Abstract
The pharmacokinetics, oral bioavailability, and ex vivo antimalarial activity of mirincamycin isomers in a healthy rhesus monkey model were assessed to support lead optimization of novel nonhemolytic drugs for radical cure and causal prophylaxis of malaria. Fourteen male rhesus monkeys were randomized to four groups, which included cis and trans isomers by the oral and intravenous routes, with vehicle-only controls for each dosing route. Concentration-time data were collected for 7 days and were analyzed by noncompartmental analysis. cis-Mirincamycin had an absolute oral bioavailability of 13.6%, which was slightly higher than that of trans-mirincamycin (11.7%), but this difference was not statistically significant. There was a statistically significant difference between the area under the concentration-time curve from zero to 48 h (AUC0–48) of cis-mirincamycin and that of trans-mirincamycin after oral dosing. When cultured in vitro with the W2 clone of Plasmodium falciparum, the 50% inhibitory concentrations for cis-mirincamycin, trans-mirincamycin, and dihydroartemisinin were 11,300, 12,300, and 2.30 nM, respectively. However, when dosed primate plasma was cultured ex vivo against the W2 clone, both isomers had much greater relative potencies than their in vitro activities relative to results for dihydroartemisinin, an increase of approximately 100-fold for the cis isomer and 150-fold for the trans isomer. Further, oral ex vivo activity was significantly higher than intravenous activity for both isomers, particularly during the first 90 min following dosing, suggesting the first-pass formation of one or more metabolites with blood-stage antimalarial activity. Identification of the metabolic pathways and metabolites may help to further delineate the properties of this class of drugs with previously demonstrated liver-stage antimalarial activity.
INTRODUCTION
The U.S. Army antimalarial drug development program is currently focusing on development of drugs able to eliminate the merozoite and hypnozoite forms of liver-stage malaria parasites that could serve, respectively, as agents for causal (liver-stage) antimalarial chemoprophylaxis or the “radical cure” of blood- and liver-stage Plasmodium vivax. However, the discovery of novel liver-stage agents without hemolytic liability in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency lags far behind the development of safe, effective blood-stage agents due to limitations in preclinical in vitro and in vivo models (22).
Mirincamycin was investigated as a promising lead candidate as a causal prophylactic and/or radical curative antimalarial drug. Both Powers and Schmidt et al. showed that mirincamycin, a lincosamide antibiotic, administered orally as a single agent had intrinsic radical cure and causal prophylactic activity in the rhesus monkey Plasmodium cynomolgi model (13, 16). When administered orally in a causal prophylaxis model, clindamycin did not completely protect any animal up to a dose of 80 mg/kg of body weight/day for 9 days, while mirincamycin protected 3 of 6 animals at 40 mg/kg/day for 9 days. Mirincamycin was likewise superior as a radical cure agent in this model; while clindamycin did not cure any animal at up to 80 mg/kg/day for 7 days, mirincamycin cured 2 of 3 animals at 40 mg/kg/day for 7 days. A dose-related delay in parasite patency occurred with both drugs for both indications. In 1985, Schmidt showed that 2.5 mg/kg mirincamycin, 0.375 mg/kg primaquine, and 2.5 mg/kg chloroquine for 7 days potentiated the efficacy of primaquine's antihypnozoite activity following an inoculum of 8.5 × 103 sporozoites of Plasmodium cynomolgi (15).
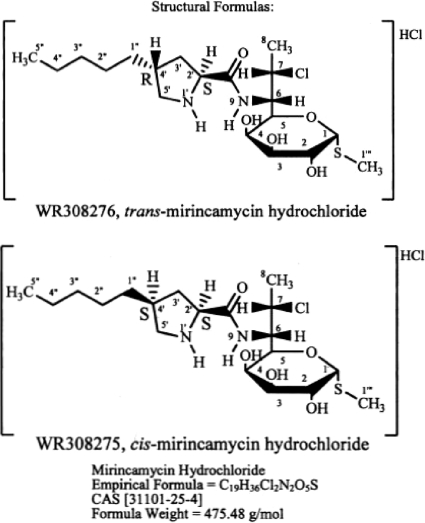
Mirincamycin is a mixture of stereoisomers, cis-mirincamycin and trans-mirincamycin (Fig. 1). Apparent causal prophylactic activity of both isomers was demonstrated in Plasmodium berghei-infected mice at subcutaneous doses of 1.1, 3.3, 10, and 40 mg/kg/day for 3 days (2, 3). Mirincamycin isomers were also found to be safe and to have acceptable pharmacokinetic parameters in rodent models. The pharmacokinetic and ex vivo blood-stage antimalarial pharmacodynamic relationships of the two isomers were evaluated in a healthy rhesus monkey model. This was one of a series of experiments performed in support of the U.S. Army and the Medicines for Malaria Venture's shared critical path to develop new treatments for relapsing malaria.
Fig. 1.
Chemical structures of cis-mirincamycin and trans-mirincamycin.
MATERIALS AND METHODS
Animals.
Fourteen male Indian-origin rhesus monkeys (Macaca mulatta) were studied. Monkeys were randomized to 4 parallel groups of 3 animals (cis isomer, oral [p.o.] and intravenous [i.v.] administration; trans isomer, p.o. and i.v.), with an additional control animal in each dosing-route group (vehicle only). Each animal was dosed, and plasma samples were collected into heparin-coated syringes at 0, 15, 30, and 60 min, 2, 4, 6, 8, 12, and 24 h, and 2 and 7 days, with an additional specimen collected at 5 min for animals dosed i.v. All plasma samples were frozen at −80°C before analysis by liquid chromatography/mass spectrometry (LC/MS). All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committees of AFRIMS and the Walter Reed Army Institute of Research (WRAIR).
Drugs.
Mirincamycin synthesis has been described previously (7). Mirincamycin isomers were synthesized by Bridge Organics Co. (Vicksburg, MI) and provided to the WRAIR by Maldevco, Inc. Separation and purity of the isomers were confirmed by SRI International using infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy, high-performance liquid chromatography (HPLC), and mass spectrometry. Both cis-mirincamycin (optical rotation, +148.69°; c = 1.011, H2O) and trans-mirincamycin (optical rotation, +144.99°; c = 0.6867, H2O) were dissolved in 0.45% saline at 5 mg/ml for intravenous and oral administration, and 0.45% saline was used as a control for each dosing route. Dihydroartemisinin (DHA) powder for in vitro and in vivo studies was synthesized by Starks Associates, Buffalo, NY, and provided to WRAIR.
Sample preparation.
Mirincamycin isomers in plasma samples were extracted by protein precipitation. Briefly, a 100-μl plasma sample was added into 200 μl of cold acetonitrile and mixed vigorously for 60 s. Then, the mixture was centrifuged at 3,000 × g for 10 min, and the supernatant was collected into a tube for LC/MS analysis.
LC/MS analysis of biological samples.
Mirincamycin in extracted samples was separated by a liquid chromatography system (Waters 2695 Separations; Waters Corp., Massachusetts) equipped with an XTerra MS C18 column. Chromatographic resolution was obtained by running a 6-min acetonitrile–0.1% (vol/vol) formic acid gradient from 20 to 78% acetonitrile within 3 min and held for 3 min at a 0.6-ml/min flow rate. Both isomers eluted at 3.8 min at the same m/z ratio of 439. The mass of both isomers was acquired on a single-quadrupole mass spectrometer (Waters Micromass ZQ; Waters Corp., Massachusetts), operated using electrospray ionization in positive ion mode. The voltages applied to both isomers for the cone, capillary, extractor, and radio frequency (RF) lens were 30 V, 2 kV, 2 V, and 0.5 V, respectively. The source cone and desolvation temperatures were set at 20°C and 350°C, respectively. Nitrogen gas was produced by a nitrogen generator, and the gas flows were applied at 40 liters/h for cone and 450 liters/h for desolvation. The gas cell Pirani pressure was <10−4 mbar. The data were recorded in the single-ion recording mode. The intra-assay accuracy and precision for analyzing cis-mirincamycin at 1.80 (lower limit of quantitation [LLOQ]), 3.61, and 923 μg/liter were within ±12.1%, and the coefficients of variation were less than 14%. For trans-mirincamycin, the intra-assay accuracy and precision at 1.95 (LLOQ), 3.91, and 1,000 μg/liter were within ±18.1% and the coefficients of variation were less than 6.4%. The interassay accuracy and precision for cis-mirincamycin and trans-mirincamycin at the same three concentrations (15.6, 125, and 500 μg/liter) tested were within ±5.11 and ±11.2% error, and coefficients of variation were <4.8% and <8.5%, respectively. Both cis- and trans-mirincamycin were stable in plasma (15.6 and 500 μg/liter) at room temperature (25°C) for up to 4 and 6 h, with the percent difference from time zero within ±11.2% and ±12.8%, respectively.
Measurement of antimalarial activity.
In vitro drug susceptibility was measured by incubating serial dilutions of mirincamycin isomers with a W2 Plasmodium falciparum clone for 72 h using a nonisotopic histidine-rich protein 2 (HRP2) enzyme-linked immunosorbent assay (ELISA) method (11). The Plasmodium falciparum-based bioassay for the measurement of antimalarial activity in plasma has been described previously (19). Briefly, by modifying a method for an in vitro drug susceptibility test, the antimalarial activity in plasma containing an unknown concentration of drug can be equated to known concentrations of DHA required to inhibit parasite growth. Primate plasma at each pharmacokinetic time point from animals dosed with cis- or trans-mirincamycin by each dosing route was incubated with a W2 P. falciparum clone, and growth inhibition was measured using a nonisotopic HRP2 ELISA method (12). Growth inhibition activity of primate plasma ex vivo was compared to a standard curve generated for DHA as a reference control. Relative potency was determined by comparing both in vitro and ex vivo pharmacodynamic area under the concentration-time curve from zero to 12 h (AUC0–12) and maximum drug concentration (Cmax) measurements with corresponding pharmacokinetic measurements.
Data analysis.
Concentration-time data collected during 48 h after dosing were analyzed by noncompartmental analysis using PK Solutions 2.0 software (Summit Research Services, Montrose, CO). Comparisons of pharmacokinetic data between experimental groups were made by using the Mann-Whitney U test, with P < 0.05 used as a criterion for statistical significance. Absolute bioavailability was calculated using the following formula for each isomer: F = 100 × (AUCp.o. × dosei.v.)/(AUCi.v. × dosep.o.).
RESULTS
Pharmacokinetics.
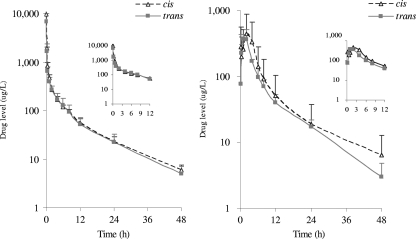
It was found that the plasma concentrations of mirincamycin isomers decreased rapidly for the first 30 min following i.v. dosing, while oral plasma concentrations increased gradually and peaked at 0.5 to 1 h after dosing (Fig. 2). cis-Mirincamycin had an absolute oral bioavailability of 13.6%, which was slightly higher than that of trans-mirincamycin (11.7%), but this difference was not statistically significant (Table 1). There was a statistically significant difference between the AUC0–48 of cis-mirincamycin and that of trans-mirincamycin after oral dosing (30% higher for cis), but this was not the case for the Cmax. There were not significant differences in tissue distribution between the two isomers, with mean adjusted volume of distribution (V/F) of 10 liters/kg by the oral route and 15 to 20 liters/kg in intravenous groups. Clearance was slow, with a mean terminal elimination half-life of 9 to 15 h depending on the route and isomer; concentrations were detectable at up to 48 h. The half-life by the oral route was longer for cis-mirincamycin than for trans-mirincamycin, and cis-mirincamycin had a slower clearance than trans-mirincamycin. No biologically significant differences between isomers were observed in terms of pharmacokinetics or toxicity.
Fig. 2.
Plasma concentration-time courses of intravenous (left panel) or oral (right panel) cis-mirincamycin (triangle) and trans-mirincamycin (rectangle) after dosing for 48 h. Each time point represents the mean value for three animals.
Table 1.
Pharmacokinetic parameters of cis-mirincamycin and trans-mirincamycin after intravenous and oral dosing in healthy rhesus monkeysh
| Parameter | Value for drug and dosing |
|||
|---|---|---|---|---|
|
cis-Mirincamycin |
trans-Mirincamycin |
|||
| i.v. (4 mg/kg) | p.o. (20 mg/kg) | i.v. (4 mg/kg) | p.o. (20 mg/kg) | |
| AUC0–48 (μg·h/liter)a | 4,597 ± 666 | 3,050 ± 198* | 3,998 ± 1,404 | 2,347 ± 481 |
| AUC0–∞ (μg·h/liter)b | 4,705 ± 684 | 3,193 ± 222* | 4,082 ± 1,426 | 2,389 ± 504 |
| Cmax (μg/liter)c | 9,693 ± 1,690 | 554 ± 161 | 6,890 ± 3,156 | 430 ± 156 |
| Tmax (h)d | NA | 2.08 ± 1.88 | NA | 0.50 ± 0.00 |
| V/F (liters/kg)e | 15.0 ± 3.30 | 10.1 ± 1.77 | 18.4 ± 8.82 | 10.2 ± 2.28 |
| t1/2 (h)f | 12.3 ± 1.41 | 15.4 ± 2.13* | 11.5 ± 1.37 | 9.43 ± 0.28 |
| Cl/F (liters/h/kg)g | 0.84 ± 0.12 | 0.46 ± 0.03* | 1.08 ± 0.44 | 0.76 ± 0.18 |
| Absolute bioavailability (%) | 100 | 13.6 | 100 | 11.7 |
Area under curve from time zero to 48 h.
Area under curve from time zero to infinity.
Maximum concentration.
Time to maximum concentration (not applicable [NA] for iv infusions).
Adjusted volume of distribution.
Terminal elimination half-life.
Adjusted clearance.
Data are mean value for three animals. *, statistical significance between isomers (P < 0.05).
Toxicity.
Acute toxicity was limited to loose stools of 1 to 3 days' duration following a single oral dose with the cis isomer and 1 to 2 days with the trans isomer. There were mild transient elevations in alanine transaminase (ALT); 1 of 6 animals experienced a 30% rise above the upper limit of normal (ULN) in the cis group, while the control animal had a 10% rise. For aspartate transaminase (AST), 3 animals in each of the cis and trans groups had a rise of 1.5× to 2× ULN at 12 h; however, control animals had similar elevations. All elevations resolved within 48 h after dosing.
Pharmacodynamics.
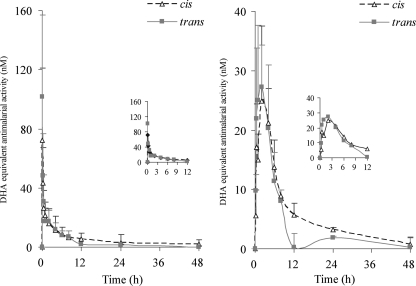
In the in vitro system, the 50% inhibitory concentrations (IC50s) of cis-mirincamycin, trans-mirincamycin, and DHA against W2 clones expressing HRP2 were 11,300, 12,300, and 2.30 nM, respectively, after 72 h of incubation (Table 2). In the ex vivo system, both isomers showed peak antimalarial activity against W2 clones of Plasmodium falciparum 1 h following oral dosing and 5 to 15 min following intravenous dosing (Fig. 3). There were no statistically significant differences in ex vivo antimalarial activity between the two isomers. While DHA was approximately 5,000-fold more potent against P. falciparum than mirincamycin in the in vitro model, the ex vivo potency of both isomers increased dramatically relative to the in vitro DHA reference measurements, with an approximate 100-fold increase for cis and 150-fold for trans (Table 3). Further, oral ex vivo activity was significantly higher than intravenous activity for both isomers, particularly during the first 90 min following dosing, suggesting the first-pass formation of one or more metabolites with blood-stage antimalarial activity. cis-Mirincamycin given orally was approximately 2-fold more potent ex vivo than in vitro against P. falciparum compared to the i.v. route in a Cmax and AUC0–12 comparison. This difference was slightly less pronounced with trans-mirincamycin, which was only 1.2× more potent at Cmax and 1.9× more potent by AUC comparison.
Table 2.
Relative in vitro potencies of cis-mirincamycin and trans-mirincamycin compared to that of DHA against W2 clones of P. falciparum
| Agent | IC50 (nM) | Relative potencya |
|---|---|---|
| Dihydroartemisinin | 2.30 | 1.00 |
| cis-Mirincamycin | 11,300 | 2.03 × 10−4 |
| trans-Mirincamycin | 12,300 | 1.87 × 10−4 |
Relative potencies are reported as DHA equivalent values.
Fig. 3.
Pharmacodynamic profiles expressed as DHA equivalents (nM) after subtracting baseline activity of cis-mirincamycin (triangle) and trans-mirincamycin (rectangle) following intravenous 4-mg/kg (left panel) and oral 20-mg/kg (right panel) dosing. Each time point represents the mean value for three animals.
Table 3.
Ex vivo pharmacodynamic activities of cis-mirincamycin and trans-mirincamycin against W2 clones of Plasmodium falciparumg
| Agent | Emaxa (nM) | Cmaxb (nM) | PD/PK relative potencyc | Ex vivo/in vitro potency by Emax | AUC0–12 PDd (nM·h) | AUC0–12 PKe (nM·h) | PD/PK relative potencyf | Ex vivo/in vitro potency by AUC |
|---|---|---|---|---|---|---|---|---|
| cis-Mirincamycin (intravenous) | 178 | 22,100 | 8.06 × 10−3 | 39.7 | 145 | 8,530 | 1.70 × 10−2 | 83.7 |
| cis-Mirincamycin (oral) | 27.7 | 1,260 | 2.19 × 10−2 | 108 | 164 | 5,300 | 3.10 × 10−2 | 153 |
| trans-Mirincamycin (intravenous) | 406 | 15,700 | 2.59 × 10−2 | 139 | 144 | 7,410 | 1.94 × 10−2 | 104 |
| trans-Mirincamycin (oral) | 29.8 | 980 | 3.04 × 10−2 | 163 | 149 | 4,010 | 3.72 × 10−2 | 199 |
Maximum ex vivo antimalarial activity expressed in DHA equivalents.
Maximum concentration of mirincamycin from pharmacokinetic study.
Emax divided by Cmax. PD, pharmacodynamic; PK, pharmacokinetic.
Area under curve of ex vivo antimalarial activity expressed as DHA equivalents.
Area under curve of mirincamycin from pharmacokinetic study.
AUCPD divided by AUCPK.
Data are mean values for three monkeys.
DISCUSSION
Mirincamycin is a member of the lincosamide antibacterial class, and in addition to mirincamycin, other members of this class have been found to have antimalarial properties, particularly clindamycin (14, 18). We have demonstrated that in the rhesus monkey model, both isomers of mirincamycin have low absolute oral bioavailability, which is similar to that seen with lincomycin (∼20%) but substantially lower than that of clindamycin, which was previously shown to have 90% bioavailability in humans (9). The time to maximum concentration (Tmax) of both isomers after oral dosing is approximately 0.5 to 2 h, which is not substantially different from those of other lincosamides. Both isomers of mirincamycin have high apparent volumes of distribution, suggesting extensive tissue distribution. Our results correlate well with those of a previous report, where the pharmacokinetic data following a single 150-mg/kg dose of mirincamycin in human subjects could not be fitted to a single-compartment model due to the large volume of distribution (10).
We found that the elimination half-life of both isomers varied between 10 and 15 h, which is similar to rodent data reported from WRAIR in 2008 (2). In contrast, pharmacokinetic studies of mirincamycin mixtures in the 1970s and 1980s reported a half-life of 2 to 3 h in both mice and monkeys (20). This discrepancy is thought to be due to the availability of more-sensitive assay methods incorporating LC/MS analysis. The higher method sensitivity and longer period of sample collection in this study may have resulted in a longer apparent terminal elimination half-life in our pharmacokinetic analysis and also enabled a better understanding of metabolism in vivo (8). trans-Mirincamycin had a shorter half-life and more-rapid clearance than cis-mirincamycin by the oral route. The lower AUC and bioavailability of trans-mirincamycin may have resulted from faster metabolism and/or clearance compared to results for cis-mirincamycin. Several reports have determined that the major metabolic pathway of lincosamides is via hepatic phase I biotransformation enzymes (5). Wynalda reported that CYP3A4 is the major enzyme responsible for degradation of clindamycin and CYP3A5 is a minor pathway (24). Two major metabolites, clindamycin sulfoxide and n-desmethyl clindamycin, are formed following hepatic transformation with antimicrobial activity against Helicobacter pylori (23). Therefore, it is possible that metabolic transformation of mirincamycin also occurs via this enzyme superfamily, which may be responsible for generating active metabolites with increased antimalarial activity. However, current understanding is limited, and it is thought that all lincosamides are metabolized by multiple enzymatic pathways, so the major routes of metabolism and excretion remain poorly understood.
Among lincosamide derivatives, it is likely that lincomycin is the most potent drug against both the D6 and W2 clones of Plasmodium falciparum in vitro (10). However, lincomycin does not appear to be active in animal models of malaria (6). Meanwhile, clindamycin demonstrated antimalarial efficacy in the mouse model but not in the primate P. cynomolgi model, while mirincamycin showed efficacy in both the mouse and primate models (2). In the present experiment, dosed monkey plasma with both mirincamycin isomers showed antimalarial activity against the W2 P. falciparum clone ex vivo, with slightly greater pharmacodynamic activity and relative potency observed with cis-mirincamycin. A recent report by Held (4) found that the cis-mirincamycin isomer had a slightly higher median 50% inhibitory concentration (IC50) than the trans isomer against clinical isolates of Plasmodium falciparum from Gabon. Of note, both mirincamycin isomers appeared to be much more potent using a prolonged 6-day incubation method. However, this could be explained by differences in technical methods related to the in vitro assay. As previously reported (19), the method we used here may not be optimal for drugs with relatively low potencies due to limits of detection. Longer incubations with low-potency drugs against malaria have shown increased potency at 96 h and beyond when 2 life cycles are covered. A recent study by Wein et al. (21) suggested that drug mechanism of action may have a significant influence on the outcomes and reliability of the HRP2 in vitro method.
There was greater relative potency by the oral route than by the i.v. route for both cis and trans isomers at Cmax and over the first 90 min after dosing, suggesting hepatic biotransformation leading to active metabolites. However, this difference diminished over the course of dosing, with only a 2-fold difference by AUC0–12 for cis and trans. While it is possible that low absorption may contribute to the low apparent oral bioavailability of mirincamycin, it is likely that first-pass metabolism with production of metabolites unmeasured in this experiment may also have been an important factor in the apparently low exposure measured. Despite the potential formation of active metabolites and greater P. falciparum activity ex vivo, it should be noted that mirincamycin's blood-stage activity is quite modest compared to that of DHA and other antimalarials in the ex vivo bioassay. Although the pharmacodynamic experiment here does not provide direct evidence of liver-stage antimalarial activity, it supports the likelihood that previously demonstrated antimalarial efficacy of mirincamycin in primates (15) is attributable to liver-stage activity given very low observed activity against the blood stage in vitro and ex vivo in this experiment. However, clindamycin is known to have blood-stage activity both in vitro and in vivo (18), so it is unclear whether liver-stage activity is predominant across this class. The healthy rhesus monkey model allows efficient screening of pharmacokinetic parameters, and efficient preliminary pharmacokinetic-pharmacodynamic assessments to optimize experiments in the rhesus monkey relapsing P. cynomolgi model, which will be reported in a future experiment.
The potential for differential toxicities and/or improvements in efficacies of isomers over racemates are of interest and a proven strategy for improving the therapeutic index of drugs. Schmidt et al. previously demonstrated reduced toxicity and a substantial increase in the therapeutic index of the d-primaquine isomer over the l form, with no reduction in therapeutic efficacy compared to that of the racemate (17). The most common adverse events related to lincosamide derivatives are gastrointestinal disturbances, especially acute diarrhea (5). Both isomers caused transient loose stools in this experiment after a single dose, with a nonsignificantly longer duration (2 to 3 days) among animals treated with the cis isomer. None of the animals suffered from prolonged diarrhea, nor was there any evidence of Clostridium difficile-associated colitis, which represents the greatest potential liability with this class. There were no clinically significant changes in hematological parameters or kidney function after a single dose of either isomer, but there were non-clinically significant elevations in AST and ALT, which were also observed in vehicle-only control animals. These physiologic responses in primates during experimental studies have been observed previously (1).
In conclusion, cis-mirincamycin had a slightly more favorable pharmacokinetic profile than trans-mirincamycin, and both compounds had ex vivo P. falciparum blood-stage activity that, while modest compared to that of DHA, increased dramatically compared to in vitro activity. The longer exposure and smaller clearance of cis-mirincamycin may result in higher antimalarial activity in vivo. This will be explored in upcoming efficacy experiments using a rhesus monkey Plasmodium cynomolgi relapsing malaria model. The low apparent bioavailability of both isomers may have resulted from first-pass metabolism and production of active metabolites. Identification of metabolic pathways and potential active metabolites will be useful to better understand the antimalarial properties of this drug class and develop analogues to support future drug development efforts to further optimize the mirincamycin lead compound.
ACKNOWLEDGMENTS
Raveewan Siripokasupkul, Roongnapha Apinan, and Kuntida Tangthongchaiwiriya of the AFRIMS Department of Immunology and Medicine performed laboratory pharmacokinetic and pharmacodynamic analysis; Montip Gettyacamin and Yvonne van Gessel of the AFRIMS Department of Veterinary Medicine managed the primate colony and developed standard operating procedures. Ian Bathurst and Carl Kraft of MMV provided resources, program direction, and oversight of this project. Richard Westerman of Maldevco, Inc., provided mirincamycin isomers synthesized by Bridge Organics (Vicksburg, MS). Compound purity was certified by Peter Lim, Ronald Spanggord, Patrick Mcauley, and Jennifer Wang of SRI International. Bryan Smith, Qiqui Li, Mike Kozar, and Geoff Dow provided consultation as part of the WRAIR Integrated Project Team.
This study was supported by the U.S. Army Research and Materiel Command and the Medicines for Malaria Venture (MMV).
The opinions or assertions contained herein are the views of the authors and are not to be construed as official or as reflecting the true views of the Department of the Army or Department of Defense.
Footnotes
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Fortman J., Hewett T., Bennett B. 2001. The laboratory nonhuman primate, p 17–19 CRC Press, Boca Raton, FL [Google Scholar]
- 2. Fracisco S., et al. 2009. Mirincamycin: reassessment of a promising antimalarial agent with potential in a P. cynomolgi relapsing malaria monkey model, abstr. ASTMH09-298. Abstr. 58th Annu. Meet. Am. Soc. Trop. Med. Hygiene. [Google Scholar]
- 3. Fracisco S., Gettayacamin Y. R. M., Westerman R., Ohrt C. 2008. Anti-malarial activity of mirincamycin and its analogs in vitro and in an in vivo presumptive causal prophylactic mouse model, abstr. ASTMH08-758. Abstr. 57th Annu. Meet. Am. Soc. Trop. Med. Hygiene. [Google Scholar]
- 4. Held J., Westerman R., Kremsner P., Mordmuller B. 2010. In vitro activity of mirincamycin (U24729A) against Plasmodium falciparum isolates from Gabon. Antimicrob. Agents Chemother. 54:540–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacy C., Armstrong L., Goldman M., Lance L. 2008. Drug information handbook, 17th ed., p. 354–357 Lexi-Comp Information Management System, Hudson, OH [Google Scholar]
- 6. Lewis C. 1968. Antiplasmodial activity of 7-halogenated lincomycins. J. Parasitol. 54:169–170 [PubMed] [Google Scholar]
- 7. Magerlein B. J. 1972. Lincomycin. 14. An improved synthesis and resolution of the antimalarial agent, 1′-demethyl-4′-depropyl-4′-(R)- and -(S)-pentylclindamycin hydrochloride (U-24, 729A). J. Med. Chem. 15(12):1255–1259 [DOI] [PubMed] [Google Scholar]
- 8. Mahmood I. 2007. Application of allometric principles for the prediction of pharmacokinetics in human and veterinary drug development. Adv. Drug Deliv. Rev. 59:1177–1192 [DOI] [PubMed] [Google Scholar]
- 9. McEvoy G., Litvak K., Welsh O., Snow E. 1999. AHFS drug information. American Society of Health-System Pharmacists, Bethesda, MD [Google Scholar]
- 10. Medicines for Malaria Venture 2008. Mirincamycin Collaborative Project, MMV08/0084 2008. Walter Reed Army Institute of Research, Silver Spring, MD [Google Scholar]
- 11. Noedl H., et al. 2006. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am. J. Trop. Med. Hyg. 75:1205–1208 [PubMed] [Google Scholar]
- 12. Noedl H., Teja-Isavadharm P., Miller R. 2004. Nonisotopic, semi-automated Plasmodium falciparum bioassay for measurement of antimalarial drug levels in serum or plasma. Antimicrob. Agents Chemother. 48:4485–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Powers K. 1969. Activity of chlorinated lincomycin analogues against Plasmodium cynomolgi in rhesus monkeys. Am. J. Trop. Med. Hyg. 18:458–490 [DOI] [PubMed] [Google Scholar]
- 14. Powers K., Aikawa M., Nugent K. 1976. Plasmodium knowlesi: morphology and course of infection in rhesus monkeys treated with clindamycin and its N-demethyl-4′-pentyl analog. Exp. Parasitol. 40:13–24 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt L. 1985. Enhancement of the curative activity of primaquine by concomitant administration of mirincamycin. Antimicrob. Agents Chemother. 27:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt L., Harrison J., Ellison R., Worcester P. 1970. The activities of chlorinated lincomycin derivatives against infections with Plasmodium cynomolgi in Macaca mulatta. Am. J. Trop. Med. Hyg. 19:1–11 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt L., Alexander S., Allen L., Rasco J. 1977. Comparison of the curative antimalarial activities and toxicities of primaquine and its d and l isomers. Antimicrob. Agents Chemother. 12:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spizek J., Rezanka T. 2004. Lincomycin, clindamycin and their applications. Appl. Microbiol. Biotechnol. 64:455–464 [DOI] [PubMed] [Google Scholar]
- 19. Teja-Isavadharm P., et al. 2004. Plasmodium falciparum-based bioassay for measurement of artemisinin derivatives in plasma or serum. Antimicrob. Agents Chemother. 48:954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Upjohn Co. Ltd 1973. Mirincamycin clinical brochure, issue 3. The Upjohn Co. Ltd., Kalamazoo, MI [Google Scholar]
- 21. Wein S., et al. 2010. Reliability of antimalaria sensitivity tests depends on drug mechanisms of action. J. Clin. Microbiol. 48:1651–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wells T., Burrows J., Baird J. 2010. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 26(3):145–151 [DOI] [PubMed] [Google Scholar]
- 23. Westblom T., Midkiff B., Czinn S. 1993. In vitro susceptibility of Helicobacter pyroli to trospectomycin, pirlimycin (U-57930E), mirincamycin (U-24729A) and N-demethyl clindamycin (U-26767A). Eur. J. Clin. Microbiol. Infect. Dis. 12:560–562 [DOI] [PubMed] [Google Scholar]
- 24. Wynalda M., Hutzler J., Koets M., Podoll T., Wienkers L. 2003. In vitro metabolism of clindamycin in human liver and intestinal microsomes. Drug Metab. Dispos. 31:878–887 [DOI] [PubMed] [Google Scholar]