Abstract
Tenofovir (TFV) is effective in preventing simian immunodeficiency virus (SIV) transmission in a macaque model, is available as the oral agent tenofovir disoproxil fumarate (TDF), and may be useful in the prevention of mother-to-child transmission of human immunodeficiency virus (HIV). We conducted a trial of TDF and TDF-emtricitabine (FTC) in HIV-infected pregnant women and their infants. Women received a single dose of either 600 mg TDF, 900 mg TDF, or 900 mg TDF-600 mg FTC at labor onset or prior to a cesarean section. Infants received no drug or a single dose of TDF at 4 mg/kg of body weight or of TDF at 4 mg/kg plus FTC at 3 mg/kg as soon as possible after birth. All regimens were safe and well tolerated. Maternal areas under the serum concentration-time curve (AUC) and concentrations at the end of sampling after 24 h (C24) were similar between the two doses of TDF; the maximum concentrations of the drugs in serum (Cmax) and cord blood concentrations were higher in women delivering via cesarean section than in those who delivered vaginally (P = 0.04 and 0.046, respectively). The median ratio of the TFV concentration in cord blood to that in the maternal plasma at delivery was 0.73 (range, 0.26 to 1.95). Without TDF administration, infants had a median TFV concentration of 12 ng/ml 12 h after birth. Following administration of a single dose of TDF at 4 mg/kg, infant TFV concentrations fell below the targeted level, 50 ng/ml, by 24 h postdose. In HIV-infected pregnant women and their infants, 600 mg of TDF is acceptable as a single dose during labor. Low concentrations at birth support infant dosing as soon after birth as possible. Rapidly decreasing TFV levels in infants suggest that multiple or higher doses of TDF will be necessary to maintain concentrations that are effective for viral suppression.
INTRODUCTION
Worldwide, the majority of human immunodeficiency virus (HIV) perinatal transmissions occur during labor and delivery. Zidovudine (ZDV), as administered in ACTG 076, substantially reduced HIV perinatal transmission (6, 30) but was too complex and expensive for use in resource-limited settings. HIVNET 012 demonstrated that single peripartum maternal and infant nevirapine (NVP) doses can provide effective single-agent therapy for prevention of mother-to-child transmission (PMTCT) of HIV in resource-limited areas of the world (13). This agent appeared optimal because of its strong potency, easy storage, and oral administration. However, there are concerns over the development of NVP resistance in women and infants who received this drug alone for prevention of perinatal transmission (8–11, 27). While development of NVP resistance can be mitigated by the use of short “tail” regimens in mothers (5, 21, 34) and the use of triple antiretroviral PMTCT regimens is becoming widespread, even in the developing world (28), knowledge about alternative peripartum antiretroviral strategies is needed. This is especially important for women who register late or not at all for prenatal care or who are diagnosed in active labor and for their infants, who may benefit from aggressive intrapartum and postpartum interventions.
Tenofovir (TFV), a potent inhibitor of retroviral transcriptase, has demonstrated significant prophylactic efficacy against simian immunodeficiency virus (SIV) infection in macaques, a primate model of AIDS, in multiple studies, including a perinatal transmission model (32, 35, 36, 38–40). In addition, the emergence in reverse transcriptase (RT) of the K65R substitution mutation, the main mutation associated with TDF resistance, occurs infrequently (31). An understanding of the disposition of these agents in pregnant women and the transfer to their fetuses is an essential first step in determining a role in PMTCT. The availability of tenofovir disoproxil fumarate (TDF), an oral prodrug of TFV, supports the investigation of tenofovir as a potential agent for use in PMTCT. TDF is currently approved for the treatment of HIV infection as part of a combination antiretroviral therapy (cART) at a dose of 300 mg once daily and is also available as the fixed drug combination tenofovir-emtricitabine (TDF-FTC; Truvada), coformulated with 200 mg emtricitabine (FTC). PACTG 394 was developed to determine the safety, tolerance, and pharmacokinetics (PK) of TDF, administered either as a single agent or as TDF-FTC in HIV-infected pregnant women and their infants. The findings of this study complement those of the ANRS 12109 (14–16, 33, 34) and HPTN 057 (23, 24) trials, studies that administered TDF to pregnant women and their infants in various dosing regimens and employed complementary analysis techniques.
MATERIALS AND METHODS
Study population.
HIV-infected pregnant women at ≥34 weeks of gestation were recruited at clinical trial sites in the United States and Puerto Rico. Women and their infants were allowed to receive any antiretroviral agents, except TDF, as part of a PMTCT regimen. They were excluded from participation if they had serious illness, laboratory results indicating grade 3 or higher underlying toxicity (based on the Division of AIDS 1994 Toxicity Table, available at http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf), a creatinine clearance of <70 ml/min/1.73 m2, or urine protein levels of ≥300 mg/24 h. They were also excluded if they had clinical or sonographic evidence of fetal growth retardation or major congenital anomalies, use of highly nephrotoxic drugs, or therapeutic use of heparin. Breast-feeding was not permitted.
Study procedures.
Two groups of mothers administered TDF in dosages of 600 mg and 900 mg were initially planned for the study. Dose escalation from a dose of 600 mg TDF in the first group to 900 mg TDF in the second group was dependent on preestablished safety and PK criteria. Safety criteria to increase the dose (or for any dose to be considered safe) required that there were no life-threatening toxicities and that the incidence of non-life-threatening toxicities (grade 3 or 4) was <30%, as well as that no adverse pregnancy or neonatal outcomes could be attributable to study treatment. PK criteria to escalate the maternal dose included median TFV concentrations in either cord blood or infants at 24 h of life of less than 50 ng/ml, the typical 24-h trough concentration in adults receiving TDF therapeutically (4). The maternal area under the serum concentration-time curve (AUC) was also required to be <1.5 times the value for nonpregnant adults given a single 600-mg dose (2). In the first group, no TDF or FTC was administered to infants. The infant dose and the timing of postdelivery dosing were determined by PK findings in the initial group of women and infants studied. In all groups, infants received standard ZDV prophylaxis until 6 weeks of age.
In the first group, women received a single oral dose of 600 mg TDF, administered as two 300-mg TDF tablets, with two ounces of milk or nutritional supplement at the onset of active labor or 4 to 8 h prior to scheduled delivery by cesarean section. All women received standard intravenous ZDV prophylaxis. The women's PK samples were obtained predose, at 1, 2, 4, 8, 12, and 24 h postdose, and at the time of delivery. Cord blood and infant samples were collected at 12, 24, and 36 h of life. Accrual continued until 10 evaluable mother/infant pairs were enrolled. An evaluable pair was defined as a mother and infant with a complete set of PK evaluations, with infant delivery occurring between 2 and 24 h after the maternal dosing of TDF. In this group, a minimum of five women were expected to deliver vaginally.
In the second group, women received 900 mg TDF, administered as three TDF tablets, and infants received 4 mg/kg of body weight of a TDF suspension (investigational new drug [IND] number 68947) as soon as possible after delivery. TDF for oral suspension is an investigational product and consists of a white to off-white powder that is constituted with water to yield a TDF suspension with a concentration of 20 mg/ml. The PK samples for women in the second group were obtained as for the first group. Infant PK samples were obtained predose and at 4, 12, 24, and 36 h postdose. Cord blood was also collected. A maximum of 10 evaluable mother/infant pairs was to be included in this group, with no restrictions on mode of delivery.
Following a protocol amendment, a third group of women received a single dose of 900 mg TDF-600 mg FTC, administered as three tenofovir-emtricitabine tablets, and infants received a single dose of 4 mg/kg of the TDF suspension and 3 mg/kg of an FTC solution as soon as possible after delivery. The PK sampling was the same as for the second group. A minimum of 6 evaluable mother/infant pairs was targeted for this third group, and the maximum for the second group was modified to include 6 evaluable mother/infant pairs to achieve a total of 10 to 12 evaluable mother/infant pairs in the second and third groups combined. TDF, TDF-FTC, and FTC were provided by Gilead Sciences.
Women were followed until 12 weeks postpartum. Those with a detectable virus level (>1,000 copies/ml) at labor and delivery or 1, 6, and/or 12 weeks postpartum were subjected to viral resistance testing. If targeted TFV resistance mutations were detected, repeat assessments were performed over 1 year. Infants were followed until 2 years of age. Mother and infant adverse events were monitored by the study team on biweekly or monthly teleconference calls, during which the final relationship of the study drugs to the outcome was determined. All women and infants exposed to TDF, tenofovir-emtricitabine, or FTC in this study were evaluated for safety.
Informed consent was obtained from all mothers, and the study and all subsequent amendments were approved by each clinical trial site's institutional review board before implementation.
Laboratory procedures.
TFV and FTC concentrations were measured by Gilead Sciences using a validated liquid chromatography/mass spectrometry (LC/MS) assay as previously described (20). The analytic laboratory conforms to international standards of good laboratory practices (GLP) and GLP-like analyses for clinical samples and is overseen by Gilead Sciences, Inc. Briefly, samples were deproteinized using methanol with an internal standard added (lamivudine [3TC] for FTC and adefovir for TFV). After extraction, the analytes were resolved using isocratic reverse-phase chromatography and detected using mass spectrometry with lower limits of 5 ng/ml for FTC and 10 ng/ml for TFV. The selected reaction-monitoring (SRM) mode was used with atmospheric pressure chemical ionization (APCI) with positive polarity. The ion transitions were 288 → 176 m/z for TFV, 248 → 130 m/z for FTC, 274 → 162 m/z for adefovir, and 230 → 112 m/z for 3TC. Inter- and intra-assay performance, as determined during validation, had a precision percent coefficient of variation (%CV) of <13% and an accuracy within 16% (20). The viral loads were measured using the Roche UltraSensitive Monitor assay, version 1.5 (Roche Molecular Diagnostics, Pleasanton, CA). Bulk sequencing for the detection of resistance mutations was performed using the Siemens Trugene HIV-1 genotyping assay. Viral loads and genotyping were performed at the University of North Carolina at Chapel Hill laboratory, which was certified by the Division of AIDS Virology Quality Assurance program for both viral load and genotyping assays. For the sensitive real-time PCR resistance assays, HIV-1 genomic RNA from the blood plasma samples was extracted using Qiagen BioRobot M48 from 200 μl patient plasma and tested at the Centers for Disease Control and Prevention in Atlanta, GA. A region of the HIV-1 template that included nucleotide 58 to nucleotide 777 of the gene for RT was amplified by RT-PCR as previously described (17, 18). The RNA samples were tested for two mutations in RT, K65R and K70E, using established assay cutoffs equivalent to 0.4% to 1% of the mutant virus, which are above the background reactivity observed when testing wild-type virus.
Pharmacokinetic and statistical analysis.
Noncompartmental pharmacokinetic parameters were calculated using two commercial computer programs, WinNonlin (Pharsight Corp., Palo Alto, CA) and Microsoft Excel (Redmond, WA). The maximum concentration (Cmax), time to maximum concentration (tmax), and concentration at the end of sampling (24 h) (C24) were determined by inspection of concentration-time curves. The area under the serum concentration-time curve (AUC) was calculated by the trapezoidal rule. Terminal elimination half-lives (t1/2s) were determined by a weighted least-squares fitting of the terminal elimination phase of the concentration-time curves. As a measure of placental transfer, the median ratio of the TFV concentration in cord blood to that in the maternal plasma at delivery (CB/M TFV concentration ratio) was calculated using the maternal TFV plasma concentration obtained within 90 min of delivery and the measured cord blood concentration. The Wilcoxon rank sum test was used for a comparison of PK parameters by group using SAS version 9.1 software (SAS Institute, Inc., Cary, NC), with a two-sided P value of <0.05 considered to indicate statistical significance.
RESULTS
Three groups of women and their infants were included in the study. Following review of the safety and PK findings in group 1, maternal dose escalation was warranted based on unacceptable infant concentrations at 24 h of life. Group 2 included women and their infants who received TDF only, and group 3 included those who received both TDF and FTC. The characteristics of women who received the study drug and their infants are shown in Table 1. Fifteen women were enrolled in group 1, and 14 received the study drug. The subject who did not receive the study drug was not considered further. One woman had only one sample with detectable TFV at 8 h after dosing and was excluded from the PK analysis. In addition, two women delivered more than 24 h after the dose of TDF and one woman delivered within 2 h after the dose of TDF. These 3 women, but not their infants, completed PK evaluations and are included in the PK analysis. Thus, there are 13 women and 10 infants from group 1 included in the PK analysis.
Table 1.
Characteristics of mothers who received the study drug and their infants
| Characteristic of mothers or infantsd | Value (range) for: |
||
|---|---|---|---|
| Group 1a | Group 2b | Group 3c | |
| Mothers | |||
| Median age (yr) | 23.5 (19–44) | 27.5 (19–37) | 26 (22–37) |
| Race (no.) | |||
| Black | 11 | 6 | 5 |
| Hispanic | 3 | 2 | 2 |
| White | 0 | 0 | 2 |
| Median viral load (log10 copies/ml) | 1.83 (1.70–3.95) | 1.70 (1.70–3.17)e | 1.70 (1.70–2.32) |
| Median wt (kg) | 87.5 (65.3–124.7) | 82.8 (67.1–113.5) | 90.7 (45–111.3) |
| Median time from dose to delivery (h) | 5.4 (0.3–35.7) | 7 (1.6–12)e | 7.3 (4.7–70.1) |
| No. who delivered vaginally | 6 | 5 | 5 |
| No. who received background ART that was: | |||
| PI based | 4 | 6 | 8 |
| NNRTI based | 2 | ||
| NRTI based | 7 | 2 | 1 |
| Other | 1 | ||
| Infants | |||
| Median birth wt (kg) | 3.1 (2.4–4.2) | 3.4 (2.8–4.1)e | 3.2 (2.8–3.7) |
| Median gestational age (wk) | 40 (38–42) | 40 (38–42)e | 39 (37–41) |
| Median time from birth to study drug dose (h) | Not applicable | 6 (4–7.9) | 4.7 (1.8–11) |
Group 1 consisted of mothers (n = 14) given 600 mg TDF.
Group 2 consisted of mothers (n = 8) given 900 mg TDF and infants (n = 8) given 4 mg/kg TDF.
Group 3 consisted of mothers (n = 9) given 900 mg TDF and 600 mg FTC and infants (n = 9) given 4 mg/kg TDF and 3 mg/kg FTC.
PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor. All NNRTI-based regimens contained nevirapine. All NRTI-based regimens consisted of zidovudine, lamivudine, and abacavir. The “other” ART regimen contained efavirenz and lopinavir-ritonavir.
n = 7.
Nine women were enrolled in group 2. Two mother/infant pairs were excluded from PK analysis, one where the mother developed a medical complication before the study drug was administered and the second where the mother received a commercial preparation of the study drug instead of the study product, and neither she nor her infant had PK sampling performed. The second woman and her infant are included in safety but not PK evaluations. Thus, seven women and their infants in group 2 were included in the PK analysis.
Nine women were enrolled in group 3. One woman was discovered to be in false labor and was discharged following study drug administration with no PK sampling performed. However, her infant received the study drug and completed PK sampling following birth. Also in group 3, one infant received TDF only. This resulted in eight women and nine infants being included in the TFV PK analysis in group 3.
Tenofovir pharmacokinetics.
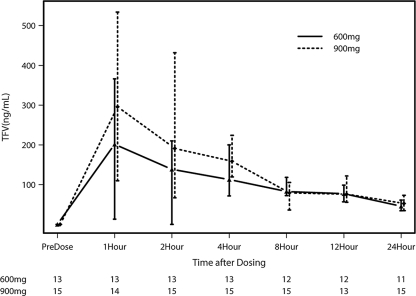
Maternal PK parameters for TFV are shown in Table 2, and maternal TFV levels over the 24-h sampling period are depicted in Fig. 1. Values for PK parameters for groups 2 and 3 (900 mg TDF alone and 900 mg TDF in combination with FTC, respectively) were similar and were therefore combined. No statistically significant differences in AUC, Cmax, t1/2, tmax, C24, or concentration at delivery were observed between the 600-mg and 900-mg groups. In the entire cohort, the Cmax was greater in women who delivered via cesarean section than in those who delivered vaginally (P = 0.04). Substantial interpatient variability was seen with both doses.
Table 2.
TFV PK parametersa
| PK parameter | Value (range) for mothers receivingb: |
Range for HIV-infected patients receiving 300 mg TDF dailyc | |||||
|---|---|---|---|---|---|---|---|
| 600 mg TDF |
900 mg TDF |
||||||
| All women (n = 13) | Delivering by cesarean section (n = 7) | Delivering vaginally (n = 6) | All women (n = 15) | Delivering by cesarean section (n = 6) | Delivering vaginally (n = 9) | ||
| Median TFV AUC (ng·h/ml) | 2,361 (1,344–4,431) | 2,361 (1,507–4,431) | 2,211 (1,344–2,962) | 2,519 (823–5,677) | 3,006 (2,261–4,207) | 1,878 (823–5,677) | 2,550–3,100 |
| Median Cmax (ng/ml) | 234 (83–595) | 282 (168–595) | 199 (83–528) | 456 (80–839) | 511 (432–839) | 191 (80–714) | 276–355 |
| Median tmax (h) | 1.1 (0.9–12.7) | 1.1 (0.9–12.7) | 2.6 (1–7.9) | 2 (1–12) | 1 (1–2) | 4 (1–12) | |
| Median t1/2 (h) | 15.9 (3.3–187.6) (n = 12) | 19.1 (3.3–187.6) (n = 6) | 15.6 (9.6–32.7) | 16.9 (10.2–33.7) (n = 13) | 19.1 (14–22.8) (n = 6) | 14.2 (10.2–33.7) (n = 7) | |
| Median C24 (ng/ml) | 46 (23–95) | 41 (23–95) | 47 (28–61) | 53 (12–90) | 62 (21–78) | 48 (12–90) | |
| Median concentration at delivery (ng/ml)d | 78 (0–252) (n = 10) | 72 (48–237) (n = 5) | 83 (0–252) (n = 5) | 109 (0–381) (n = 13) | 157 (101–381) (n = 6) | 80 (0–150) (n = 7) | |
Observed following 600-mg and 900-mg TDF administration in HIV-infected pregnant women and 300-mg, steady-state, daily doses in nonpregnant, HIV-infected patients.
Exceptions to the stated numbers of women are noted.
Ranges shown are based on steady-state PK parameters following dosing with 300 mg daily over 12 to 48 weeks of continuous dosing (19).
Maternal concentrations at delivery were obtained within 90 min of delivery.
Fig. 1.
Median maternal TFV concentrations following a single dose of 600 mg or 900 mg TDF. Vertical bars represent interquartile ranges (IQRs), and the numbers along the bottom are the numbers of available data points at each time point for each dose level.
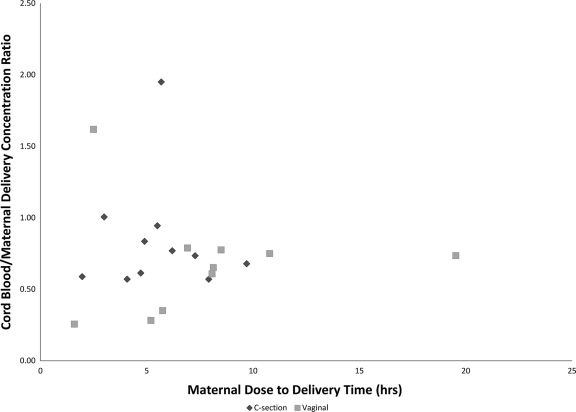
Cord blood concentrations are shown in Table 3. The median cord blood concentration was 76 ng/ml in group 1 (600 mg) and 68 ng/ml in groups 2 and 3 (900 mg), demonstrating similar concentrations regardless of maternal dose (P = 0.5). Higher cord blood concentrations were observed in infants delivered via cesarean section than in infants delivered vaginally (P = 0.046). For the entire study population, the CB/M concentration ratio was 0.73 (range, 0.26 to 1.95). The relationship between the time from maternal dose to delivery and the CB/M concentration ratio is presented graphically in Fig. 2 for the entire study population.
Table 3.
Cord blood TFV PK parameters
| PK parameter | Value (range) for infants of mothers receivinga: |
|||||
|---|---|---|---|---|---|---|
| 600 mg TDF |
900 mg TDF |
|||||
| All infants (n = 10) | Delivered by cesarean section (n = 5) | Delivered vaginally (n = 5) | All infants (n = 15) | Delivered by cesarean section (n = 6) | Delivered vaginally (n = 9) | |
| Median cord blood concn (ng/ml) | 76 (0–309) | 94 (41–223) | 64 (0–309) | 68 (0–224) | 110 (68–224) | 49 (0–114) |
| No. with cord blood concn of <50 ng/ml | 4 | 2 | 2 | 5 | 0 | 5 |
| CB/M TFV ratio | 0.78 (0.35–1.95) (n = 9) | 0.94 (0.57–1.95) | 0.76 (0.35–1.62) (n = 4) | 0.63 (0.26–0.84) (n = 12) | 0.71 (0.59–0.84) | 0.63 (0.26–0.79) (n = 6) |
Observed following 600-mg and 900-mg TDF administration in HIV-infected pregnant women. Exceptions to the stated numbers of infants are noted.
Fig. 2.
Comparison of CB/M TFV concentration ratios and the intervals between maternal doses and deliveries.
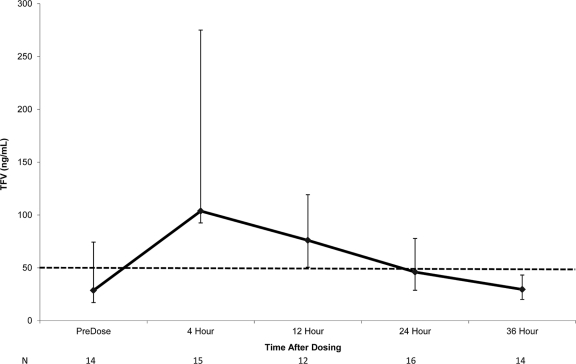
In group 1, the infant TFV concentrations at all measured time points (12, 24, and 36 h of life) were below the 50-ng/ml target. At 12 h of life, the median TFV concentration was 12 ng/ml. In groups 2 and 3, infants received TDF at a median of 5.5 h of life (range, 1.8 to 11.1 h). Median infant TFV concentrations following the dose are shown in Fig. 3. At the time of TDF dosing, the median TFV concentration in the infants was 29 ng/ml (range, 0 to 91 ng/ml) and was below the targeted minimum concentration of 50 ng/ml in 9 of 15 infants. The median tmax was 4 h (range, 4 to 24 h), and the median Cmax was 101 ng/ml (range, 40 to 621 ng/ml) (Table 4). The Cmax was less than 50 ng/ml in 3 of 16 infants. By 24 h after dosing, the median TFV concentration was 46 ng/ml (range, 17 to 120 ng/ml).
Fig. 3.
Median infant TFV concentrations following a single 4-mg/kg dose administered shortly after birth. Vertical bars represent IQRs. The dashed line represents a TFV concentration of 50 ng/ml, the targeted C24 concentration. The numbers along the bottom are the numbers of available data points at each testing time.
Table 4.
Infant TFV PK parametersa
| PK parameter | Value (range) for all infants (n = 16) |
|---|---|
| Median TFV AUC (ng·h/ml) | 1,841 (883–7,931) |
| Median Cmax (ng/ml) | 101 (40–621) |
| Median tmax (h) | 4.0 (3.8–23.8) |
| Median t1/2 (h) | 16.9 (8.5–27.0) |
| Median C24 (ng/ml) | 46 (17–120) |
| Median C36 (ng/ml) | 30b (15-78) |
Observed following 4-mg/kg TDF administration to infants.
n = 14.
Emtricitabine pharmacokinetics.
PK samples from all eight women who received TDF-FTC and from 6 of their infants were evaluable. None of the study mothers received emtricitabine as part of their cART regimen. Infants were dosed with FTC at a median of 5.3 h of life (range, 1.5 to 7.7 h). FTC PK parameters for mothers and infants are presented in Table 5.
Table 5.
Maternal, cord blood, and infant FTC PK parameters in group 3a
| PK parameter | Value (range) |
|---|---|
| Mothers (600 mg FTC) (n = 8) | |
| Median FTC AUC (ng·h/ml) | 13,794 (5,322–36,268) |
| Median Cmax (ng/ml) | 1,795 (393–4,820) |
| Median tmax (h) | 3.0 (2.0–12.6) |
| Median t1/2 (h) | 4.7 (2.7–10.0) |
| Median C24 (ng/ml) | 81 (26–238) |
| Median concn at delivery (ng/ml) | 822 (22–1,161) |
| Cord blood (n = 8) | |
| Median amount of cord blood (ng/ml) | 717 (21–1,072) |
| CB/M FTC ratio | 0.85 (0.45–1.07) |
| Infants (3 mg/kg FTC) (n = 6) | |
| Median FTC AUC (ng·h/ml) | 17,852 (9,525–22,898) |
| Median Cmax (ng/ml) | 1,068 (769–1,538) |
| Median tmax (h) | 4.0 (3.8–12.0) |
| Median t1/2 (h) | 9.2 (8.1–10.1) |
Observed after administration of three tablets of TDF-FTC (900 mg TDF plus 600 mg FTC in mothers and 4 mg/kg TDF plus 3 mg/kg FTC in infants).
TFV resistance.
The viral load measurements for 19 women were greater than 1,000 copies/ml in 23 samples over the course of the study. One woman had a K65R mutation at baseline; no new K65R or K70E mutations were detected in any of the study samples when tested either by bulk sequencing or in a sensitive real-time PCR assay.
Safety and tolerance.
The mothers and infants tolerated the study drug well. There were multiple adverse events reported, mostly consisting of laboratory abnormalities considered unrelated to the study agents. There was one grade 3 hemoglobin value for an infant that was thought to possibly be related to the drawing of blood for the study. There were no grade 4 events or adverse maternal or neonatal outcomes. There were no HIV MTCT cases in this study.
DISCUSSION
This study demonstrates that TDF administration at the time of active labor can achieve TFV concentrations that can suppress HIV replication and can have potential utility for the prevention of HIV MTCT. Although substantial interpatient variability exists, we have confirmed that TDF doses of 600 mg administered during labor result in TFV exposure consistent with that of the standard 300-mg doses used in treatment of HIV-infected nonpregnant adults and achieve targeted levels in most women and in cord blood (2, 29). We saw no further increase in TFV AUC, C24, or cord blood concentration when the TDF dose was increased to 900 mg, either as TDF alone or as TDF coformulated with FTC. Although the number of subjects was modest, these data suggest that increasing the intrapartum TDF dose to 900 mg does not significantly increase TFV exposure in mother or infant. This was unexpected, because the TFV Cmax and AUC were dose proportional following single doses of TDF between 75 mg and 600 mg (2). This confirms that 600 mg should be the recommended TDF dose for intrapartum use (14). We also identified that women delivering via cesarean section had increased TFV Cmax compared to those of women delivering vaginally. Many of the cesarean sections performed on women in this study were planned, and women did not enter active labor. It is possible that TDF absorption may be impaired during active labor. The clinical significance of this difference is not known. However, since TFV exposure in the infant seems to be determined more by infant than maternal dosing, the significance is expected to be small.
As predicted by animal models, substantial amounts of TFV cross the placenta. Studies of pregnant rhesus monkeys demonstrated fetal/maternal TFV ratios of 17% and 60% following daily subcutaneous doses and a single dose administered to near-term macaques, respectively (32, 38). Although TFV crossed the placenta well in our subjects, with a CB/M TFV concentration ratio of 0.73, 4 of 10 infants in group 1 and 5 of 15 infants in groups 2 and 3 failed to exceed the target concentration of 50 ng/ml in cord blood. In previous studies of intrapartum administration of nevirapine and tenofovir, the time between maternal dosing and delivery played an important role in determining fetal exposure to a maternally administered drug (14, 16, 22). Hirt et al. and the TEmAA ANRS 12109 Study Group recommend repeat dosing of women with an additional 600 mg TDF if more than 12 h have elapsed since dosing and the woman has not yet delivered the infant (14, 34). Pharmacokinetic findings from repeat dosing in two women have demonstrated concentrations comparable to those in women who had received a single dose as well as no significant safety issues (16, 34).
A very surprising finding in our study, corroborated by others, is the very rapid decline in infant TFV concentrations immediately after birth (14, 16, 23). Infant rhesus monkeys demonstrate a 65% decrease in the clearance of TFV, which is thought to be due to immature renal function and which suggests that TFV elimination would also be decreased in neonates (37). In contrast, elimination of transplacentally acquired TFV appeared brisk in our newborns in group 1, with no infant having a TFV concentration above 14 ng/ml by 12 h of life. Based on these findings of rapid decline of infant TFV concentrations after birth in the first group studied, we opted to dose infants as soon as possible after delivery in the second and third groups. Despite this plan, TDF was administered to infants in our study at a median of 5.5 h of life due to practical reasons. If TDF is to be given to infants as part of a successful PMTCT regimen, infant dosing should be given sooner after birth in order to maintain targeted levels for viral suppression. The optimal length of time necessary to maintain targeted levels for viral suppression in newborns remains unknown. However, based on nevirapine pharmacokinetics in newborns (25) and recent data from the HPTN 040/PACTG 1043 trial, where 1 to 2 weeks of additional antiretroviral agents was added to the standard 6 weeks of ZDV in infants born to women who received no antepartum therapy, resulting in reduced transmission (26), a minimum of 1 to 2 weeks seems reasonable.
Differential clearance of plasma TFV has been observed between doses administered as parenteral TFV and doses administered as oral TDF. In macaques, the plasma half-life of subcutaneous TFV was 3.9 h, compared to 15.3 h for TDF. Additionally, TDF administration resulted in higher concentrations of the active metabolite TFV-diphosphate (TFV-DP) in peripheral blood mononuclear cells (7). Because infants in groups 2 and 3 were exposed to circulating TFV via the placenta and received TDF orally, their pharmacokinetic parameters likely represent a combination of both parenteral- and oral-administration PK patterns. This also suggests that despite low plasma concentrations following oral administration of TDF, substantial intracellular concentrations of the active metabolite TFV-DP may be present. TFV-DP was assessed by Hirt et al. in 20 cord blood specimens following maternal intrapartum dosing of 600 mg and in 22 infants 10 to 45 h old following a dose of 13 mg/kg of TDF suspension (16). Only 2 of the cord blood specimens had quantifiable TFV-DP present, but the infant concentrations were comparable to those in adults at steady state. Although there was no sampling between birth and the assessment 10 to 45 h after infant dosing, a lag in the appearance of TFV-DP is proposed. The role of TFV-DP concentrations in adult pre- and postexposure prophylaxis regimens is under investigation and may provide additional insight (1, 12).
Our study is one of the first to report the pharmacokinetics of infant dosing with TDF in the first week of life. After receiving 4 mg/kg at a median of 5.5 h after birth, these infants had low TFV AUC and Cmax (but similar t1/2) compared to those of uninfected adults receiving standard 300-mg doses (19, 29) as well as to those of the mothers in the current study who received 600 mg or 900 mg during labor. By 24 h after the dose, the median infant concentration was 46 ng/ml, which is below the 50-ng/ml target. These data suggest that a larger infant dose will be needed to maintain infant TFV concentrations above the target concentration in the first days of life. Hirt et al. had proposed an infant dose of 13 mg/kg based on a simulation of data from maternal intrapartum TDF doses and the washout of transplacentally acquired drugs from infants (14). They have recently reported their findings using a population PK approach (16). The median infant TFV AUCs and Cmax were approximately twice those seen with our 4-mg/kg dose (3,730 versus 1,841 ng·h/ml and 290 versus 101 ng/ml, respectively). Additionally, the safety of this dose in infants seems acceptable (34). A clinical study using a 6-mg/kg dose with repeated dosing is under way (24), and findings from this study will help determine the optimal infant TDF dosing regimen.
Eight of our study mothers also received 600-mg intrapartum doses of FTC in the form of TDF-FTC, the fixed-dose combination of TDF and FTC. FTC PK parameters in these subjects were compared to those seen in non-HIV-infected adults receiving standard FTC doses of 200 mg; the AUC was increased by roughly 40%, while the Cmax, C24, and t1/2 were similar (29). However, the FTC AUC, Cmax, and C24 values in our subjects were similar to those seen following intrapartum 400-mg doses in another study (15). This similarity suggests that intrapartum administration of 2 TDF-FTC tablets, which provide 600 mg TDF and 400 mg FTC, should provide an exposure to both drugs that is equivalent to that seen in nonpregnant adults receiving standard doses of 300 mg TDF and 200 mg FTC.
The median CB/M FTC concentration ratio was 0.85, confirming that transfer of FTC from mother to infant is substantial (3, 15). Our data are the first to describe FTC pharmacokinetics following administration to neonates. We chose a conservative dose of 3 mg/kg, one-half of the usual pediatric dose of 6 mg/kg (41). The FTC AUC in our infants exceeded that seen following 200-mg doses in uninfected adults and 120-mg/m2 doses in HIV-infected children, while the Cmax was lower in our infants (29, 41). However, our sampling schedule may have resulted in an underestimation of the Cmax, as our first sample was collected 4 h after the dose but the FTC tmax averages 1.5 h in older infants and children (41). The median t1/2 in our neonates was 9.2 h, similar to that observed in older children and adults (29, 41). Our data suggest that an FTC dose of 3 mg/kg as soon as possible after birth provides FTC exposure that exceeds the average in vitro FTC 90% inhibitory concentration (IC90) for wild-type HIV-1 virus replication of 51 ng/ml for at least 24 h after the dose. A smaller dose of 2 mg/kg may be adequate for prophylaxis of HIV MTCT in neonates, consistent with the recommendation from a simulation of neonatal FTC dosing (15).
In summary, TDF, alone or with FTC, at doses of 600 mg and 900 mg, was safe and well tolerated in HIV-infected women both in active labor and prior to a cesarean section and resulted in TFV exposure similar to that seen in HIV-infected persons taking 300 mg TDF daily. No significant maternal or infant adverse events, including the development of new 65R or 70E mutations, were observed with the administration of TDF to pregnant women. Maternal FTC administration was also well tolerated, and FTC exposure with 600-mg intrapartum doses was greater than that seen with 200-mg doses in nonpregnant adults and children but not different than that seen with 400-mg intrapartum doses. Based on these data, we recommend intrapartum TDF dosing of 600 mg, with 400 mg FTC recommended if the fixed-dose combination product is used. Infant doses of 4 mg/kg TDF resulted in targeted TFV concentrations, but these concentrations were not maintained over 24 h, suggesting that TDF should be given to infants immediately after birth and that doses higher than the 4 mg/kg used in this study and/or more frequent dosing intervals will be required to maintain targeted TFV levels in the first days of life.
ACKNOWLEDGMENTS
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (grant U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (grant AI068632). This work was supported by the Statistical and Data Analysis Center at the Harvard School of Public Health under National Institute of Allergy and Infectious Diseases cooperative agreement number 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and cooperative agreement number 1 U01 AI068616 with the IMPAACT Group. Support for the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network, funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Gilead Sciences, Inc., provided the study drugs TDF, tenofovir-emtricitabine (Truvada), and FTC and performed the drug concentration assays.
We thank Kimberly Hudgens for assistance with protocol development and management. We would also like to acknowledge Jeff Johnson and Jonathan Lipscomb from the Centers for Disease Control and Prevention for performing the sensitive real-time PCR resistance assays.
The following clinical trial sites and personnel participated in the conduct of this study: at San Juan City Hospital, Elvia Perez-Hernandez, Antonio Rodriguez-Mimoso, Midnela Acevedo-Flores, and Luis Marquez-Babilonia; at the Regional Medical Center at Memphis, Edwin M. Thorpe, Jr., and Nina K. Sublette; at the New Jersey Medical School, Arry Dieudonne, Charmane Calilap-Bernardo, Juliette Johnson, and Lisa Monti; at St. Jude Children's Research Hospital, Katherine Knapp, Nehali Patel, Jill Utech, and Sandra J. Boyd; at the Children's Hospital of Michigan, Ernestine Brown, Tamika Watson, and Theodore B. Jones; at Bronx-Lebanon Hospital, Mahboobullah Mirza-Baig, Mavis Dummitt, Stefan Hagmann, and Murli Purswani; and at the University of Miami, Amanda Cotter, Gwendolyn B. Scott, Erika Lopez, and Sergio Jordan.
Footnotes
Published ahead of print on 6 September 2011.
REFERENCES
- 1. Anderson P. L., et al. 2011. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J. Antimicrob. Chemother. 66(2): 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barditch-Crovo P., et al. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45: 2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Best B. M., et al. 2008. High-dose lopinavir and standard-dose emtricitabine pharmacokinetics during pregnancy and postpartum, abstr. 629. Abstr. 15th Conf. Retrovir. Oppor. Infect., Boston, MA [Google Scholar]
- 4. Blum M. R., Chittick G. E., Begley J. A., Zong J. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J. Clin. Pharmacol. 47: 751–759 [DOI] [PubMed] [Google Scholar]
- 5. Chi B. H., et al. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370: 1698–1705 [DOI] [PubMed] [Google Scholar]
- 6. Connor E. M., et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group N. Engl. J. Med. 331: 1173–1180 [DOI] [PubMed] [Google Scholar]
- 7. Durand-Gasselin L., et al. 2009. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol. Pharm. 6: 1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eshleman S. H., et al. 2006. Development of nevirapine resistance in infants is reduced by use of infant-only single-dose nevirapine plus zidovudine postexposure prophylaxis for the prevention of mother-to-child transmission of HIV-1. J. Infect. Dis. 193: 479–481 [DOI] [PubMed] [Google Scholar]
- 9. Eshleman S. H., et al. 2001. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012). AIDS 15: 1951–1957 [DOI] [PubMed] [Google Scholar]
- 10. Flys T. S., et al. 2006. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J. Acquir. Immune Defic. Syndr. 42: 610–613 [DOI] [PubMed] [Google Scholar]
- 11. Flys T., et al. 2005. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J. Infect. Dis. 192: 24–29 [DOI] [PubMed] [Google Scholar]
- 12. Grant R. M., et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363: 2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guay L. A., et al. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354: 795–802 [DOI] [PubMed] [Google Scholar]
- 14. Hirt D., et al. 2009. ANRS 12109. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin. Pharmacol. Ther. 85: 182–189 [DOI] [PubMed] [Google Scholar]
- 15. Hirt D., et al. 2009. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob. Agents Chemother. 53: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirt D., et al. 2011. Plasma and intracellular tenofovir pharmacokinetics in the neonate (ANRS 12109 Trial, step 2). Antimicrob. Agents Chemother. 55: 2961–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson J. A., et al. 2005. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J. Infect. Dis. 192: 16–23 [DOI] [PubMed] [Google Scholar]
- 18. Johnson J. A., et al. 2007. Simple PCR assays improve the sensitivity of HIV-1 subtype B drug resistance testing and allow linking of resistance mutations. PLoS One 2: e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kearney B. P., Flaherty J. F., Shah J. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43(9): 595–612 [DOI] [PubMed] [Google Scholar]
- 20. Mathias A. A., et al. 2007. Bioequivalence of efavirenz/emtricitabine/tenofovir disoproxil fumarate single tablet regimen. J. Acquir. Immune Defic. Syndr. 46: 167–173 [DOI] [PubMed] [Google Scholar]
- 21. McIntyre J. A., et al. 2009. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial. PLoS Med. 6(10): e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mirochnick M., et al. 2003. Predose infant nevirapine concentration with the 2 dose intrapartum-neonatal nevirapine regimen: association with timing of maternal intrapartum nevirapine dose. J. Acquir. Immune Defic. Syndr. 33: 153–156 [DOI] [PubMed] [Google Scholar]
- 23. Mirochnick M., et al. 2009. Pharmacokinetics of tenofovir disoproxil fumarate after administration to HIV-1-infected pregnant women and their newborns, abstr. 940. Abstr. 16th Conf. Retrovir. Oppor. Infect., Montreal, Canada [Google Scholar]
- 24. Mirochnick M., et al. 2010. Tenofovir disoproxil fumarate (TDF) pharmacokinetics (PK) with increased doses in HIV-1 infected pregnant women and their newborns (HPTN 057), abstr. 3. Abstr. 11th Int. Workshop Clin. Pharmacol. HIV Ther., Sorrento, Italy [Google Scholar]
- 25. Mirochnick M., et al. 2008. Nevirapine concentrations in newborns receiving an extended prophylactic regimen. J. Acquir. Immune Defic. Syndr. 47: 334–337 [PubMed] [Google Scholar]
- 26. Nielsen-Saines K., et al. 2011. Phase III randomized trial of the safety and efficacy of 3 neonatal ARV regimens for prevention of intrapartum HIV-1 transmission: NICHD HPTN 040/PACTG 1043, abstr. 124LB. Abstr. 18th Conf. Retrovir. Oppor. Infect., Boston, MA [Google Scholar]
- 27. Palumbo P., et al. 2010. Antiretroviral treatment for children with peripartum nevirapine exposure. N. Engl. J. Med. 363: 1510–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panel on Treatment of HIV-Infected Pregnant Women Prevention of Perinatal Transmission 14 September 2011, posting date 2 November 2011, accession date Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Department of Health and Human Services, NIH, Bethesda, MD. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf
- 29. Ramanathan S., Shen G., Cheng A., Kearney B. P. 2007. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J. Acquir. Immune Defic. Syndr. 45: 274–279 [DOI] [PubMed] [Google Scholar]
- 30. Sperling R. S., et al. 1996. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group N. Engl. J. Med. 335: 1621–1629 [DOI] [PubMed] [Google Scholar]
- 31. Stephan C. 2008. Experience with tenofovir disoproxil fumarate for antiretroviral therapy. Expert Opin. Pharmacother. 9(7): 1197–1209 [DOI] [PubMed] [Google Scholar]
- 32. Tarantal A. F., Marthas M. L., Shaw J. P., Cundy K., Bischofberger N. 1999. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): safety and efficacy studies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20: 323–333 [DOI] [PubMed] [Google Scholar]
- 33. TEmAA ANRS 12109 Study Group 2009. Tolerance and viral resistance after single-dose nevirapine with tenofovir and emtricitabine to prevent vertical transmission of HIV-1. AIDS 23: 825–833 [DOI] [PubMed] [Google Scholar]
- 34. TEmAA ANRS 12109 Study Group 2010. Maternal and neonatal tenofovir and emtricitabine to prevent vertical transmission of HIV-1: tolerance and resistance. AIDS 24: 2481–2488 [DOI] [PubMed] [Google Scholar]
- 35. Van Rompay K. K., et al. 1998. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS 12: F79–F83 [DOI] [PubMed] [Google Scholar]
- 36. Van Rompay K. K. A., et al. 2004. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob. Agents Chemother. 48: 1469–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Rompay K. K., et al. 2006. Evaluation of oral tenofovir disoproxil fumarate and topical tenofovir GS-7340 to protect infant macaques against repeated oral challenges with virulent simian immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 43: 6–14 [DOI] [PubMed] [Google Scholar]
- 38. Van Rompay K. K., et al. 1998. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res. Hum. Retroviruses 14: 761–773 [DOI] [PubMed] [Google Scholar]
- 39. Van Rompay K. K., et al. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184(4): 429–438 [DOI] [PubMed] [Google Scholar]
- 40. Van Rompay K. K. A., et al. 2000. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 74: 1767–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L. H., et al. 2004. Pharmacokinetics and safety of single oral doses of emtricitabine in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 48: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]