antimicrobial susceptibility of Neisseria gonorrhoeae isolated from Bangladesh during 1997–2006: emergence and pattern of drug-resistant isolates. Health Popul. Nutr. 28:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amano A., Nakagawa I., Kataoka K., Morisaki I., Hamada S. 1999. Distribution of Porphyromonas gingivalis strains with fimA genotypes in periodontitis patients. J. Clin. Microbiol. 37:1426–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amorena B., et al. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J. Antimicrob. Chemother. 44:43–55 [DOI] [PubMed] [Google Scholar]
- 4. Azakami H., et al. 2006. Involvement of N-acetyl-d-galactosamine-specific lectin in biofilm formation by the periodontopathogenic bacterium, Eikenella corrodens. Biosci. Biotechnol. Biochem. 70:441–446 [DOI] [PubMed] [Google Scholar]
- 5. Banin E., Brady K. M., Greenberg E. P. 2006. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 72:2064–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute (CLSI). 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard 7th edition. M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 8. Equi A., Balfour-Lynn I. M., Bush A., Rosenthal M. 2002. Long term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trial. Lancet 360:978–984 [DOI] [PubMed] [Google Scholar]
- 9. Foulds G., Shepard R. M., Johnson R. B. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25:73–82 [DOI] [PubMed] [Google Scholar]
- 10. Gomi K., et al. 2007. Effects of full-mouth scaling and root planing in conjunction with systemically administered azithromycin. J. Periodontol. 78:422–429 [DOI] [PubMed] [Google Scholar]
- 11. Gomi K., et al. 2007. Drug concentration in inflamed periodontal tissues after systemically administered azithromycin. J. Periodontol. 78:918–923 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez R., et al. 2010. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int. J. Dermatol. 49:1003–1007 [DOI] [PubMed] [Google Scholar]
- 13. Hattori N., Nakajima M. O., O'Hara K., Sawai T. 1998. Novel antibiotic susceptibility tests by the ATP-bioluminescence method using filamentous cell treatment. Antimicrob. Agents Chemother. 42:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ichimiya T., et al. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186–191 [DOI] [PubMed] [Google Scholar]
- 15. Ito A., Taniuchi A., May T., Kawata K., Okabe S. 2009. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 75:4093–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaramillo A., et al. 2005. Clinical and microbiological characterization of periodontal abscesses. J. Clin. Periodontol. 32:1213–1218 [DOI] [PubMed] [Google Scholar]
- 17. Kastner U., Guggenbichler J. P. 2001. Influence of macrolide antibiotics on promotion of resistance in the oral flora of children. Infection 29:251–256 [DOI] [PubMed] [Google Scholar]
- 18. Kato N., Kato H., Tanaka K., Watanabe K., Ueno K. 1995. The in vitro and in vivo activity of azithromycin, a new macrolide, against anaerobic bacteria and ureaplasms. Jpn. J. Chemother. 43:31–39 [Google Scholar]
- 19. Kolenbrander P. E. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54:413–437 [DOI] [PubMed] [Google Scholar]
- 20. Kosakai N., et al. 1979. The measurement method for minimum inhibitory concentration (MIC) of anaerobic bacteria. Jpn. J. Chemother. 27:559–561 [Google Scholar]
- 21. Kudoh S., Azuma A., Yamamoto M., Izumi T., Ando M. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829–1832 [DOI] [PubMed] [Google Scholar]
- 22. Larsen T. 2002. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol. Immunol. 17:267–271 [DOI] [PubMed] [Google Scholar]
- 23. Lo Bue A. M., Rossetti B., Cali G., Nicoletti G., Condorelli F. 1997. Antimicrobial interference of a subinhibitory concentration of azithromycin on fimbrial production of Porphyromonas gingivalis. J. Antimicrob. Chemother. 40:653–657 [DOI] [PubMed] [Google Scholar]
- 24. Miura M., Hamachi T., Fujise O., Maeda K. 2005. The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitis. J. Periodontal Res. 40:147–152 [DOI] [PubMed] [Google Scholar]
- 25. Miyake Y., Fujiwara S., Usui T., Suginaka H. 1992. Simple method for measuring the antibiotic concentration required to kill adherent bacteria. Chemotherapy 38:286–290 [DOI] [PubMed] [Google Scholar]
- 26. Molloy A., et al. 2010. First report of Salmonella enterica serotype paratyphi A azithromycin resistance leading to treatment failure. J. Clin. Microbiol. 48:4655–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagino K., Kobayashi H. 1997. Influence of macrolides on mucoid alginate biosynthetic enzyme from Pseudomonas aeruginosa. Clin. Microbiol. Infect. 3:432–439 [DOI] [PubMed] [Google Scholar]
- 28. Nakagawa I., et al. 2002. Identification of a new variant of fimA gene of Porphyromonas gingivalis and its distribution in adults and disabled populations with periodontitis. J. Periodontal Res. 37:425–432 [DOI] [PubMed] [Google Scholar]
- 29. Nakayama K. 2003. Molecular genetics of Porphyromonas gingivalis: gingipains and other virulence factors. Curr. Protein Pept. Sci. 4:389–395 [DOI] [PubMed] [Google Scholar]
- 30. Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noguchi N., Noiri Y., Narimatsu M., Ebisu S. 2005. Identification and localization of extraradicular biofilm-forming bacteria associated with refractory endodontic pathogens. Appl. Environ. Microbiol. 71:8738–8743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noiri Y., Ozaki K., Nakae H., Matsuo T., Ebisu S. 1997. An immunohistochemical study on the localization of Porphyromonas gingivalis, Campylobacter rectus and Actinomyces viscosus in human periodontal pockets. J. Periodontal Res. 32:598–607 [DOI] [PubMed] [Google Scholar]
- 33. Noiri Y., et al. 2003. Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J. Periodontol. 74:1647–1651 [DOI] [PubMed] [Google Scholar]
- 34. Noiri Y., Li L., Yoshimura F., Ebisu S. 2004. Localization of Porphyromonas gingivalis-carrying fimbriae in situ in human periodontal pockets. J. Dent. Res. 83:941–945 [DOI] [PubMed] [Google Scholar]
- 35. Rocas I. N., Siqueira J. F., Jr. 2010. Distribution of Porphyromonas gingivalis fimA genotypes in primary endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109:474–478 [DOI] [PubMed] [Google Scholar]
- 36. Sandoe J. A., Wysome J., West A. P., Heritage J., Wilcox M. H. 2006. Measurement of ampicillin, vancomycin, linezolid and gentamicin activity against enterococcal biofilms. J. Antimicrob. Chemother. 57:767–770 [DOI] [PubMed] [Google Scholar]
- 37. Starner T. D., Shrout J. D., Parsek M. R., Appelbaum P. C., Kim G. 2008. Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob. Agents Chemother. 52:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamura A., Ara T., Imamura Y., Fujii T., Wang P. L. 2008. The effects of antibiotics on in vitro biofilm model of periodontal disease. Eur. J. Med. Res. 13:439–445 [PubMed] [Google Scholar]
- 39. Tateda K., Standiford T. J., Pechere J. C., Yamaguchi K. 2004. Regulatory effects of macrolides on bacterial virulence: potential role as quorum-sensing inhibitors. Curr. Pharm. Des. 10:3055–3065 [DOI] [PubMed] [Google Scholar]
- 40. van Winkelhoff A. J., Herrera D., Oteo A., Sanz M. 2005. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J. Clin. Periodontol. 32:893–898 [DOI] [PubMed] [Google Scholar]
- 41. Wang Q., et al. 2010. Distribution of Porphyromonas gingivalis fimA genotypes in chronic apical periodontitis associated with symptoms. J. Endod. 36:1790–1795 [DOI] [PubMed] [Google Scholar]
- 42. Wolter J., et al. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]