Abstract
A recent in vitro study showed that the three compounds of antiviral drugs with different mechanisms of action (amantadine, ribavirin, and oseltamivir) could result in synergistic antiviral activity against influenza virus. However, no clinical studies have evaluated the efficacy and safety of combination antiviral therapy in patients with severe influenza illness. A total of 245 adult patients who were critically ill with confirmed pandemic influenza A/H1N1 2009 (pH1N1) virus infection and were admitted to one of the intensive care units of 28 hospitals in Korea were reviewed. Patients who required ventilator support and received either triple-combination antiviral drug (TCAD) therapy or oseltamivir monotherapy were analyzed. A total of 127 patients were included in our analysis. Among them, 24 patients received TCAD therapy, and 103 patients received oseltamivir monotherapy. The 14-day mortality was 17% in the TCAD group and 35% in the oseltamivir group (P = 0.08), and the 90-day mortality was 46% in the TCAD group and 59% in the oseltamivir group (P = 0.23). None of the toxicities attributable to antiviral drugs occurred in either group of our study, including hemolytic anemia and hepatic toxicities related to the use of ribavirin. Logistic regression analysis indicated that the odds ratio for the association of TCAD with 90-day mortality was 0.58 (95% confidence interval, 0.24 to 1.42; P = 0.24). Although this study was retrospective and did not provide virologic outcomes, our results suggest that the treatment outcome of the triple combination of amantadine, ribavirin, and oseltamivir was comparable to that of oseltamivir monotherapy.
INTRODUCTION
Pandemic influenza A/H1N1 2009 (pH1N1) virus was a considerable public health concern worldwide. Most infected people experienced mild and uncomplicated illnesses, but some patients developed rapidly progressive pneumonia that led to respiratory failure, shock, and death (8, 24, 32). The Centers for Disease Control and Prevention (CDC) recommended that all patients with severe pH1N1 illness be treated with oseltamivir or zanamivir as soon as possible (11). Oseltamivir and zanamivir are neuraminidase inhibitors (NAIs), which interfere with the release of progeny influenza virus from infected cells, thereby preventing new rounds of infection (29). Oseltamivir is readily available in oral formulation, and zanamivir is available in inhalation or intravenous form. Rimantadine and amantadine are closely related adamantanes (also called M2 inhibitors), and they target the M2 protein of influenza A virus, which forms a proton channel in the viral membrane that is essential for efficient viral replication (37). Ribavirin is approved for the treatment of respiratory syncytial virus and in combination with interferon or peginterferon for hepatitis C virus. In vitro inhibitory activity against both viral RNA and DNA polymerase may imply a reduced potential of ribavirin for the drug resistance of influenza viruses (28). Ribavirin is available in oral, aerosolized, and intravenous formulations, although intravenous form is not currently approved in the United States.
Previous studies on antiviral therapies for influenza virus were conducted primarily in healthy outpatients with uncomplicated illnesses (14, 31). Limited data are available on antiviral use in patients with severe influenza infection, and recommendations are based mostly on expert opinions or observational studies (1, 26). Treatment can be complicated if NAI-resistant viral strains are suspected (6, 41). Recent studies reported the emergence of oseltamivir-resistant viruses after short-term drug therapy (18, 27). The prevalence of drug-resistant strains could undermine the clinical benefit of antiviral drugs when utilized as monotherapy, especially for critically ill patients, when the development of antiviral resistance is rapid.
The present limitations of monotherapy to treat severe influenza illness have renewed interest in antiviral therapies that combine multiple drugs with different mechanisms of action (17, 30, 34). Nguyen et al. evaluated the three compounds of antiviral drugs (amantadine, ribavirin, and oseltamivir), called a triple-combination antiviral drug (TCAD) regimen, using an in vitro infection model (30). They reported that TCAD had a strong effect on drug-resistant viruses, including pH1N1 strains. Such combination therapy might be clinically useful in the treatment of influenza viruses that are resistant to one or more antivirals. The CDC reported that 1,143 of 1,148 (99.6%) seasonal H1N1 viruses isolated in the period from 2008 to 2009 and 10 of 1,497 (0.6%) pH1N1 isolates tested in the United States were resistant to oseltamivir (6). In Korea, from May 2009 through January 2010, a total of 740,835 patients were reported with pH1N1 virus infection, and 11 of 67 (16%) patients who were suspected of having drug-resistant pH1N1 strains were identified as having oseltamivir-resistant strains (33). To date, rapid diagnostic tests to determine the susceptibility profile of influenza viruses are not available. The availability of a broad-spectrum antiviral therapy that would be effective regardless of the susceptibility to each antiviral drug could be of high clinical utility. However, no clinical studies have demonstrated the efficacy and safety of TCAD for patients with severe influenza illness.
In this study, we analyzed the efficacy and safety of TCAD for patients with pH1N1 infection whose illnesses were severe enough for admission to an intensive care unit (ICU) with ventilator support.
MATERIALS AND METHODS
Patient selection.
We initially reviewed the data of a cohort from the Korean Society of Critical Care Medicine (KSCCM) H1N1 Collaborative. The cohort is composed of critically ill adult patients who were at least 15 years old, had confirmed pH1N1 infection, and were admitted to one of the ICUs of the 28 participating tertiary or referral hospitals of Korea from September 2009 to February 2010. Pandemic influenza A/H1N1 2009 virus was confirmed by a positive result from a probe-based reverse transcriptase PCR test from a nasopharyngeal swab or bronchoalveolar lavage (5). From this cohort, we included in analysis patients who (i) required ventilator support (invasive or noninvasive) and (ii) received either TCAD or oseltamivir monotherapy as the initial antiviral treatment.
Antiviral treatment protocol.
During the 2009 pandemic, the KSCCM established the management protocol for new H1N1 influenza pneumonia with respiratory failure (23). The KSCCM also invited the study investigators to participate in this study and encouraged them to follow the protocol. With respect to the use of antivirals, the protocol states that a triple-combination antiviral regimen (150 mg oseltamivir twice daily, 100 mg amantadine twice daily, and 300 mg ribavirin three times daily) may be considered in case of severe influenza illness. The drug dosage for amantadine was based on data from product labels, and a reduction in dosage was recommended for patients ≥65 years old or with creatinine clearance (CLCR) of <50 ml/min/1.73 m2. The dosage for oral ribavirin was based on previously published studies (7, 25), and the drug was used with caution in patients with CLCR of <50 ml/min/1.73 m2. As for oseltamivir, we suggested doubling the dose (150 mg twice daily) when used in a triple-combination regimen to treat severe influenza illness. For patients with CLCR of <30 ml/min/1.73 m2, a reduction in dosage was recommended. The antiviral drugs were administered via a nasogastric (NG) or gastric tube when the patients were on ventilator support.
Study design.
We performed a retrospective cohort analysis to compare the clinical outcomes of patients given TCAD therapy or oseltamivir therapy. The primary outcome of this study was death. To assess the safety of antiviral agents, we identified any possible adverse events related to antiviral therapy and reviewed serum liver enzymes and creatinine at the time of ICU admission (baseline) and at 3, 7, and 14 days after the initiation of drug treatment. We also analyzed factors associated with death, including TCAD treatment. This study was approved by the local Institutional Review Board (IRB) of each hospital. Informed consent was not necessary, because this was not an interventional study.
Definitions.
A nosocomial infection of pH1N1 was defined as one in which symptoms or signs suggestive of influenza illness newly developed 3 days or more after hospital admission and pH1N1 virus was confirmed as a causative agent. Cardiovascular disease included hypertension, ischemic heart disease, arrhythmia, and congestive heart failure. An immunosuppressive condition was diagnosed if there was an underlying disease that affected the immune system (chronic liver disease, chronic renal disease, human immunodeficiency virus infection, or malignancy) or if immunosuppressive therapy was being administered at the time of infection. Acute respiratory distress syndrome (ARDS) was diagnosed by consensus definition (4). Severity of illness was assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II score (22) and the Sequential Organ Failure Assessment (SOFA) score (40) on the day of ICU admission.
Statistical analysis.
Data are presented as the numbers ± standard deviations (SD) or percentages of patients unless indicated otherwise. Student's t test or the Kruskall-Wallis test was used to compare continuous data, and the chi-square or Fisher's exact test was used to compare categorical data as appropriate. In order to identify factors associated with 90-day mortality, all prespecified covariables, which are listed in Table 1 and Table 2, were included in the multiple logistic regression analysis by using stepwise backward selection procedures with P values of <0.10. To prevent multicollinearity, variables with a high correlation with each other were strictly controlled. Model calibration was assessed with the Hosmer-Lemeshow test (χ2 = 4.07, df = 8, P = 0.85). All tests of significance were two tailed, and P values of less than 0.05 were considered significant. All analyses were performed with SPSS version 18.0K for Windows (SPSS Inc., Chicago, IL).
Table 1.
Baseline characteristics of the TCAD group and oseltamivir group
| Variableb | Resulta |
P | |
|---|---|---|---|
| TCAD (n = 24 patients) | Oseltamivir (n = 103 patients) | ||
| Age, years (mean ± SD) | 63.5 ± 18.9 | 55.7 ± 17.7 | 0.046 |
| Male | 14 (58) | 58 (56) | 0.86 |
| Body mass index, kg/m2 (mean ± SD) | 22.5 ± 4.3 | 23.3 ± 4.9 | 0.39 |
| Nosocomial H1N1 infection | 4 (17) | 16 (16) | >0.99 |
| Chronic coexisting conditions | |||
| Cardiovascular disease | 9 (38) | 54 (52) | 0.19 |
| COPD/asthma | 6 (25) | 15 (15) | 0.23 |
| Diabetes | 4 (17) | 28 (27) | 0.29 |
| Chronic liver disease | 2 (8) | 5 (5) | 0.62 |
| Chronic renal disease | 2 (8) | 15 (15) | 0.53 |
| Cerebral vascular disease | 3 (13) | 18 (18) | 0.76 |
| Solid tumor | 5 (21) | 29 (28) | 0.47 |
| Hematologic malignancy | 3 (13) | 3 (3) | 0.08 |
| Pregnancy | 0 | 1 (1) | >0.99 |
| No underlying diseases | 3 (13) | 13 (13) | >0.99 |
| Immunosuppressive conditions | 11 (46) | 45 (44) | 0.85 |
| Shock | 10 (42) | 50 (49) | 0.54 |
| ARDS | 16 (67) | 63 (61) | 0.62 |
| Severity of illness at ICU admission | |||
| APACHE II score (mean ± SD) | 22.5 ± 6.7 | 22.1 ± 7.5 | 0.84 |
| SOFA score (mean ± SD) | 8.1 ± 2.3 | 9.2 ± 3.5 | 0.14 |
| Baseline values at ICU admission (mean ± SD) | |||
| Temp (°C) | 37.8 ± 1.0 | 37.7 ± 2.1 | 0.60 |
| Heart rate (beats/min) | 120.1 ± 26.5 | 127.8 ± 32.0 | 0.28 |
| PaO2/FiO2 | 145.9 ± 80.9 | 129.3 ± 68.6 | 0.53 |
| Mean arterial pressure (mmHg) | 73.7 ± 14.1 | 72.5 ± 17.9 | 0.76 |
| White cell count/mm3 | 1,3791 ± 10,066 | 10,655 ± 7,363 | 0.19 |
| Hemoglobin (g/dl) | 11.2 ± 2.0 | 11.7 ± 2.9 | 0.56 |
| Platelet count/mm3 | 150,100 ± 113,758 | 166,770 ± 118,327 | 0.47 |
| Total bilirubin (mg/dl) | 1.4 ± 2.5 | 1.2 ± 2.0 | 0.46 |
| Creatinine (mg/dl) | 1.6 ± 1.8 | 1.9 ± 2.3 | 0.49 |
| Antibiotic treatment | 24 (100) | 103 (100) | |
| Steroid treatment | 14 (58) | 53 (52) | 0.54 |
| Time from symptom onset to ICU admission, days (mean ± SD) | 5.6 ± 5.7 | 5.2 ± 5.6 | 0.94 |
| Time from symptom onset to antivirals, days (mean ± SD) | 4.7 ± 5.4 | 5.0 ± 4.3 | 0.14 |
| Antiviral dose, mg/day (mean ± SD) | |||
| Oseltamivir | 0.01 | ||
| ≤150 mg/day | 9 (38) | 68 (67) | |
| ≥300 mg/day | 15 (63) | 34 (33) | |
| Amantadine | 188.3 ± 48.2 | NA | |
| Ribavirin | 587.5 ± 211.2 | NA | |
| Antiviral duration, days (mean ± SD) | 11.1 ± 7.0 | 6.2 ± 3.6 | <0.001 |
Data are presented as the numbers ± SD or percentages of patients unless indicated otherwise.
TCAD, triple combination antiviral drug; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; NA, not applicable.
Table 2.
Clinical outcomes of the TCAD group and oseltamivir group
| Variable | Resulta |
P | |
|---|---|---|---|
| TCAD (n = 24 patients) | Oseltamivir (n = 103 patients) | ||
| Secondary bacterial pneumonia | |||
| Community acquired | 3 (13) | 10 (10) | 0.71 |
| Hospital acquired | 13 (54) | 51 (50) | 0.82 |
| Barotrauma | 1 (4) | 5 (5) | >0.99 |
| Renal replacement therapyb | 2 (8) | 21 (20) | 0.24 |
| Rescue therapy | |||
| Neuromuscular blocker | 16 (70) | 59 (58) | 0.30 |
| Nitric oxide | 3 (13) | 2 (2) | 0.04 |
| Prone position | 3 (13) | 12 (12) | >0.99 |
| Extracorporeal membrane oxygenator | 0 | 5 (5) | 0.58 |
| No. of ventilator-free days (mean ± SD) | 9.7 ± 12.2 | 8.6 ± 11.5 | 0.74 |
| Time from symptom to death | |||
| 14 days | 4 (17) | 36 (35) | 0.08 |
| 30 days | 8 (33) | 51 (50) | 0.15 |
| 90 days | 11 (46) | 61 (59) | 0.23 |
| Cause of death (n = 72) | 0.36 | ||
| Septic shock/multiorgan failure | 3 (27) | 12 (20) | |
| Pneumonia/ARDS | 8 (73) | 38 (62) | |
| Otherc/undetermined | 0 | 11 (18) | |
Data are presented as the numbers ± SD or percentages of patients unless indicated otherwise.
Patients who were dialyzed due to previous renal failure were excluded.
Other causes of death include aggravation of underlying conditions, bleeding, liver failure, and infection other than pneumonia.
RESULTS
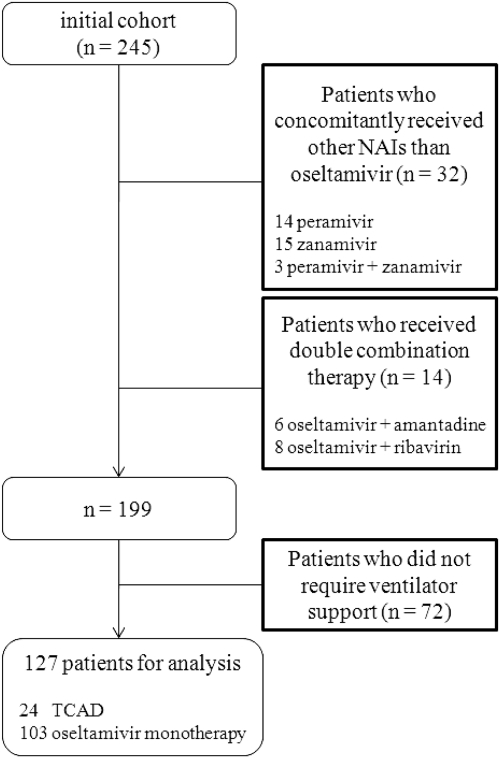
There were 245 patients in the initial cohort, and 127 of these patients were ultimately included in our analysis. Considerable dropout occurred, due largely to patients being treated with NAIs other than oseltamivir (n = 32). A total of 24 of the included patients received TCAD, and 103 received oseltamivir monotherapy (Fig. 1).
Fig. 1.
Disposition of pH1N1-infected patients included in the analysis of the impact of TCAD versus oseltamivir monotherapy. NAIs, neuraminidase inhibitors; TCAD, triple-combination antiviral drug.
Table 1 shows the baseline characteristics of the TCAD group and the oseltamivir group. Patients given TCAD were older (mean age of 63.5 ± 18.9 years versus 55.7 ± 17.7 years; P = 0.046), but there was no difference in sex or body mass index. There were no differences between the two groups in comorbidities, immunosuppressive conditions, severity of illness, and baseline values measured at ICU admission. The proportions of patients with shock and ARDS were also similar between the two groups. Adjuvant corticosteroids were administered to about half of the patients in each group. There were no differences between the groups in mean times from onset of symptoms to antiviral drug administration. In the TCAD group, higher doses of oseltamivir were administered (oseltamivir at ≥300 mg/day; 63% versus 33%; P = 0.01). The mean treatment duration was longer (11.1 ± 7.0 days versus 6.2 ± 3.6 days; P < 0.001) in the TCAD group, although in patients who survived (n = 55), there was no significant difference (9.6 ± 5.4 days versus 7.3 ± 3.1 days; P = 0.15). The mean doses of amantadine and ribavirin in the TCAD group were less than the recommended doses of the KSCCM protocol.
Table 2 shows the clinical outcomes of the two groups. There were three cases (13%) of community-acquired pneumonia in the TCAD group and 10 cases (10%) in the oseltamivir group. During the course of illness, hospital-acquired pneumonia occurred, and the proportions of patients were similar between the two groups. Within 90 days from the onset of symptoms, 11 patients (46%) died in the TCAD group and 61 (59%) in the oseltamivir group. The deaths occurred within a 14-day period in 36% (4/11 patients) of the TCAD group and 59% (36/61 patients) of the oseltamivir group. There was a trend toward lower 14-day, 30-day, and 90-day mortality rates in the TCAD group, but this was not statistically significant. All 11 patients in the TCAD group and 50 patients (82%) in the oseltamivir group died due to aggravation of septic shock or pneumonia. In the oseltamivir group, three patients died due to uncontrolled liver failure, two due to intraabdominal infection, two due to exacerbation of underlying disease, and four due to undetermined causes. There were no differences between the two groups in mean numbers of ventilator-free days.
The results of additional analysis of the study group stratified by duration of ICU stay are shown in Table 3. Most of the baseline characteristics were similar between the two groups, but patients with ICU stays of more than 7 days were more immunocompromised (52% versus 34%; P = 0.04) and had longer mean durations of symptoms before antiviral administration (6.3 ± 5.1 days versus 3.5 ± 3.1 days; P = 0.001). Secondary bacterial (hospital-acquired) pneumonia occurred more in the group with longer ICU stays (69% versus 31%; P < 0.001); however, more deaths occurred in the group of patients with ICU stays less than or equal to 7 days (66% versus 48%; P = 0.04).
Table 3.
Baseline characteristics and clinical outcomes of ICU patients stratified by duration of ICU stay
| Variable | Resulta |
P | |
|---|---|---|---|
| ICU stay ≤7 days (n = 59 patients) | ICU stay >7 days (n = 67 patients) | ||
| Age, years (mean ± SD) | 57.0 ± 20.3 | 57.4 ± 16.2 | 0.74 |
| Nosocomial H1N1 infection | 8 (14) | 12 (18) | 0.51 |
| Immunosuppressive conditions | 20 (34) | 35 (52) | 0.04 |
| Shock | 31 (53) | 29 (43) | 0.30 |
| ARDS | 36 (61) | 42 (63) | 0.85 |
| APACHE II score (mean ± SD) | 22.1 ± 7.6 | 22.1 ± 7.1 | 0.98 |
| SOFA score (mean ± SD) | 9.4 ± 3.6 | 8.6 ± 3.0 | 0.17 |
| Steroid treatment | 28 (48) | 39 (58) | 0.23 |
| Type of antivirals | 0.31 | ||
| TCAD | 9 (15) | 15 (22) | |
| Oseltamivir monotherapy | 50 (85) | 52 (78) | |
| Symptom to antivirals, days (mean ± SD) | 3.5 ± 3.1 | 6.3 ± 5.1 | 0.001 |
| Oseltamivir dose of ≥300 mg/day | 22 (38) | 26 (39) | 0.92 |
| Hospital-acquired secondary bacterial pneumonia | 18 (31) | 46 (69) | <0.001 |
| Death | 39 (66) | 32 (48) | 0.04 |
| Cause of death | 0.61 | ||
| Septic shock/multiorgan failure | 10 (26) | 5 (16) | |
| Pneumonia/ARDS | 23 (59) | 22 (69) | |
| Otherb/undetermined | 6 (15) | 5 (16) | |
Data are presented as the numbers ± SD or percentages of patients unless indicated otherwise.
Other causes of death include aggravation of underlying conditions, bleeding, liver failure, and infection other than pneumonia.
Table 4 shows the safety measures related to antiviral agents. There were neither hematologic (e.g., hemolytic anemia) nor neurologic (e.g., seizure) events in any of the 127 patients. In the TCAD group, the mean difference (posttreatment value − baseline value) for aspartate transaminase (AST) was −12.8 ± 38.7 IU/liter, for alanine transaminase (ALT) it was −2.9 ± 21.3 IU/liter, and for total bilirubin (TB) it was −0.2 ± 3.0 mg/dl. In the oseltamivir group, seven cases had severe liver enzyme elevation (see Table S1 in the supplemental material), and the mean differences of AST and ALT were very high. There was no significant difference in the mean differences of serum creatinine between the two groups. Three patients, all in the oseltamivir group, died due to uncontrolled liver failure, although the relationship between toxicity and drug use was unclear.
Table 4.
Safety assessments of antiviral agents
| Variablec | Resulta |
P | |
|---|---|---|---|
| TCAD | Oseltamivir | ||
| Δ (posttreatment − baseline)b | |||
| AST (IU/liter) | −12.8 ± 38.7 | 518.0 ± 1,993.0 | 0.03 |
| ALT (IU/liter) | −2.9 ± 21.3 | 283.2 ± 1,116.9 | 0.08 |
| TB (mg/dl) | −0.2 ± 3.0 | 0.9 ± 2.5 | 0.46 |
| Creatinine (mg/dl) | −0.4 ± 1.4 | −0.3 ± 1.5 | 0.55 |
| Hemolytic anemia | None | None | |
| Seizure | None | None | |
Data are presented as mean values ± SD unless indicated otherwise.
The baseline values (at ICU admission) were subtracted from values measured after initiation of drugs (day 3, 7, or 14) for each case.
AST, aspartate transaminase; ALT, alanine transaminase; TB, total bilirubin.
Table 5 shows the results of our univariate and multivariate analysis of risk factors associated with 90-day mortality. APACHE II score and mean arterial pressure were excluded in further analysis due to multicollinearity with SOFA score and shock, respectively. Multivariate analysis that adjusted for variables associated with 90-day mortality rate indicated that increasing age, nosocomial infection of the virus, higher SOFA score, and prone position were significantly associated with increased mortality rates. On the other hand, larger doses of oseltamivir, longer duration of antiviral therapy, and TCAD therapy were not significantly associated with increased survival.
Table 5.
Unadjusted (univariate) and adjusted (multivariate) logistic regression models of factors associated with 90-day mortalitya
| Variable | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Age | 1.04 (1.01–1.06) | 0.002 | 1.04 (1.01–1.07) | 0.005 |
| Body mass index | 1.08 (0.98–1.18) | 0.12 | ||
| Nosocomial H1N1 infection | 5.36 (1.48–19.36) | 0.01 | 4.26 (1.01–18.03) | 0.049 |
| Chronic coexisting conditions | ||||
| Diabetes | 1.98 (0.85–4.63) | 0.11 | ||
| Hematologic malignancy | 4.03 (0.46–35.53) | 0.21 | ||
| No underlying diseases | 0.41 (0.14–1.21) | 0.11 | ||
| Immunosuppressive conditions | 2.30 (1.11–4.76) | 0.03 | ||
| Shock | 2.19 (1.07–4.49) | 0.03 | ||
| APACHE II scoreb | 1.07 (1.02–1.13) | 0.01 | ||
| SOFA score | 1.23 (1.09–1.38) | <0.001 | 1.21 (1.05–1.40) | 0.01 |
| Mean arterial pressureb | 0.99 (0.97–1.01) | 0.19 | ||
| Hemoglobin | 0.74 (0.63–0.87) | <0.001 | 0.83 (0.68–1.01) | 0.06 |
| Platelet count | 0.997 (0.99–1.00) | 0.08 | ||
| Total bilirubin | 1.27 (0.92–1.75) | 0.15 | ||
| Steroid treatment | 1.68 (0.83–3.41) | 0.15 | ||
| Hospital-acquired secondary bacterial pneumonia | 1.42 (0.70–2.87) | 0.33 | ||
| Renal replacement therapy | 4.57 (1.46–14.36) | 0.01 | 3.10 (0.84–11.51) | 0.09 |
| Nitric oxide | 3.06 (0.33–28.19) | 0.32 | ||
| Prone position | 5.62 (1.21–26.08) | 0.03 | 9.95 (1.89–52.33) | 0.01 |
| Oseltamivir dose of ≥300 mg/day | 0.60 (0.29–1.24) | 0.17 | ||
| Antiviral duration | 0.94 (0.87–1.02) | 0.13 | ||
| TCAD | 0.58 (0.24–1.42) | 0.24 |
The variables with P values of less than 0.10 (in univariate analysis) were included in the multivariate analysis by using stepwise backward selection procedures. OR, odds ratio; CI, confidence interval.
The APACHE II score and mean arterial pressure were not considered for the multivariate analysis to prevent multicollinearity.
DISCUSSION
The present results indicate that use of the TCAD regimen to treat pH1N1 illness with ventilator support was well tolerated, with no significant adverse drug reactions. Although the use of TCAD was not associated with decreased mortality rates, its treatment outcome was comparable to that of oseltamivir monotherapy.
The prevalence of the oseltamivir-resistant pH1N1 viruses were identified worldwide (6, 33). The prevalence of drug-resistant strains is a concern to patients with severe influenza illness, especially when the development of antiviral resistance occurs within a few days of patients' exposure to antivirals (18, 27). Most cases of the oseltamivir-resistant pH1N1 virus have been associated with the H275Y mutation in the neuraminidase gene, and they are susceptible to zanamivir. However, zanamivir in inhalation form is not recommended for patients with asthma or chronic obstructive pulmonary disease due to the possibility of bronchospasm (13). More importantly, the use of a zanamivir disc inhaler is impossible for intubated patients, because the medication cannot be properly delivered to infection sites. Also, a nebulized preparation is not recommended, because it can clog the ventilator tube (20). Recent clinical data of patients with oseltamivir-resistant pH1N1 infection have indicated that intravenous zanamivir is effective (12, 21), but most of them are case reports of a single patient. Recently, Hernandez et al. described 31 hospitalized patients who received peramivir, another parenteral antiviral agent, and showed moderate efficacy and safety (16). However, most of the patients concomitantly received oseltamivir, no comparison group was available, and no virologic outcomes were provided.
An alternative drug regimen to treat severe influenza infection, such as combining agents with different mechanisms of action, is possible. To date, two clinical studies evaluated combination therapy but have failed to prove its efficacy (10, 19). Several preclinical studies, both in vitro and in vivo, support the effectiveness of combination therapy (17, 30, 34). In double combinations, however, the additive or synergistic effects varied according to combination type, dosage of drugs, and virus strain. For example, in the animal study by Ilyushina et al. (17), double combination of amantadine and other antivirals provided greater protection against amantadine-sensitive strains than did monotherapy. In contrast, no benefit was noted with combination therapy when the infecting virus was resistant to amantadine (17, 34). Such combinations with amantadine would not be effective in patients with pH1N1 virus, because the World Health Organization confirmed that the pandemic virus is resistant to the M2 inhibitors (42). In a recent in vitro study, Nguyen et al. tested the activity of TCAD against six amantadine-resistant viruses, including three strains of pH1N1 virus, and two oseltamivir-resistant viruses (30). Surprisingly, they found that the TCAD was highly synergistic against resistant viruses, and the synergism from a triple combination was greater than that from any double combination. The pH1N1 virus is intrinsically resistant to amantadine, which had no activity as a single agent in the study by Nguyen et al. However, when it was added to ribavirin and oseltamivir, amantadine significantly contributed to the synergism of the TCAD. This may suggest that the TCAD regimen has a broad-spectrum antiviral activity for seasonal and pandemic influenza viruses compared to that of double combination or monotherapy. Moreover, the synergism of the triple combination occurred at concentrations that are achievable in plasma for the recommended doses of each antiviral drug, implicating for the clinical use of TCAD therapy.
In addition to potential antiviral effect, oral combination antiviral drugs can be easily administered via an NG tube without clogging the ventilator tube. The absorption of oral agents in a patient with decreased bowel movement associated with the ventilator may be a concern. However, several clinical studies indicated that enteric absorption of oseltamivir via an NG tube in ventilated patients achieved plasma levels that were comparable to those in ambulatory patients (2, 38).
TCAD appears to be an effective treatment modality for critically ill influenza patients, but more data are needed before it can be recommended in a clinical setting. To date, our study is the first clinical study in which the efficacy of triple-combination antiviral therapy is compared to single oseltamivir therapy in patients with severe influenza illness. However, our statistical analysis of mortality appears to prove that TCAD was not superior to the standard oseltamivir therapy against pH1N1 infection, in contrast with previous preclinical studies. There are several possible explanations for this discrepancy. Many of our study patients were severely diseased. As a result, the treatment outcomes might have not been satisfactory despite the use of multiple antiviral drugs. Insufficient antiviral dosage may also have been the problem. Ribavirin, although its use for the treatment of influenza is investigational, showed a promising effect when used in a high-dose or intravenous formulation (15, 36). In the recent observation by van der Vries et al., pH1N1 virus titer significantly dropped when ribavirin was added to oseltamivir and zanamivir in a dually NAI-resistant virus-infected patient (39). In our study, the actual dose of ribavirin administered was much less than the dose used in the previous studies or the dose recommended by the KSCCM. The treatment outcomes might have been different for the TCAD group if a higher dose of ribavirin was administered.
Toxicity can be a significant problem when using three different drugs simultaneously. Oseltamivir is well tolerated and safe at the recommended doses, with few side effects (9). On the other hand, ribavirin has teratogenic properties and can cause hematologic, gastrointestinal, and hepatic toxicities. An increased incidence of seizures has been reported in patients given amantadine (3). During the study period, none of the toxicities attributable to amantadine or ribavirin occurred. Hemolytic anemia, the primary toxicity of ribavirin, usually occurs within 1 to 2 weeks of the initiation of therapy. In our case, the median (range) duration of ribavirin treatment was 7 (2 to 24) days, and 6 of 24 (25%) patients received ribavirin treatment for longer than 14 days. The mean dose of ribavirin was less than 600 mg/day. In spite of relatively long treatment durations in some of our study patients, there is a chance that serious anemia could have been avoided due to the small dosage of ribavirin administered. There was no remarkable elevation of serum liver enzymes and creatinine in the TCAD group. On the other hand, seven patients in the oseltamivir group experienced severe liver enzyme elevation. We believe that oseltamivir played at most a minor role in elevation of liver enzymes, because oseltamivir is known to be adequately metabolized in hepatically impaired individuals (35), and most of these patients were in uncontrolled sepsis with organ failure at the time of liver enzyme elevation.
The present study has several limitations. First, the study was retrospective and underpowered. Also, a relatively small sample size was used for the TCAD group compared to that of the oseltamivir group. We could not adjust unmeasured confounders, and the matching process to compensate the sample size difference between the two groups was not feasible; hence, our results cannot be considered conclusive and must be assessed for consistency with other studies. Second, we did not monitor the duration of viral shedding and emergence of drug resistance. As a result, we could not clarify the relationship between antiviral effect and clinical outcome or evaluate the efficacy of TCAD in association with viral resistance. Third, we did not measure the serum concentrations of the antiviral agents during treatment and hence could not establish their pharmacokinetic profiles. Fourth, antiviral dose, timing of antiviral commencement, and duration of antiviral treatment were not standardized among centers. The proportion of patients who received the double dose of oseltamivir and antiviral duration varied between the two groups. However, in the regression analysis, the oseltamivir dose and antiviral duration were not associated with increased or decreased survival. Last, there is a possibility that secondary bacterial pneumonia, especially hospital-acquired pneumonia, might have significantly contributed to mortality. We performed an additional analysis of the study group stratified by duration of ICU stay. From the results, we assume that the bacterial pneumonia by itself was less likely to be the major determinant of increased mortality. Also, the presence of bacterial pneumonia was not associated with increased mortality in the regression model. Further studies are needed to evaluate the efficacy and safety of TCAD in severe influenza illness. However, as prospective clinical trials are not available in the near future, we believe that this observational study can provide a certain aspect regarding selection of antiviral agents in severe influenza infection with mechanical ventilation and, possibly, a useful background for planning further clinical trials.
In conclusion, our comparison of TCAD therapy with oseltamivir monotherapy for patients who were critically ill on mechanical ventilation with pH1N1 virus indicates that TCAD was well tolerated and the treatment outcome was comparable to that of oseltamivir monotherapy.
Supplementary Material
ACKNOWLEDGMENTS
All the authors have no conflicts of interest to disclose and no funding source and are indebted to all who participated in this study. All the authors read and approved the final manuscript.
We also thank Eun Mi Cho and Na Yeon Lee (clinical research nurses) for assistance in the chart review.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 3 October 2011.
REFERENCES
- 1. Abdel-Ghafar A. N., et al. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261–273 [DOI] [PubMed] [Google Scholar]
- 2. Ariano R. E., et al. 2010. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ 182:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkinson W. L., et al. 1986. Amantadine prophylaxis during an institutional outbreak of type A (H1N1) influenza. Arch. Intern. Med. 146:1751–1756 [PubMed] [Google Scholar]
- 4. Bernard G. R., et al. 1994. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 149:818–824 [DOI] [PubMed] [Google Scholar]
- 5. CDC 2010. Interim recommendations for clinical use of influenza diagnostic tests during the 2009-10 influenza season. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/h1n1flu/guidance/diagnostic_tests.htm [Google Scholar]
- 6. CDC 2009. 2008-2009 influenza season: week 36 ending September 12, 2009. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly36.htm [Google Scholar]
- 7. Cohen A., Togo Y., Khakoo R., Walderman R., Sigel M. 1976. Comparative clinical and laboratory evaluation of the prophylactic capacity of ribavirin, amantadine hydrochloride, and placebo in induced human influenza type A. J. Infect. Dis. 133(Suppl.):A114–A120 [DOI] [PubMed] [Google Scholar]
- 8. Dominguez-Cherit G., et al. 2009. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 302:1880–1887 [DOI] [PubMed] [Google Scholar]
- 9. Dutkowski R., et al. 2003. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 26:787–801 [DOI] [PubMed] [Google Scholar]
- 10. Duval X., et al. 2010. Efficay of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 7:e1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiore A. E., et al. 2011. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 60:1–24 [PubMed] [Google Scholar]
- 12. Gaur A. H., et al. 2010. Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza. N. Engl. J. Med. 362:88–89 [DOI] [PubMed] [Google Scholar]
- 13. Glaxo Wellcome, Inc 2009. Relenza (zanimivir for inhalation) package insert. Glaxo Wellcome, Inc., Research Triangle Park, NC [Google Scholar]
- 14. Hayden F. G., et al. 1997. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N. Engl. J. Med. 337:874–880 [DOI] [PubMed] [Google Scholar]
- 15. Hayden F. G., Sable C. A., Connor J. D., Lane J. 1996. Intravenous ribavirin by constant infusion for serious influenza and parainfluenzavirus infection. Antivir. Ther. 1:51–56 [PubMed] [Google Scholar]
- 16. Hernandez J. E., et al. 2011. Clinical experience in adults and children treated with intravenous peramivir for 2009 influenza A (H1N1) under an emergency IND program in the United States. Clin. Infect. Dis. 52:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ilyushina N. A., Hoffmann E., Salomon R., Webster R. G., Govorkova E. A. 2007. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir. Ther. 12:363–370 [PubMed] [Google Scholar]
- 18. Inoue M., et al. 2010. Emergence of oseltamivir-resistant pandemic (H1N1) 2009 virus within 48 hours. Emerg. Infect. Dis. 16:1633–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ison M. G., et al. 2003. Safety and efficacy of nebulized zanamivir in hospitalized patients with serious influenza. Antivir. Ther. 8:183–190 [PubMed] [Google Scholar]
- 20. Kiatboonsri S., Kiatboonsri C., Theerawit P. 2010. Fatal respiratory events caused by zanamivir nebulization. Clin. Infect. Dis. 50:620. [DOI] [PubMed] [Google Scholar]
- 21. Kidd I. M., et al. 2009. H1N1 pneumonitis treated with intravenous zanamivir. Lancet 374:1036. [DOI] [PubMed] [Google Scholar]
- 22. Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818–829 [PubMed] [Google Scholar]
- 23. Korean Society of Critical Care Medicine 2010. KSCCM H1N1 registry. Korean Society of Critical Care Medicine, Seoul, Republic of Korea: http://www.ksccm.org [Google Scholar]
- 24. Kumar A., et al. 2009. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302:1872–1879 [DOI] [PubMed] [Google Scholar]
- 25. Magnussen C. R., Douglas R. G., Jr., Betts R. F., Roth F. K., Meagher M. P. 1977. Double-blind evaluation of oral ribavirin (Virazole) in experimental influenza A virus infection in volunteers. Antimicrob. Agents Chemother. 12:498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGeer A., et al. 2007. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin. Infect. Dis. 45:1568–1575 [DOI] [PubMed] [Google Scholar]
- 27. Memoli M. J., et al. 2010. Rapid selection of a transmissible multidrug-resistant influenza A/H3N2 virus in an immunocompromised host. J. Infect. Dis. 201:1397–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moscona A. 2008. Medical management of influenza infection. Annu. Rev. Med. 59:397–413 [DOI] [PubMed] [Google Scholar]
- 29. Moscona A. 2005. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 353:1363–1373 [DOI] [PubMed] [Google Scholar]
- 30. Nguyen J. T., et al. 2010. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One 5:e9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicholson K. G., et al. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 355:1845–1850 [DOI] [PubMed] [Google Scholar]
- 32. Rello J., et al. 2009. Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1)v in Spain. Crit. Care 13:R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin S. Y., et al. 2011. Drug-resistant pandemic (H1N1) 2009, South Korea. Emerg. Infect. Dis. 17:702–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smee D. F., Hurst B. L., Wong M. H., Bailey K. W., Morrey J. D. 2009. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob. Agents Chemother. 53:2120–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snell P., et al. 2005. Lack of effect of moderate hepatic impairment on the pharmacokinetics of oral oseltamivir and its metabolite oseltamivir carboxylate. Br. J. Clin. Pharmacol. 59:598–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein D. S., et al. 1987. Oral ribavirin treatment of influenza A and B. Antimicrob. Agents Chemother. 31:1285–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takeda M., Pekosz A., Shuck K., Pinto L. H., Lamb R. A. 2002. Influenza A virus M2 ion channel activity is essential for efficient replication in tissue culture. J. Virol. 76:1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taylor W. R., et al. 2008. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS One 3:e3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Vries E., Stelma F. F., Boucher C. A. 2010. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N. Engl. J. Med. 363:1381–1382 [DOI] [PubMed] [Google Scholar]
- 40. Vincent J. L., et al. 1996. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22:707–710 [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization 2009. Antiviral use and the risk of drug resistance—pandemic (H1N1) 2009 briefing note 12. WHO, Geneva, Switzerland: http://who.int/csr/disease/swineflu/notes/h1n1_antiviral_use_20090925/en/index.html [Google Scholar]
- 42. World Health Organization 2009. Pandemic (H1N1) 2009—update 65, September 11, 2009. WHO, Geneva, Switzerland: http://www.who.int/csr/don/2009_09_11/en/index.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.