Abstract
Atazanavir (Reyataz; ATV) is a well-tolerated protease inhibitor (PI) that is indicated as a once-daily treatment for HIV infections. These features of ATV, combined with its virologic potency, make it particularly desirable for the treatment of HIV-infected pediatric patients. The objective of this study was to use a model-based approach to recommend body weight-based ATV capsule doses for pediatric patients. ATV concentration-time data from three adult studies and one pediatric study were described by a C0-delinked one-compartment model to guard against introducing bias in pharmacokinetic (PK) parameter estimates due to the potential nonadherence in outpatient studies. The apparent clearance (CL/F) and apparent volume of distribution (V/F) were determined to increase with body weight, and CL/F was 40.9% lower in patients receiving ATV comedication with ritonavir (RTV). The relative bioavailability (Frel) of ATV was 132% higher with RTV comedication and was 35.5% lower for the ATV powder formulation than the capsule formulation. Model-based simulations were used to recommend weight-based ATV capsule doses of 150 to 300 mg boosted with 100 mg RTV for pediatric patients weighing ≥15 kg, such that the exposures in these patients are similar to those obtained in HIV-infected adults treated with the recommended ATV/RTV dose of 300/100 mg.
INTRODUCTION
There is an unmet medical need for the development of potent, safe, well-tolerated, and simplified once-a-day therapies for children infected with HIV. Virologic failure and long-term drug-related toxicities have become increasingly common in children, and thus there is a need for drugs that offer improved tolerability and resistance to viral mutations, with convenient dosing schedules. Atazanavir (Reyataz; ATV) is an azapeptide protease inhibitor (PI) that is approved in the United States for the treatment of HIV-infected adults and children in combination with other antiretrovirals (3). The safety profile of ATV is notable for causing fewer perturbations in lipids and less gastrointestinal intolerance than the other PIs. In adult and pediatric patients, the most common side effect of ATV is indirect bilirubin elevation due to inhibition of the UDP-glucuronil transferase enzyme, which is usually well tolerated and does not lead to treatment discontinuation (9). Additionally, ATV combines virologic potency with a high barrier for development of cross-resistance to other PIs (16). On these grounds, ATV represents an ideal PI for the treatment of HIV-infected pediatric patients.
The safety, efficacy, and pharmacokinetics (PK) of ATV in HIV-infected pediatric patients, with or without ritonavir (RTV), were studied in trial PACTG1020 (ClinicalTrials.gov identifier NCT00006604). The ATV doses in this study were body surface area (BSA) normalized and adjusted to target exposures and safety criteria prespecified in the protocol. A BSA-based ATV dose of 205 mg/m2 met the prespecified criteria for patients older than 6 years taking ATV capsules with RTV. However, given the available ATV capsule dose strengths (100, 150, 200, and 300 mg), it is not possible to administer the exact mg dose equivalent to the BSA dose. The BSA dose was converted to the recommended body weight-tiered ATV pediatric doses in the U.S. labeling requirements. This report describes the analyses performed to further optimize the body weight-based pediatric ATV doses and to obtain approval of these doses in the European Union.
Conventional approaches to dose selection for pediatric patients have utilized size-related allometric scaling, as suggested for physiological processes across species of different sizes (1, 11). These approaches assume PK parameters to be a function of the allometric exponent of body weight, without appreciation of drug-specific PK or PK/pharmacodynamic (PD) data that are available for the adult population studied previously. Regulatory authorities, in particular the European Medicines Agency (EMA) and the U.S. FDA, have advocated model-based pediatric drug development, which offers the advantages of integrating adult and pediatric data or data from different pediatric age groups to support the use of a previously approved drug in a new target patient population (8, 19). Numerous scientific publications have provided successful examples of the added value of modeling and simulation (M&S) in pediatric drug development (5, 12, 13).
In this study, we utilized M&S as a tool to optimize the ATV capsule doses for pediatric patients by integrating available pediatric and adult data. Steady-state ATV PK data from three adult studies and one pediatric study were pooled and used to develop a population PK model. Model-based simulation was then applied to determine body weight-based ATV/RTV doses for pediatric HIV patients 6 years and older that produce ATV exposures similar to those known to be efficacious in HIV-infected adults.
MATERIALS AND METHODS
Study data.
Data from 4 clinical studies (3 adult and 1 pediatric) were included in the analysis. A summary of the study designs is shown in Table 1.
Table 1.
Summary of clinical studies
| Study | Study population | Study design | Study drug dosage regimena | No. of patients | PK assessment |
|---|---|---|---|---|---|
| AI424008 | Adult HIV patients | Phase 2/3 active-controlled, 3-arm parallel group | ATV at 400 mg QD or 600 mg QD (not included here) or NFV at 1,250 mg BID (not included here) | 13 | 0, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h on day 29 |
| AI424089 (ClinicalTrials.gov identifier NCT00084253) | Adult HIV patients | Phase 4 open label, 2-arm parallel group | ATV at 400 mg QD or ATV/RTV at 300/100 mg QD | 27 | 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, and 24 h on day 29 |
| AI424137 (ClinicalTrials.gov identifier NCT00162149) | Adult HIV patients | Phase 1 open label, two-cohort sequential design | ATV/RTV at 300/100 mg QD (2nd cohort only) | 11 | 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, and 24 h on day 10 |
| PACTG1020 (ClinicalTrials.gov identifier NCT00006604) | Pediatric HIV patients | Phase 1/2 open label, titration design | ATV at 100-1,200 mg QD or ATV at 48-600 mg QD + RTV at 26-100 mg QD | 176 | 0, 1, 2, 3, 4, 6, 8, 12, and 24 h at weeks 1 and 56 plus 2 weeks after a dose adjustment |
QD, once daily; BID, twice daily; ATV, atazanavir; NFV, nefinavir; RTV, ritonavir.
The pediatric study PACTG1020 investigated the PK, safety, and tolerability of ATV in antiretroviral treatment (ART)-naïve and -experienced HIV-infected infants, children, and adolescents. The eligible subjects were assigned to treatment groups that were stratified by age, ATV formulation, and RTV comedication (yes/no), as shown in Table 2. The starting ATV dose for all groups was 310 mg/m2 once a day (QD). The RTV-boosted groups also received RTV at 100 mg/m2 QD (up to 100 mg QD for liquid or a 100-mg capsule QD). ATV doses were increased or decreased based on prespecified ATV PK exposure targets and safety criteria. Intensive PK samples collected over a 24-h period from 176 patients at weeks 1 and 56 and 2 weeks after the initiation of ATV dose adjustments were included in this analysis.
Table 2.
Stratification of treatment groups in PACTG1020 pediatric study
| Formulation | Group |
Age range | |
|---|---|---|---|
| ATV without RTV | ATV with RTV | ||
| Powder | 1 | 5 | Infants (3 months to ≤2 yr) |
| 2 | 6 | Children (>2 to ≤13 yr) | |
| Capsule | 3 | 7 | Children (>2 to ≤13 yr) |
| 4 | 8 | Adolescents (>13 to ≤21 yr) | |
The plasma concentration of ATV was determined using a validated high-performance liquid chromatography-tandem mass spectrometry method (17). A total of 3,939 ATV concentrations were included in the analysis data set, including 620 from the adult studies and 3,319 from the pediatric study. Summary statistics of the patient characteristics are listed in Table 3.
Table 3.
Summary statistics of patient characteristics and covariates
| Covariate | Value |
||
|---|---|---|---|
| Adultsa | Pediatric patientsb | Total | |
| Body wt (kg) | |||
| n | 51 | 176 | 227 |
| Mean | 70.7 | 33.5 | 41.9 |
| Range | 49.7–97.1 | 2.6–122 | 2.6–122 |
| Age (yr) | |||
| n | 51 | 176 | 227 |
| Mean | 36.3 | 9.17 | 15.3 |
| Range | 22–64 | 0.33–21 | 0.33–64 |
| ART status (no. of patients) | |||
| Naïve | 40 | 80 | 120 |
| Experienced | 11 | 96 | 107 |
| RTV comedication (no. of patients) | |||
| Without RTV | 28 | 82 | 110 |
| With RTV | 23 | 94 | 117 |
| Formulation (no. of patients) | |||
| Capsule | 51 | 112 | 163 |
| Powder | 0 | 64 | 64 |
| Sex (no. of patients) | |||
| Female | 16 | 88 | 104 |
| Male | 35 | 88 | 123 |
| Race (no. of patients) | |||
| White | 15 | 37 | 52 |
| Black | 34 | 116 | 150 |
| Other | 2 | 23 | 25 |
| Region (no. of patients) | |||
| Africa | 31 | 60 | 91 |
| North America | 11 | 116 | 127 |
| Europe | 9 | 0 | 9 |
Pharmacokinetic analysis.
The PK data from adult and pediatric patients were pooled and analyzed by a nonlinear mixed-effects (population PK) model. The population PK analysis was performed using first-order conditional estimation (FOCE) as implemented in NONMEM software (version VI; Icon Development Solutions, Ellicott City, MD). Postprocessing of NONMEM output was performed using S-Plus (version 7.0 for Linux; Tibco Software Inc., Palo Alto, CA).
(i) Model development.
ATV pharmacokinetics was described by a C0-delinked one-compartment linear model with first-order absorption (10). The ATV structural model was parameterized in terms of first-order absorption rate (ka), elimination rate (ke), apparent volume of distribution (V/F), and relative bioavailability (Frel). A parameter to describe the predose concentration (C0) was estimated as a separate term in the model to guard against introducing bias in the compartmental PK parameters due to nonadherence (10). The ATV plasma concentration (C) at time t following an ATV dose (D) was described using the following expression:
| (1) |
The random-effects model includes interindividual variability (IIV) and interoccasion variability (IOV) models, which are specified by the log-normal random-effects model given by the following equation:
| (2) |
where θij is the value of a compartmental model parameter for the ith individual at the jth occasion, θTV is the typical value of the model parameter, ηi denotes the interindividual random effect accounting for the ith individual's deviation from the typical value, and κij denotes the interoccasion random effect accounting for the ith individual's deviation at the jth occasion. The ηi and κij values are assumed to be independent, with a mean of zero and covariance matrices denoted ΩIIV and ΩIOV, respectively.
Residual variability was described by the following log-normal residual error model:
| (3) |
where Yij is the observed concentration for the ith individual at time tj, Cij is the corresponding predicted concentration based on the PK model, and εij denotes the intraindividual (residual) random effect, which is assumed to have a mean of zero and a variance of σ2.
The covariates of interest were selected based on clinical and pharmacological judgment and were incorporated into the full model regardless of statistical significance. The analysis assessed the effects of several intrinsic and extrinsic covariates on ATV PK. The intrinsic covariates included body weight, age, sex, race, and ART-naïve or -experienced status, and the extrinsic covariates included formulation, RTV comedication, and study region. The covariate effects on apparent clearance (CL/F), V/F, ka, and Frel were investigated. The relationship between continuous covariates (xij) and the typical value of the PK parameters (θTV,ij) was described by a power model as follows:
| (4) |
where θREF and θx are the fixed-effects parameters and xREF is a reference value of the covariate xij. In the context of this analysis, the reference values for age and body weight are 18 years and 70 kg.
The relationship between categorical covariates (xij) and the typical value of the PK parameters (θTV,ij) was modeled as follows:
| (5) |
where θREF and θx are the fixed-effects parameters and xij is the indicator variable, which is equal to 1 or 0 depending on the category of the covariates.
The covariate model was parameterized by assuming multiplicative effects for both the continuous and categorical covariates. Wald's approximation method (WAM) was used to identify a final parsimonious model relative to the full covariate model (14). To further evaluate the degree of parsimony of the final model, forward selection followed by backward elimination was performed on the final model. A change of objective function value (OFV) equal to the logarithm of the number of observations was set as a cutoff criterion for the inclusion and exclusion of the covariates to be consistent with Schwarz's Bayesian criterion (SBC), employed by the WAM procedure.
(ii) Model evaluation.
The final population PK model was evaluated using a visual predictive check (VPC) and posterior predictive check (PPC). VPC was conducted by simulating 500 data sets, using the final model population parameter estimates. The 10th, 50th, and 90th percentiles (80% VPC interval) of the simulated data were constructed and superimposed on the observed data in the original analysis data set. The percentage of observed concentration data outside the 80% VPC interval was calculated to assess the consistency of observed and model-predicted concentration values.
PPC was conducted by assuming that the final model parameter estimates (θ, Ω, and Σ) followed a multivariate normal distribution, with the mean vector set to the population parameter estimates and the covariance matrix set to the variance-covariance matrix of the estimates (20). One thousand simulated data sets conditioned on the study designs, dose regimens, and covariates in the observed data set were generated by using 1,000 sets of population parameter values sampled from the multivariate normal distribution. For each simulated data set as well as the observed data set, the ATV trough concentration (Cmin), peak concentration (Cmax), and area under the concentration-time curve (AUC) were calculated for each simulated subject and used as PPC statistics. The observed statistics were compared to the median and the 5th and 95th percentiles of the 1,000 simulated statistics.
Model-based simulation to support pediatric dose recommendations.
Model-based simulations were conducted to evaluate a comprehensive set of weight-based dosing scenarios for pediatric patients weighing ≥15 kg given ATV capsule doses in combination with RTV. The simulation was not conducted for pediatric patients weighing <15 kg due to insufficient data on efficacy and safety. The final population PK model was used to simulate steady-state ATV concentration-time curves for 10,000 hypothetical subjects for each dosing scenario. Individual body weights for 10,000 hypothetical subjects were sampled from a uniform distribution within each weight group. Values for Cmin, Cmax, and AUC were calculated for each of the simulated steady-state ATV concentration-time profiles. These results were subsequently summarized by calculating the geometric mean (GM) across the 10,000 simulated subjects.
A bridging strategy was adopted to determine pediatric doses that produced exposures determined to be safe and efficacious in adults. Weight-based pediatric ATV capsule doses were identified by determining the doses that produced summary steady-state exposures (Cmin, Cmax, and AUC) that were similar to target exposures achieved in HIV-infected adults receiving the recommended adult dose (ATV/RTV at 300/100 mg QD). The similarity criteria for exposures in pediatric and adult patients were defined as follows: GM of pediatric Cmin of >75% of GM of adult Cmin, GM of pediatric Cmax of <150% of GM of adult Cmax, and GM of pediatric AUC within 80 to 125% of GM of adult AUC.
In addition, we determined the percentage of pediatric subjects attaining exposures within the 10th and 90th percentiles of the adult exposures produced by the adult recommended dose. A percentage of >75% in conjunction with exposure similarity was deemed to be appropriate for proposing weight-based ATV pediatric doses.
RESULTS
Pharmacokinetic analysis. (i) Base model development.
The base population PK model describing steady-state ATV concentration-time profiles was determined to be a C0-delinked one-compartment model with delayed first-order absorption and first-order elimination. This modeling approach has been shown to perform better than the conventional method (which assumes 100% treatment adherence), based on the bias and imprecision of parameter estimates and the power and type I error in the likelihood ratio test for covariate effects (10, 18). RTV coadministration was included as a fixed effect in C0 in view of the higher ATV trough concentration with an ATV/RTV regimen than with ATV alone. Under full adherence, C0 is expected to be dose proportional; hence, it was of interest to assess whether C0 was dose proportional across the wide range of ATV doses investigated in the pediatric study (48 to 1,200 mg QD). The relationship between ATV dose and C0 was investigated by incorporating a proportional dose effect on C0 for ATV and ATV/RTV regimens. The OFVs were compared to that for the model with no dose effects on C0. The incorporation of the ATV dose effect resulted in a large reduction in OFV, by 84 points, and comparison of OFVs for the ATV dose effect revealed that the reduction can be explained primarily by the dose effect on C0 for the ATV/RTV regimen.
The random-effects model incorporates IIV effects on ka, ke, and V/F and IOV effects on ke, Frel, and C0. The IOV effect on C0 was modeled as having both a PK component and a nonadherence component. The PK-related IOV component on C0 was likely due to IOV on Frel, so the same variance component was included on both C0 and Frel. Residual variability was modeled using the log-transform error model as shown in Equation 3. Separate residual variance components were included for ATV alone and ATV/RTV, to account for the differences in residual variability between these two treatment regimens.
(ii) Final model development.
Models that included all possible combinations of covariate parameters in the full model were evaluated by the WAM procedure. The top 15 WAM-ranked models, in order of decreasing SBC, were then fit in NONMEM. The model with the highest actual SBC based on the NONMEM runs corresponded to the top WAM-ranked model, but the correlation coefficient of WAM- and NONMEM-based SBCs was low (r = 0.157). Therefore, further ad hoc NONMEM-based model evaluations were undertaken to confirm the selection of the final model. A combination of forward selection and backward elimination was performed, starting with the top WAM-ranked model, using 8.279 [log(n) = log(3,939)] as the cutoff of ΔOFV for inclusion and exclusion of a single covariate parameter. The ad hoc stepwise runs did not result in the inclusion of additional covariates or the removal of existing covariates from the model, suggesting that the top WAM-ranked model was parsimonious, and therefore this model was selected as the final model.
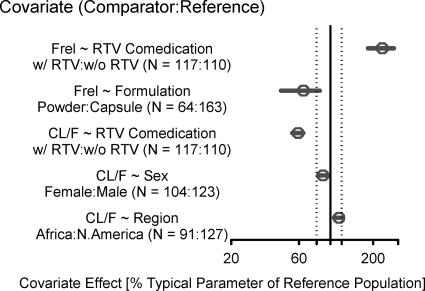
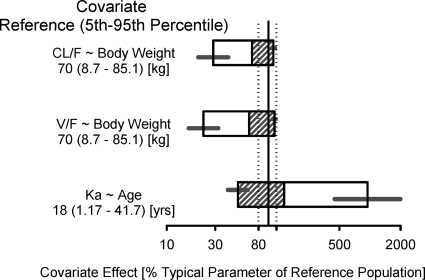
The final model contained the following covariate effects: age effect on ka; body weight effect on V/F; region (Africa), body weight, sex, and RTV comedication effects on CL/F; and formulation and RTV comedication effects on Frel. All covariates had effect magnitudes outside ±20% of reference covariate values, except for the effects of region (Africa) and sex on CL/F (Fig. 1 and 2). The final model parameter estimates are provided in Table 4. The negative exponent for the age effect on ka indicates that infants and younger children had an increased rate of absorption that resulted in a higher Cmax than that for adolescents and adults. The CL/F for a male in North America/Europe with a body weight of 70 kg receiving ATV alone was 34.6 liters/h, and the V/F for a subject with a body weight of 70 kg was 266 liters. The inclusion of an RTV comedication effect on C0, CL/F, and Frel described the substantially higher ATV exposures for patients receiving ATV/RTV than those for patients receiving ATV alone, which is consistent with the mechanism of drug interaction between ATV and RTV (15). The PK estimates of our analysis showed some discrepancy with previously reported data (CL/F of 12.9 liters/h for ATV alone and 7.0 liters/h for ATV/RTV, V/F of 88.3 liters, and ka of 0.405 h−1) (7). However, the ATV elimination half-lives (5.3 h for ATV alone and 9.0 h for ATV/RTV) were in accordance with published data (4.6 h for ATV alone and 8.8 h for ATV/RTV) (7). Finally, the model predicted an approximately 35.5% reduction in bioavailability for the powder formulation relative to the capsule formulation.
Fig. 1.
Effects of categorical covariates on ATV PK parameters (CL/F and Frel). An open circle shows the estimated covariate effect compared to the reference. Error bars show the 95% confidence interval (95% CI) for the covariate effect. The covariate effect for the reference is considered 100% (vertical solid line), and dashed vertical lines show 80% and 120% of the reference effect.
Fig. 2.
Effects of continuous covariates on ATV PK parameters (CL/F, V/F, and ka). The covariate effect (95% CI) at the 5th and 95th percentiles of the covariate is represented by the end of the horizontal box (horizontal line). The open and shaded areas of the box represent the range of covariate effects from the median to the 5th and 95th percentiles, respectively, of the covariate.
Table 4.
Final model parameter estimates
| Parametera | Mean estimate ± SE | IIV (%) | IOV (%) |
|---|---|---|---|
| Ka (1/h) | 2.04 ± 0.31 | 173 | |
| Age ∼ Ka | −0.822 ± 0.139 | ||
| Ke (1/h) | 0.130 ± 0.006 | 14.6 | 22.2 |
| V/F (liters) | 266 ± 25 | 42.5 | |
| Body wt ∼ V/F | 0.706 ± 0.082 | ||
| CL/F (liters/h) | 34.6 | ||
| Region (Africa) ∼ CL/F | 0.145 ± 0.045 | ||
| Body wt ∼ CL/F | 0.600 ± 0.083 | ||
| Sex (female) ∼ CL/F | −0.115 ± 0.035 | ||
| Comedication (with RTV) ∼ CL/F | −0.409 ± 0.026 | ||
| tlag (h)b | 0.913 ± 0.002 | ||
| C0 (ng/ml) | 118 | ||
| ATV | 158 ± 17 | ||
| ATV + RTV | 672 ± 61 | ||
| Frel | 1 | ||
| Formulation (powder) ∼ Frel | −0.355 ± 0.100 | 88.8c | |
| Comedication (with RTV) ∼ Frel | 1.32 ± 0.24 | 59.4d | |
| Residual error (%CV) | |||
| ATV | 55.2 | ||
| ATV + RTV | 35.3 |
Covariate ∼ PK parameter, covariate effect on PK parameter.
Absorption lag time.
Interoccasion variability of Frel for ATV alone.
Interoccasion variability of Frel for ATV in combination with RTV.
(iii) Final model evaluation.
The predictive performance of the final model was evaluated by VPC and PPC. The VPC plot (see Fig. S1 in the supplemental material) demonstrated that model predictions were largely in agreement with the observed data. The percentages of observations outside the 80% VPC intervals were close to the nominal value of 20%, with the exception of a few age groups that had percentages of <10% (see Table S1).
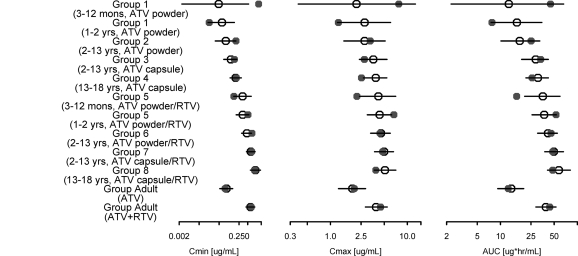
Figure 3 shows the final model posterior predictive distribution (medians with 5th and 95th percentiles) of GMs for Cmin, Cmax, and AUC overlaid with the observed GMs for ATV at week 1 in pediatric and adult subjects. The PPC of GMs (and standard deviations) of Cmin, Cmax, and AUC provided an evaluation of the model-predicted central tendency (and variability) of these exposure measures. The PPC was stratified by the 8 pediatric age/treatment groups in PACTG1020, and groups 1 and 5 were further stratified into younger (3 months to 1 year) and older (1 to 2 years) infants to assess the adequacy of the model for predictions of exposures in younger infants. Two additional stratification groups, for adults receiving ATV alone or ATV/RTV, were also included. The observed GMs for Cmin, Cmax, and AUC generally fell within the 5th and 95th percentiles of the predictive distribution for the final model across the pediatric age groups as well as the adult groups, with the exception of Cmin for the younger infants in group 1 and AUC for the younger infants in group 5. Group 1 had only one subject younger than 1 year, resulting in less precision in the prediction than that for the other groups. The GM of observed AUC in group 5 was calculated from 6 subjects and is about 20% less than the 5th percentile limit (16.3 versus 20.7 μgh/ml). The observed GMs for Cmin, Cmax, and AUC at week 56 generally fell within the 5th and 95th percentiles of the predictive distribution (see Fig. S2 in the supplemental material).
Fig. 3.
Posterior predictive check of ATV population PK model. The observed geometric means of week 1 Cmin, Cmax, and AUC in pediatric and adult patients (solid circles) are compared with the medians (open circles) and 5th and 95th percentile ranges (horizontal bars) of the geometric means for exposures calculated from 1,000 simulations.
Model-based simulation to support ATV capsule dose recommendations for pediatrics.
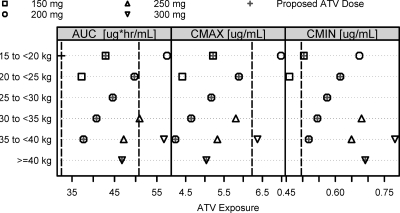
A series of ad hoc simulations were conducted to evaluate comprehensive dosing scenarios for RTV-boosted ATV capsules in HIV-infected pediatric patients in selected weight groups (≥15 kg, with 5-kg increments). The proposed weight-based pediatric ATV/RTV capsule doses are presented in Table 5. The body weight ranges in Table 5 were obtained by collapsing the weight ranges that had the same doses meeting the similarity criteria. Figure 4 presents comparisons of pediatric GMs with target values for each of the evaluated weight-based dose regimens. ATV/RTV at 200/100 mg is predicted to produce a sufficiently high Cmin for pediatric patients weighing <40 kg; however, this dose produces an excessively high Cmax and AUC for patients weighing <20 kg. Hence, ATV/RTV at 150/100 mg is proposed for children in the 15 to <20 kg weight group. We noted that ATV/RTV at 250/100 mg appeared to be a more appropriate dose than ATV/RTV at 200/100 mg for the 35 to <40 kg weight group, as it satisfied the similarity criteria while attaining higher exposures than ATV/RTV at 200/100 mg. However, this dose is not recommended because the 250 mg ATV dosage strength requires the use of two different capsule strengths (100 mg and 150 mg) and is therefore prone to dosing errors. Pediatric patients weighing ≥40 kg are recommended to take the adult, label-recommended dose. The percentage of pediatric subjects with Cmin, Cmax, and AUC values within the 10th and 90th percentiles of the adult values is either higher than or close to 75% for all the proposed ATV capsule doses (Table 5).
Table 5.
Summary of simulated ATV exposure measures at model-proposed pediatric ATV capsule doses boosted with RTV
| Body wt range (kg) | ATV/RTV dose (mg) |
Cmin |
Cmax |
AUC |
|||
|---|---|---|---|---|---|---|---|
| GM (ng/ml) | %a | GM (ng/ml) | %a | GM (ng·h/ml) | %a | ||
| 15 to <20 | 150/100 | 504 | 76.8 | 5,213 | 81.4 | 42,902 | 82.0 |
| 20 to <40 | 200/100 | 562 | 78.0 | 4,954 | 80.7 | 42,999 | 81.0 |
| ≥40 | 300/100 | 691 | 78.1 | 5,040 | 79.4 | 46,777 | 79.4 |
| Adult | 300/100 | 661 | 80 | 4,153 | 80 | 40,615 | 80 |
Percentage of pediatric subjects attaining exposures within the 10th and 90th percentiles of the target adult exposure at the adult label-recommended dose (ATV/RTV at 300/100 mg).
Fig. 4.
Geometric means of ATV exposures (AUC, Cmax, and Cmin). Model-based simulations were conducted to evaluate a comprehensive set of weight-tiered ATV capsule doses for pediatric patients weighing ≥15 kg to determine optimal RTV-boosted ATV capsule dose recommendations for pediatric patients. The proposed, weight-based ATV doses are labeled by crosses. The vertical dashed line in each panel represents the respective target adult exposure (80% and 125% of adult GM AUC, 150% of adult GM Cmax, and 75% of adult GM Cmin).
DISCUSSION
The present study illustrates the optimization of ATV/RTV capsule dose regimens for pediatric patients by utilizing M&S to bridge adult and pediatric data. The proposed ATV doses listed in Table 5 were recently approved by the European Union for use in HIV-infected pediatric patients of 6 years and older (4). These doses are predicted to achieve exposures in pediatric patients similar to those observed in adult patients receiving the recommended ATV/RTV dosing regimen (ATV/RTV at 300/100 mg once daily) that has been shown to be effective and safe. The extrapolation of efficacy from adults to pediatric patients based on similar systemic exposures was justified according to the International Conference on Harmonization (ICH) E11 guideline, in recognition that adults and children are similar in HIV pathophysiology and efficacy endpoint for drug treatment (19).
The final population PK model determined the effect magnitudes of several extrinsic and intrinsic factors on ATV PK parameters and provided adequate predictive performance of the key measures of ATV exposure (Cmin, Cmax, and AUC) that were used to evaluate and inform the pediatric dose recommendations. The population PK modeling approach (C0-delinked model) accounts for a potential lack of adherence to drug dosing recommendations by disassociating the predose concentration (which could be impacted by a lack of adherence to prior doses) from the estimation of compartmental PK model parameters. The C0-delinked model isolates the variation in exposures due to nonadherence to the C0 parameter, thereby allowing for more accurate estimation of the compartmental PK parameters (i.e., ka, V/F, and CL/F) following the known dosing time and amount of the most recent dose recorded in the clinic. Essentially, variation in C0 can be thought of as a parameter that accounts for nonadherence without requiring any imputation or additional assumptions regarding the unknown dosing history prior to the predose sample. The extent of nonadherence, quantified as the ratio of C0 under nonadherence versus full adherence, was assessed for typical individuals (body weights of 17.5, 30, and 55 kg) given the proposed, weight-based ATV/RTV capsule doses (150, 200, and 300 mg ATV, respectively, boosted with 100 mg RTV). C0 values under nonadherence were estimated to be 336, 448, and 672 ng/ml, respectively, and the corresponding C0 ratios were 65.8, 83.8, and 110%. The results suggest that higher ATV exposures translate to improved patient adherence.
To optimally balance the benefit and risk, ATV capsule dose selection for pediatric patients was targeted to achieve a GM AUC within 80 to 125% of the GM AUC in adults, while keeping the GM Cmin at >500 ng/ml (approximately 75% of the adult GM Cmin) and the GM Cmax at <6,230 ng/ml (150% of the adult GM Cmax). The determination of target exposures was supported by the efficacy and safety data observed in adult ATV clinical trials. Exposure-response analysis of data from adult studies AI424-089 and AI424-138 (ClinicalTrials.gov identifier NCT00272779) suggested that patients who achieved a Cmin of >130 ng/ml were predicted to have a >90% probability of achieving an HIV RNA reduction to <400 copies/ml and a >75% probability of achieving an HIV RNA reduction to <50 copies/ml at week 48 (2, 21). The selected target for the ATV GM Cmin (500 ng/ml) is anticipated to result in ∼90% of pediatric patients achieving a Cmin of >130 ng/ml at the proposed ATV doses. With regard to safety, it was found that Cmax-dependent asymptomatic PR prolongation was more frequent in pediatric patients than in adults. Pediatric subjects who reached a Cmax of 7,000 ng/ml or above may present with PR prolongation, mostly borderline, at higher rates than subjects with a lower Cmax (42% versus 14% of 101 pediatric subjects). Given that the PR prolongations were asymptomatic, even in pediatric subjects with Cmax values as high as 20,000 ng/ml, the ATV GM Cmax of <150% of the adult value was determined as the target for Cmax which corresponds to ∼90% of pediatric patients having a Cmax of <16,000 ng/ml at the proposed ATV doses. Since the peak-to-trough concentration ratios in pediatric patients are much more pronounced than they are in adults, the higher Cmax values in pediatric patients are inevitable in order to maintain an adequate Cmin to support efficacy. The proposed ATV pediatric doses strike a balance between Cmin and Cmax exposures so as to achieve an AUC similar to that in adults.
ATV capsule doses of 150 to 300 mg boosted with 100 mg RTV were recommended for ART-naïve or -experienced patients weighing ≥15 kg. The proposed weight-based ATV capsule doses were compared with the protocol-specified BSA-based dose (205 mg/m2) (3) that was administered in the PACTG1020 pediatric study. The results indicated that these weight-tiered ATV doses were similar to BSA-based doses except for the weight group of individuals weighing ≥40 kg, where the BSA-based dose was higher than the weight-based dose (data not shown). This is related to the fact that the patients in the PACTG1020 study were allowed to receive ATV doses higher than the label-approved 300 mg (with RTV boosting), while the weight-based doses proposed from model-based analysis were capped at the 300 mg adult dose. In addition, the model-based ATV dose recommendations were compared with the ATV pediatric doses currently approved in the United States and some other countries. The ATV dose regimens proposed by this model-based approach have the following advantages: (i) uniform dose recommendations for ART-naïve and -experienced patients; (ii) recommendation of an ATV capsule dose for ART-experienced patients weighing as little as 15 kg (according to the CDC growth chart [6], the median body weight for children at age 6 years who are able to swallow capsules is approximately 20 kg); and (iii) elimination of potential dosing errors resulting from the 250-mg dose, which requires the use of ATV capsules at two different strengths.
The RTV doses administered in the PACTG1020 study ranged from 26 mg to 100 mg. An ad hoc analysis was conducted to investigate whether the RTV dose explained additional variation in CL/F beyond the covariate-CL/F relationships determined in the final model (data not shown). A CL/F submodel with the covariate effects of body weight, RTV comedication (dichotomous effect due to the presence or absence of RTV), region (African), and sex was expanded to include the RTV dose effect. The submodel was fit to the individual post hoc CL/F estimates. The change in OFV (ΔOFV = 0.118) between the submodels with and without the RTV dose effect suggested that RTV dose explained little additional variation relative to the dichotomous RTV comedication effect after adjusting for the other covariate effects that were included in the final model. In the context of this analysis, we concluded that there were insufficient data to substantiate the identification of the optimal RTV dose for each weight group. As a result, the protocol-adopted RTV regimen (100 mg RTV capsule) was proposed in combination with ATV capsules for pediatric patients.
In conclusion, the population PK model developed in this analysis quantified the magnitudes of covariate effects on ATV PK and quantitatively described ATV PK in adult and pediatric patients. The use of model-based simulation in conjunction with the PK bridging strategy enabled optimization of ATV dosing regimens for pediatric patients. Future work will apply this modeling framework to the ATV powder formulation, which is currently being evaluated in HIV-infected pediatric patients younger than 6 years of age.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the pediatric patients and their families for participation in the PACTG1020 pediatric study. We acknowledge Jennifer J. Kiser, Richard M. Rutstein, Pearl Samson, Bobbie Graham, Grace Aldrovandi, Lynne M. Mofenson, Elizabeth Smith, Courtney V. Fletcher, and the PACTG1020 study team for providing data.
This analysis was sponsored by Bristol-Myers Squibb.
Kenneth G. Kowalski was a paid consultant to Bristol-Myers Squibb.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Anderson B. J., Holford N. H. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303–332 [DOI] [PubMed] [Google Scholar]
- 2. Bertz R., et al. 2007. Assessment of PK/PD relationships through 48 weeks from a study in HIV+, ARV-naïve subjects receiving antiretroviral regimens containing atazanavir 400 mg or atazanavir/ritonavir 300/100 mg once daily, poster 565. Abstr. 14th Conf. Retrovir. Opportunistic Infect., Los Angeles, CA [Google Scholar]
- 3. Bristol-Myers Squibb February 2011. Reyataz® (atazanavir sulfate) capsules: US prescribing information. Bristol-Myers Squibb, Princeton, NJ: http://packageinserts.bms.com/pi/pi_reyataz.pdf [Google Scholar]
- 4. Bristol-Myers Squibb 2011. Reyataz® (atazanavir sulfate) capsules: summary of product characteristics. Bristol-Myers Squibb, Princeton, NJ: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000494/WC500056380.pdf [Google Scholar]
- 5. Cella M., Gorter de Vries F., Burger D., Danhof M., Della Pasqua O. 2010. A model-based approach to dose selection in early pediatric development. Clin. Pharmacol. Ther. 87:294–302 [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2000. Centers for Disease Control and Prevention (CDC) growth charts. CDC, Atlanta, GA: http://www.cdc.gov/growthcharts/data/zscore/wtage.xls [Google Scholar]
- 7. Colombo S., et al. 2006. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 50:3801–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency 2006. Guideline on the role of pharmacokinetics in the development of medicinal products in the paediatric population. European Medicines Agency, London, United Kingdom: http://www.pharmaceutical-medicine.org/document/session3/paediiatrics_pk _guideline.pdf [Google Scholar]
- 9. Fuster D., Clotet B. 2005. Review of atazanavir: a novel HIV protease inhibitor. Expert Opin. Pharmacother. 6:1565–1572 [DOI] [PubMed] [Google Scholar]
- 10. Gupta P., Hutmacher M. M., Frame B., Miller R. 2008. An alternative method for population pharmacokinetic data analysis under noncompliance. J. Pharmacokinet. Pharmacodyn. 35:219–233 [DOI] [PubMed] [Google Scholar]
- 11. Holford N. H. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329–332 [DOI] [PubMed] [Google Scholar]
- 12. Jadhav P. R., Zhang J., Gobburu J. V. 2009. Leveraging prior quantitative knowledge in guiding pediatric drug development: a case study. Pharm. Stat. 8:216–224 [DOI] [PubMed] [Google Scholar]
- 13. Karlsson M. O., Lutsar I., Milligan P. A. 2009. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 53:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kowalski K. G., Hutmacher M. M. 2001. Efficient screening of covariates in population models using Wald's approximation to the likelihood ratio test. J. Pharmacokinet. Pharmacodyn. 28:253–275 [DOI] [PubMed] [Google Scholar]
- 15. Le Tiec C., Barrail A., Goujard C., Taburet A. M. 2005. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharmacokinet. 44:1035–1050 [DOI] [PubMed] [Google Scholar]
- 16. Pérez-Elías M. J. 2007. Atazanavir: simplicity and convenience in different scenarios. Expert Opin. Pharmacother. 8:689–700 [DOI] [PubMed] [Google Scholar]
- 17. Schuster A., et al. 2003. Quantitative determination of the HIV protease inhibitor atazanavir (BMS-232632) in human plasma by liquid chromatography-tandem mass spectrometry following automated solid-phase extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 788:377–386 [DOI] [PubMed] [Google Scholar]
- 18. Soy D., Beal S. L., Sheiner L. B. 2004. Population one-compartment pharmacokinetic analysis with missing dosage data. Clin. Pharmacol. Ther. 76:441–451 [DOI] [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration 2000. Guidance for industry. E11 clinical investigation of medicinal products in the pediatric population. U.S. Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129477.pdf [Google Scholar]
- 20. Yano Y., Beal S. L., Sheiner L. B. 2001. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J. Pharmacokinet. Pharmacodyn. 28:171–192 [DOI] [PubMed] [Google Scholar]
- 21. Zhu L., et al. 2009. Exploration of PK/PD relationships of boosted atazanavir and lopinavir over 48 weeks in HIV-infected treatment naïve patients: CASTLE Study, poster 19. Abstr. 10th Int. Workshop Clin. Pharmacol., HIV Therapy, Amsterdam, The Netherlands [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.