Abstract
Artemether-lumefantrine and nevirapine-based antiretroviral therapy (ART) are the most commonly recommended first-line treatments for malaria and HIV, respectively, in Africa. Artemether, lumefantrine, and nevirapine are metabolized by the cytochrome P450 3A4 enzyme system, which nevirapine induces, creating potential for important drug interactions. In a parallel-design pharmacokinetic study, concentration-time profiles were obtained in two groups of HIV-infected patients: ART-naïve patients and those stable on nevirapine-based therapy. Both groups received the recommended artemether-lumefantrine dose. Patients were admitted for intense pharmacokinetic sampling (0 to 72 h) with outpatient sampling until 21 days. Concentrations of lumefantrine, artemether, dihydroartemisinin, and nevirapine were determined by validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods. The primary outcome was observed day 7 lumefantrine concentrations, as these are associated with therapeutic response in malaria. We enrolled 36 patients (32 females). Median (range) day 7 lumefantrine concentrations were 622 ng/ml (185 to 2,040 ng/ml) and 336 ng/ml (29 to 934 ng/ml) in the nevirapine and ART-naïve groups, respectively (P = 0.0002). The median artemether area under the plasma concentration-time curve from 0 to 8 h [AUC(0-8 h)] (P < 0.0001) and dihydroartemisinin AUC(60-68 h) (P = 0.01) were lower in the nevirapine group. Combined artemether and dihydroartemisinin exposure decreased over time only in the nevirapine group (geometric mean ratio [GMR], 0.76 [95% confidence interval {CI}, 0.65 to 0.90]; P < 0.0001) and increased with the weight-adjusted artemether dose (GMR, 2.12 [95% CI, 1.31 to 3.45]; P = 0.002). Adverse events were similar between groups, with no difference in electrocardiographic Fridericia corrected QT and P-R intervals at the expected time of maximum lumefantrine concentration (Tmax). Nevirapine-based ART decreased artemether and dihydroartemisinin AUCs but unexpectedly increased lumefantrine exposure. The mechanism of the lumefantrine interaction remains to be elucidated. Studies investigating the interaction of nevirapine and artemether-lumefantrine in HIV-infected patients with malaria are urgently needed.
INTRODUCTION
Malaria and HIV are among the greatest global health burdens, resulting in an estimated combined mortality of 4 million deaths annually (44). Currently, the World Health Organization recommends artemisinin-based combination therapies (ACTs) as first-line treatment for uncomplicated malaria (43). Artemether-lumefantrine is a fixed-dose combination and the most widely used ACT in Africa. Treatment for HIV in these populations is often limited by cost, and therefore the relatively inexpensive antiretroviral nevirapine in combination with two nucleoside reverse transcriptase inhibitors (NRTIs) is currently the most frequently used first-line antiretroviral therapy (ART). Artemether, lumefantrine, and nevirapine are all primarily metabolized by the cytochrome P450 (CYP) isoenzyme CYP3A4, and nevirapine is a known inducer of CYP3A4 (5, 19, 41). This creates the potential for important drug interactions with concomitant therapy. The NRTIs are intracellularly phosphorylated and predominantly renally eliminated and have not been reported to interact with the hepatically eliminated nevirapine or artemether-lumefantrine (19). The therapeutic efficacy of artemether-lumefantrine largely depends on the area under the plasma concentration-time curve (AUC) above the minimum Plasmodium falciparum parasiticidal concentration. The day 7 lumefantrine plasma concentration has proved to be a useful and simple surrogate for this measurement (41). Various therapeutic thresholds have been reported for the day 7 lumefantrine concentration, ranging between 175 and 500 ng/ml, depending on the prevalence of resistance and the method used for determining this threshold; of these, 280 ng/ml is the most widely cited (3, 12, 13, 23, 27, 28, 41). Patients with uncomplicated malaria with day 7 lumefantrine concentrations below these concentrations are potentially at increased risk of treatment failure (3, 12, 13, 23, 27, 28, 41). Therapeutic pharmacokinetic thresholds have yet to be defined for the artemisinin derivatives.
To date, there have been no published studies to inform clinicians and policy makers about the interaction between artemether-lumefantrine and nevirapine-based ART. The objective of our study was to investigate the pharmacokinetics and safety of artemether-lumefantrine when given to HIV-1-infected patients on nevirapine-based ART.
MATERIALS AND METHODS
Subjects and study design.
We conducted a parallel-design, open-label, pharmacokinetic, and safety drug interaction study at the Clinical Pharmacology Research Ward, Groote Schuur Hospital, Cape Town, South Africa, between October 2008 and August 2009. HIV-1-infected adults (18 years of age or older) with CD4+ lymphocyte counts greater than 200 cells/μl were enrolled. Participants enrolled were either ART naïve and not yet eligible for ART according to the South African National HIV Treatment Guidelines at the time (30) or were stable on treatment with nevirapine-based ART for a minimum of 6 weeks.
Exclusion criteria for safety reasons were a current diagnosis of malaria, known hypersensitivity to artemether or lumefantrine, pregnancy (as confirmed by a serum beta-human chorionic gonadotrophin test), breast-feeding, recent hypersensitivity to nevirapine or clinically relevant liver dysfunction. In addition, potential participants with a clinical condition or family history of prolonged QT interval (>450 ms on ECG), symptomatic cardiac dysrhythmia, or electrolyte disturbances or currently taking any drugs known to prolong the QT interval were excluded. Exclusion criteria for potential confounding of the pharmacokinetic parameters included participants using other drugs that may interact via the CYP450 enzyme system, including drugs of abuse, current smokers, or regular alcohol users who were unwilling to abstain from alcohol intake for the trial duration. Caffeine, grapefruit juice, or strenuous exercises were not permitted within 24 h of admission to the research ward.
Ethics approval.
Patients provided written informed consent prior to enrollment. Regulatory approval was received from the University of Cape Town Research Ethics Committee and the South African Medicines Control Council. (The Clinical Trial Registration number for this study is NCT00790881 [http://www.clinicaltrials.gov].) The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Dosing and pharmacokinetic sample collection.
Both groups received the recommended 6 doses of artemether-lumefantrine (80 mg/480 mg) at 0, 8, 24, 36, 48, and 60 h after the first dose. All doses were administered with 40 ml of soya milk (0.8 g fat) and a meal containing a minimum of 6 g of fat within 1 h of each dose, with the exception of dose 2 (at 8 h), when only soya milk accompanied the dose. Subjects were admitted for rich pharmacokinetic sampling (0 to 72 h after the first artemether-lumefantrine dose). Subsequent samples were collected on an outpatient basis until day 21. Venous blood samples were collected into heparinized (LH PST II) BD Vacutainer tubes. The blood tubes were prechilled on ice for 10 min; once drawn, the blood samples were again chilled before being placed into the 4°C centrifuge for 10 min at 2,000 × g. The resulting plasma was kept on dry ice and stored at −80°C within 30 min of venipuncture.
Plasma lumefantrine concentrations were analyzed at predose (0 h) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 14, 24, 30, 36, 42, 48, 54, 60, 61.5, 62, 63, 64, 65, 66, 68, 70, 72, 96, 120, 144, 168, 336, and 504 h after the first artemether-lumefantrine dose (20). We performed rich sampling after the first (0 to 8 h) and final (60 to 68 h) doses of artemether-lumefantrine, to assess artemether and dihydroartemisinin exposure during each of these treatment periods. Plasma artemether/dihydroartemisinin concentrations were analyzed at predose (0 h) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 26, 60, 61.5, 62, 63, 64, 65, 66, 68, 70, and 72 h. Plasma nevirapine concentrations were analyzed at predose (0 h) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 14, 24, 60, 61.5, 62, 63, 64, 65, 66, 68, 70, 72, 96, 120, and 144 h.
Pharmacokinetic sample processing.
Concentrations of lumefantrine, artemether, dihydroartemisinin, and nevirapine were measured by the University of Cape Town Division of Clinical Pharmacology Laboratory.
Lumefantrine was extracted from plasma with a protein precipitation procedure, and analyzed using a liquid chromatography-tandem mass spectrometer (LC-MS/MS) assay. The precision (total assay coefficients of variation; %CV) for lumefantrine during sample analysis was less than 7% at all quality control (QC) levels, including the limit of quantification, which was 20 ng/ml.
Artemether and dihydroartemisinin were analyzed using an LC-MS/MS assay with liquid-liquid extraction; stable isotope-labeled artemether and dihydroartemisinin were used as internal standards. The precision for artemether and dihydroartemisinin during sample analysis was less than 8% at high, medium, and low QC levels and 11.5% and 8.32% at their respective lower limits of quantification. The limit of quantification was 2 ng/ml for both artemether and dihydroartemisinin.
Nevirapine was extracted from plasma with a protein precipitation procedure and was analyzed using a validated LC-MS/MS assay (8). The precision for nevirapine during sample analysis was less than 7% at all QC levels, including the limit of quantification, which was 200 ng/ml.
Safety data collection.
A clinical evaluation and full blood count, renal function tests, liver enzymes, and lactate and glucose blood tests were performed at screening and at the final safety visit 21 days after the first artemether-lumefantrine dose. CD4+ lymphocyte counts and HIV-1 viral loads and serum pregnancy tests (in all females) as well as urine tests for drugs of abuse (amphetamines, benzodiazepines, and opiates) were performed at screening. Twelve-lead electrocardiograms (ECGs) were performed at screening, predose and at the expected time of maximal lumefantrine plasma concentration (68 h postdose) (41). Independent cardiologists assessed all ECGs, and the QT interval was calculated using the Fridericia formula (17). Adverse events were solicited throughout the study, recording the onset, duration, severity, relationship to study drug, and need for treatment.
Statistical methods.
The sample size was calculated to demonstrate a 2-fold change in lumefantrine exposure (day 7 concentration or AUC), i.e., such that the 90% confidence intervals (CIs) for geometric mean ratios lie within the interval 0.5 to 2.0 (21) with a power of 80%. Thirteen participants were required in each group and a total of 18 participants were recruited for each arm to accommodate potential dropouts. Pharmacokinetic modeling was performed and data were analyzed using Stata 11 (StataCorp, College Station, TX). Concentrations below the limits of quantification were considered missing. Elimination half-life was calculated as ln(2)/λz, where λz is the first-order rate constant associated with the terminal (log-linear) portion of the curve, estimated by linear regression of time versus log concentration, using a default of the last 3 data points. Area under the concentration-time curve was calculated using the trapezoidal rule and extrapolated to infinity (AUC0-t). The 0.5- and 1-h samples after the first dose were censored to allow comparison of the exposure between the first and last dosing periods. Coefficients of variation are reported as an indication of variability, although the accuracy of this measure may be limited by the nonparametric distribution of some of the data.
Results from the lumefantrine, artemether, dihydroartemisinin, and nevirapine noncompartmental analyses were compared with various one- and two-compartment models to determine the model with the best fit. Kruskal-Wallis tests were used for continuous covariates, and chi-squared and Fisher's exact tests were used for count data, to assess between group differences in baseline characteristics, day 7 lumefantrine concentrations, nevirapine trough concentrations, and the pharmacokinetic parameters of both lumefantrine and nevirapine. A median regression analysis was performed to explore the determinants of the lumefantrine pharmacokinetic parameters.
To explore the determinants of the day 7 lumefantrine concentrations, linear regression of the log-transformed day 7 lumefantrine concentrations was used, and the geometric mean ratio (GMR) reported, after participant 30, an outlier with an extremely low lumefantrine concentration, was removed to satisfy normality criteria. Logistic regression was used to assess factors predicting concentrations below the reported therapeutic threshold of 280 ng/ml (41).
In order to account for the repeated measures per subject, mixed-effect regression models were used to assess the possible impact of dose-occasion on artemether and dihydroartemisinin exposure, where the responses analyzed were log-transformed AUC and maximal concentrations of drug in plasma (Cmax). Since both artemether and dihydroartemisinin are highly parasiticidal, their therapeutic efficacy is likely to correlate better with their combined exposures. To approximate this, we generated a cumulative total AUC expressed in dihydroartemisinin equivalents. The measured dihydroartemisinin and artemether AUCs were converted into dihydroartemisinin molar equivalents by using their molecular weights of 298 g/mol and 284 g/mol, respectively (35, 36). As dihydroartemisinin is 4 to 5 times more potent than artemether in vitro (10), the artemether-derived dihydroartemisinin equivalents were weighted one quarter of the dihydroartemisinin-derived dihydroartemisinin equivalents.
Prospectively defined covariates considered for inclusion in these multivariate models were age, weight-adjusted (mg/kg of body weight) dosage, concomitant medication (including co-trimoxazole), CD4+ lymphocyte cell count, albumin, hemoglobin, and creatinine.
Secondary safety endpoints included frequency and severity of adverse events, changes in hematological, serum biochemical, and urinalysis parameters, and vital signs between screening and follow-up. ECG parameters (P-R and QT intervals) were compared between screening and the presumed lumefantrine Cmax (68 h after the first artemether-lumefantrine dose).
RESULTS
Thirty-six clinically well adults (18 in each group) were recruited, and all were retained for the study duration. At the baseline, the groups were well matched for weight, sex, weight-adjusted (mg/kg) artemether-lumefantrine dose, and CD4+ lymphocyte count. Those on nevirapine-based ART were older than the ART-naïve patients (P = 0.002). ART-naïve patients had a median viral load of 3.76 log copies/ml, while those on nevirapine-based ART were virologically suppressed. Albumin (P = 0.017) and alkaline phosphatase (P = 0.033) were both elevated in the nevirapine group compared to those in the ART-naïve group, although the medians in both groups were within the normal range. The significantly higher mean corpuscular volume in the nevirapine group (P = 0.0001) was ascribed to stavudine and zidovudine use (Table 1). Eleven participants were on zidovudine and the remaining seven on stavudine, both with lamivudine. Six participants in the ART-naïve group and four in the nevirapine group (P = 0.46) were receiving co-trimoxazole prophylaxis.
Table 1.
Baseline characteristics in HIV-1-infected patients who were antiretroviral na ï ive or on nevirapine-based antiretroviral therapy
| Parameter | Naïve group (n = 18)a | Nevirapine group (n = 18)a | P valueb |
|---|---|---|---|
| No. (%) of females | 17 (94) | 15 (83) | 0.29c |
| Age (yr) | 27 (25–32) | 32 (29–40) | 0.002 |
| No. of black patients:patients of mixed race | 18:0 | 16:2 | 0.15d |
| No. (%) of patients on co-trimoxazole | 6 (33) | 4 (22) | 0.46d |
| Lumefantrine total dose (mg/kg) | 49.7 (43.0–52.4) | 49.3 (43.0–53.8) | 0.67 |
| Patient wt (kg) | 58 (55–67) | 58.5 (53.5–67) | 0.67 |
| Albumin (g/liter) | 40 (39–44) | 44.5 (43–47) | 0.017 |
| Creatinine clearance (umol/liter) | 101 (84–113) | 80 (70–91) | 0.003 |
| Alkaline phosphatase (U/liter) | 57.5 (50–69) | 73 (68–87) | 0.033 |
| Mean corpuscular vol (fl) | 86.7 (84.6–91) | 106.6 (100.2–109.9) | 0.0001 |
| CD4+ count (×106/liter) | 355 (260–507) | 343.5 (263–470) | 0.83 |
| HIV viral load | 3.76 (3.04–4.08) log copies/μl | <50 copies/μl | 0.0001 |
Values are medians (interquartile ranges [IQRs]) unless specified.
Reported P values were calculated using the Kruskal-Wallis test unless otherwise indicated.
P value was calculated using chi-squared tests for categorical variables.
P value was calculated using the Fisher's exact test.
Pharmacokinetic results. (i) Effect of nevirapine-based ART on lumefantrine plasma concentrations.
Lumefantrine plasma concentrations on day 7 were highly correlated with lumefantrine AUC (Pearson r2 = 0.94). Median (range) concentrations were 336 (29 to 934) ng/ml and 622 (185 to 2,040) ng/ml in the ART-naive and nevirapine groups, respectively (P = 0.0002). In the univariate linear regression model of the log-transformed day 7 concentrations (excluding the outlier patient 30), the geometric mean ratio (GMR) for the nevirapine group versus the ART-naïve group was 1.86 (95% CI, 1.33 to 2.59; P = 0.001). Results were similar when patient 30 was included in a median regression analysis.
Adjusting this model for covariates, the day 7 lumefantrine concentration in the nevirapine group was double that in the ART-naïve group (adjusted GMR, 2.09 [95% CI, 1.52 to 2.86]; P < 0.0001) and higher for subjects on co-trimoxazole (adjusted GMR, 1.57 [95% CI, 1.12 to 2.22], P = 0.011). There was a trend toward anemia increasing lumefantrine concentrations, with a 1-unit increase in hemoglobin decreasing lumefantrine concentrations by 11% (adjusted GMR, 0.89 [95% CI, 0.78 to 1.01]; P = 0.076). Neither zidovudine nor stavudine was associated with day 7 lumefantrine concentrations.
A third (6/18) of the ART-naïve participants had day 7 lumefantrine concentrations below the recommended therapeutic concentration (280 ng/ml), compared with 1/18 of those in the nevirapine group (odds ratio [OR] = 8.5 [95% CI, 0.9 to 80.02] P = 0.061).
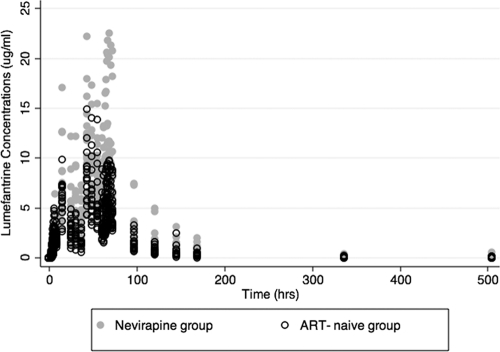
The lumefantrine plasma concentration-time curves (0 to 504 h) are depicted in Fig. 1. In the univariate analysis of the effect of treatment group on pharmacokinetic parameters, the median AUC and Cmax were higher in the nevirapine group than the ART-naïve group (AUC, 692.8 versus 445.1 μg·h/ml [P = 0.0011], and Cmax, 10.9 versus 8.8 μg/ml [P = 0.06]). The median elimination half-lives (t1/2s) and Tmaxs were similar between groups (Table 2).
Fig. 1.
Lumefantrine plasma concentrations (μg/ml) over time in HIV-1-infected patients who are antiretroviral naïve or on nevirapine-based antiretroviral therapy. ART, antiretroviral therapy. The time (in hours) after the first dose of artemether-lumefantrine is shown.
Table 2.
Lumefantrine pharmacokinetic parameters in HIV-1-infected patients who were antiretroviral-na ï ive or on nevirapine-based antiretroviral therapy
| Parametera | Value for patients who were: |
P value | |
|---|---|---|---|
| Antiretroviral naïve (n = 18) | On nevirapine therapy (n = 18) | ||
| Day 7 lumefantrine concn | |||
| Median (IQR) (μg/ml) | 0.34 (0.23–0.4) | 0.62 (0.47–0.72) | 0.0002 |
| CV (%) | 54.0 | 61.2 | |
| Cmax | |||
| Median (IQR) (μg/ml) | 8.76 (7.8–9.84) | 10.9 (8.21–14.4) | 0.06 |
| CV (%) | 26.3 | 39.9 | |
| Tmax | |||
| Median (IQR) (h) | 42.02 (41.98–66.0) | 48.0 (42.02–66.0) | 0.53 |
| CV (%) | 35.5 | 29.4 | |
| AUC(0–inf) | |||
| Median (IQR) (μg·h/ml) | 445.12 (356.6–552.9) | 692.8 (594.2–884.2) | 0.0011 |
| CV (%) | 33.5 | 45.8 | |
| t1/2 | |||
| Median (IQR) (days) | 4.1 (2.7–4.4) | 4.1 (3.5–4.4) | 0.68 |
| CV (%) | 25.7 | 18.1 | |
CV, coefficient of variation; Cmax, maximal concentration; Tmax, time to maximum concentration; AUC(0-inf), area under the plasma concentration-time curve, from 0 h to infinity; t1/2, elimination half-life.
After we adjusted for covariates in a multivariate median regression analysis, the median AUC values were 241.4 μg·h/ml higher in the nevirapine group than in the ART-naïve group (P = 0.001) and 213.9 μg·h/ml higher for subjects on co-trimoxazole (P = 0.007). The median Cmaxs were 2.14 μg/ml higher in the nevirapine group than in the ART-naïve group (P = 0.008) and were also 2.06 μg/ml higher for those on co-trimoxazole (P = 0.02). The median Cmaxs were 0.004 μg/ml higher for each 1-unit increase in the CD4 cell count (P = 0.033).
(ii) Effect of nevirapine-based ART on artemether and dihydroartemisinin exposure.
The artemether and dihydroartemisinin plasma concentration-time curves (0 to 8 h and 60 to 68 h) are depicted in Fig. 2, and pharmacokinetic parameters are summarized by treatment group and dosing time (Table 3).
Fig. 2.
Artemether and dihydroartemisinin plasma concentrations (ng/ml) over time after the first (0 to 8 h) and last (60 to 68 h) dosing occasion in HIV-1-infected patients who are antiretroviral naïve or on nevirapine-based antiretroviral therapy. ART, antiretroviral therapy; DHA, dihydroartemisinin. The time (in hours) after the first dose of artemether-lumefantrine is shown. (A) Points indicating concentration over time of artemether from 0 to 8 h after the initial dose of artemether-lumefantrine. (B) Points indicating concentration over time of artemether from 60 to 68 h after the initial dose of artemether-lumefantrine. (C) Points indicating concentration over time of dihydroartemisinin from 0 to 8 h after the initial dose of artemether-lumefantrine. (D) Points indicating concentration over time of dihydroartemisinin from 60 to 68 h after the initial dose of artemether-lumefantrine.
Table 3.
Artemether and dihydroartemisinin pharmacokinetic parametersa
| Parameterb | 0–8 h |
60–68 h |
||||
|---|---|---|---|---|---|---|
| Naive group | Nevirapine group | P value | Naïve group | Nevirapine group | P value | |
| Artemether | ||||||
| Cmax | ||||||
| Median (IQR) (ng/ml) | 59.7 (37.8–88.9) | 5.8 (3.9–21.7) | <0.0001 | 11.9 (8.2–17.5) | 5.2 (4.5–11.0) | 0.015 |
| CV (%) | 52.9 | 101.3 | 124.7 | 93.2 | ||
| Tmax | ||||||
| Median (IQR) (h) | 1.5 (1.5–2.0) | 2.0 (1.5–2.0) | 0.52 | 61.5 (61.5–62.0) | 61.5 (61.5–62.0) | 0.79 |
| CV (%) | 55.8 | 58.3 | 2.1 | 1.6 | ||
| AUC(0–inf) | ||||||
| Median (IQR) (ng·h/ml) | 151.0 (110.7–220.6) | 38.9 (25.5–98.7) | <0.0001 | 71.1 (45.5–114.2) | 31.9 (16.3–88.7) | 0.12 |
| CV (%) | 55.1 | 76.9 | 78.4 | 99.4 | ||
| t1/2 | ||||||
| Median (IQR) (h) | 1.5 (1.1–1.7) | 1.3 (0.9–1.9) | 0.52 | 2.9 (1.8–5.4) | 2.5 (1.9–3.7) | 0.61 |
| CV (%) | 44.2 | 77.6 | 96.7 | 43.8 | ||
| Dihydroartemisinin | ||||||
| Cmax | ||||||
| Median (IQR) (ng/ml) | 42.2 (31.8–63.1) | 47.3 (35.4–60.5) | 0.24 | 40.0 (31.2–66.7) | 34.5 (23.8–43.0) | 0.08 |
| CV (%) | 48.8 | 35.3 | 82.9 | 35.4 | ||
| Tmax | ||||||
| Median (IQR) (h) | 2.0 (1.5–4.0) | 2.0 (1.5–2.0) | 0.08 | 61.5 (61.5–62.0) | 61.5 (61.5–63.0) | 0.81 |
| CV (%) | 47.6 | 38.9 | 1.8 | 2.0 | ||
| AUC(0–inf) | ||||||
| Median (IQR) (ng·h/ml) | 123.8 (101.3–235.6) | 150.9 (126.1–208.9) | 0.21 | 165.7 (143.7–246.5) | 125.0 (94.6–165.1) | 0.01 |
| CV (%) | 46.8 | 29.5 | 66.4 | 44.4 | ||
| t1/2 | ||||||
| Median (IQR) (h) | 1.6 (1.3–2.1) | 1.6 (1.4–1.9) | 0.77 | 2.0 (1.8–2.7) | 1.5 (1.2–2.4) | 0.13 |
| CV (%) | 74.0 | 20.8 | 38.9 | 33.4 | ||
Values for HIV-1-infected patients who are antiretroviral na ï ive or on nevirapine-based antiretroviral therapy, after the 1st dose (0 to 8 h) and 6th dose (60 to 68 h). IQR, interquartile range.
CV, coefficient of variation; Cmax, maximal concentration; Tmax, time to maximum concentration; AUC(0-inf), area under the plasma concentration-time curve, from 0 h to infinity; t1/2, elimination half-life.
Between 0 and 8 h, the nevirapine group had a 4-fold lower artemether median (range) AUC(0-8 h) (38.9 [18.0 to 142.0] ng·h/ml) than the ART-naïve group (151.0 [41.1 to 474.0] ng·h/ml) (P < 0.0001). Median (range) Cmaxs were 10-fold lower in the nevirapine group (5.8 [2.89 to 47.8] ng/ml) than the ART-naïve group (59.7 [13.5 to 140] ng/ml) (P < 0.0001). Artemether t1/2s and Tmaxs were similar between treatment groups. For dihydroartemisinin at 0 to 8 h, all parameters were similar between groups (Table 3).
After the last artemether-lumefantrine dose (60 to 68 h), the artemether median (range) AUC(60-68 h) remained lower in the nevirapine group (31.9 [13.5 to 146.3] ng·h/ml) compared with the ART-naïve group (71.2 [16.2 to 301.0] ng·h/ml), although this effect was no longer statistically significant (P = 0.12). The median (range) Cmax remained significantly lower in the nevirapine group (5.2 [2.3 to 29.9] ng/ml), compared with the ART-naïve group (11.9 [2.9 to 107.0] ng/ml) (P = 0.015). The median (range) dihydroartemisinin AUC(60-68 h) was also significantly lower in the nevirapine group (125.0 [48.3 to 290.3] ng·h/ml) than in the ART-naïve group (165.7 [72.4 to 728.7] ng·h/ml) (P = 0.01).
The mixed-effect model exploring the determinants of artemether exposure indicated significant effects of the nevirapine group, dose occasion, and co-trimoxazole. There were interactions between these effects, with the impact of nevirapine differing by dose occasion and in subjects on co-trimoxazole and the dose occasion effect differing in the naïve and nevirapine groups. For the dose occasion effect, in the ART-naive group, both the artemether AUCs and Cmaxs were significantly lower in the 60- to 68-h period than in the 0 to 8 h period (GMR [95% CI)] values: AUC, 0.41 [0.31 to 0.55] ng·h/ml; Cmax, 0.23 [0.17 to 0.32] ng/ml]. In the nevirapine group, this decrease over time was less pronounced and was statistically significant only for Cmax (GMR [95% CI] values: AUC, 0.84 [0.56 to 1.26] ng·h/ml; Cmax, 0.71 [0.51 to 0.98] ng/ml).
Overall, subjects in the nevirapine group had lower artemether AUCs and Cmaxs than those in the ART-naïve group, but over time, this effect became less pronounced. For those subjects not on co-trimoxazole, the nevirapine effect on artemether was not statistically significant during the 60- to 68-h dosing period. Co-trimoxazole similarly decreased artemether exposure, and this effect did not vary over time. However, lower AUCs and Cmaxs were observed for subjects on both nevirapine and co-trimoxazole than for subjects on nevirapine alone, irrespective of dose occasion. The following covariates were significantly inversely related to artemether exposure: albumin, age, and CD4 cell count.
In the mixed-effect model for dihydroartemisinin exposure (Table 4) during the 0- to 8-h period, the nevirapine group tended to have higher dihydroartemisinin AUC(0-8 h)s and Cmaxs than the ART-naïve group (with P values of 0.089 and 0.06, respectively). This relationship changed for the 60- to 68-h period, when the nevirapine group had a significantly lower AUC(60-68 h) than the ART-naïve group (P = 0.037), but Cmaxs were similar between treatment groups.
Table 4.
Mixed-effects model of effects of dose occasion, treatment group, and covariates
| Parameter (variable) | GMR (95% CI) [P]a |
||
|---|---|---|---|
| Dihydroartemisinin exposure |
Combined artemether and dihydroartemisinin exposure (AUC, dihydroartemisinin mol eq)b | ||
| AUC | Cmax | ||
| Dose occasion effect (last/first dose occasion) | |||
| Naive group | 1.30 (1.11–1.51) [0.001] | 1.12 (0.89–1.40) [0.33] | 1.12 (0.95–1.31) [0.17] |
| Nevirapine group | 0.797 (0.685–0.927) [0.003] | 0.690 (0.550–0.865) [0.001] | 0.76 (0.65–0.90) [<0.0001] |
| Treatment group effect (naive group/nevirapine group) | |||
| 0–8 h | 1.244 (0.968–1.599) [0.09] | 1.362 (0.987–1.880) [0.06] | 1.24 (0.95–1.62) [0.11] |
| 60–68 h | 0.765 (0.595–0.984) [0.037] | 0.840 (0.609–1.160) [0.29] | 0.84 (0.65–1.11) [0.22] |
| Covariate | |||
| Unit increase in albumin (g/liter) | ND | 0.971 (0.941–1.002) [0.06] | 0.97 (0.95–1.00) [0.054] |
| Unit increase in age (yr) | 0.978 (0.963–0.993) [0.003] | 0.981 (0.965–0.998) [0.026] | 0.976 (0.962–0.991) [0.001] |
| Each mg/kg increase in wt-adjusted dose | 1.126 (1.033–1.227) [0.007] | 1.085 (0.988–1.192) [0.09] | 2.12 (1.31–3.45) [0.002] |
ND, not determined.
The area under the plasma concentration-time curve of combined artemether and dihydroartemisinin exposure, expressed as dihydroartemisinin molar equivalents after adjusting for relative bioactivities, in HIV-infected patients who are antiretroviral naï ive or on nevirapine-based antiretroviral therapy.
Dihydroartemisinin AUC values for both groups changed significantly between the 1st and 6th dose, increasing in the ART-naïve group but decreasing in the nevirapine group. Dihydroartemisinin Cmaxs in the nevirapine group decreased significantly between the 1st and 6th doses (P = 0.01), but no significant change over time was observed for the ART-naïve group. Dihydroartemisinin AUC was inversely associated with age and weight-adjusted dose (P values of 0.003 and 0.007, respectively), and Cmax was inversely associated with age (P = 0.026) and possibly with albumin (P = 0.063) and weight-adjusted dose (P = 0.088) (Table 4).
The results of the mixed-effects model for the combined artemether and dihydroartemisinin AUCs, expressed as dihydroartemisinin equivalents after adjusting for their relative bioactivities, are summarized in Table 4. The dose occasion (“autoinduction”) effect was reduced, and this was statistically significant only in the nevirapine group (GMR [95% CI] values: 0.76 [0.62 to 0.90]; P < 0.0001). Importantly, increasing each artemether dose by 1 mg/kg was associated with a doubling of the dihydroartemisinin-equivalent AUC (GMR [95% CI] values: 2.12 [1.31 to 3.45]; P = 0.002). The trend toward a negative correlation of albumin concentration with AUC (in dihydroartemisinin equivalents) improved the model, while this was not seen for the area under the plasma dihydroartemisinin concentration time curve.
(iii) Nevirapine concentrations.
The nevirapine median AUC was 117.16 mg·h/liter (range, 82.45 to 481.59 mg·h/liter), the median Cmax was 6.26 mg/liter (range, 4.43 to 21.21 mg/liter), and the median Tmax was 4.35 mg/liter (range, 2.00 to 24.69 mg/liter). The median 12-hour trough concentration was 4.8 mg/liter (range, 3.16 to 21.21 mg/liter).
Adverse events.
Artemether-lumefantrine was well tolerated, and there were no serious adverse events. There were 95 adverse events overall, 46 in the nevirapine group and 49 in the ART-naïve group. Thirty-two were considered possibly related to study drug, 17 and 15 in the naïve and nevirapine groups, respectively. All events were classified as mild. The most common adverse events considered possibly related to artemether-lumefantrine were dyspepsia (n = 4), nausea (n = 4), flatulence (n = 3), abdominal bloating (n = 3), and headaches (n = 5). There were no clinically relevant changes in the biochemical or hematological parameters between baseline and study completion. There was no difference in median QTcF intervals between treatment groups at the presumed Tmax for lumefantrine concentration (P = 0.51), nor was there a difference in the median QTcF intervals between screening and presumed Tmax (P = 0.8). The difference between the baseline and 68-h PR interval was not associated with either lumefantrine concentration at 68 h (P = 1.0) or nevirapine group (P = 0.5).
DISCUSSION
We report the first prospective clinical data that nevirapine may both increase and decrease exposure to concomitant medications. Our HIV-1-infected patients on nevirapine-based ART had significantly higher concentrations of lumefantrine and lower concentrations of both artemether and dihydroartemisinin than CD4-count-matched controls. We had expected that nevirapine would decrease both lumefantrine and artemether concentrations, as both are CYP3A4 substrates, which are induced by CYP3A4 enzymes (5, 16, 19).
It is concerning that one-third of our ART-naïve group had day 7 lumefantrine concentrations below the threshold considered therapeutic for successful treatment of uncomplicated malaria (280 ng/ml). Patients with subtherapeutic concentrations are generally at increased risk of treatment failure and may fuel the spread of lumefantrine resistance (9, 40). This effect would become more apparent as lumefantrine resistance emerges and spreads (6).
The day 7 lumefantrine concentrations in the nevirapine group were not only higher than our ART-naïve group but also higher than those previously reported in adult malaria patients (7, 9, 12, 25, 27, 28, 41), so our finding cannot be explained as lower lumefantrine bioavailability in untreated HIV-infected patients. Despite these high plasma lumefantrine concentrations, our safety results are reassuring. Adverse effects did not increase in frequency or severity, nor was the QTcF or PR interval prolonged in the nevirapine group.
There are some prior data indicating that nevirapine may act as an inhibitor of concomitant medication reportedly metabolized by CYP3A4. Compared with historical controls, concomitant nevirapine increased the Cmax and AUC of darunavir (29) and maraviroc (26). Nevirapine has also been reported to increase nelfinavir exposure when added to boosted nelfinavir treatment in a healthy volunteer study (1). Given that both the maximum concentrations and AUC increased, this may reflect either increased bioavailability or decreased metabolism. Competitive inhibition of enzyme binding sites or variability in drug transporters, such as P-glycoprotein or the organic anion transporting polypeptide family of enzymes, may account for the differences we found (33, 42). Our study was not designed to explore the mechanisms of the interaction between nevirapine and lumefantrine. Further research into possible mechanisms of these nevirapine interactions are warranted, as this may indicate other clinically important interactions that require prospective study.
The expected induction effect of nevirapine was seen with artemether and dihydroartemisinin. Fortunately, artemether is rapidly converted to its active metabolite dihydroartemisinin, and the resulting increased dihydroartemisinin exposure in the critical initial 8 h of treatment compensated for the lower artemether exposure in the nevirapine group. This is shown with the combined exposure to artemether and dihydroartemisinin in the mixed-effects model of area under the dihydroartemisinin-molar-equivalent time curve. However, after the last dose, this combined exposure was significantly lower only in the nevirapine group. Another important finding in this combined analysis is the significance of mg/kg dose, showing that an 80-kg patient given the same recommended adult dose would have less than half the dihydroartemisinin-molar-equivalent AUC of a 40-kg patient given the same recommended adult dose. A trend toward an increased risk of treatment failure has previously been reported for patients weighing over 65 kg (18). In that study, subgroup analysis according to body weight category showed 28-day parasitological cure rates of 100% (95% CI, 92.5% to 100.0%) in patients of body weight 65 kg or below and 93.4% (95% CI, 85.3% to 97.8%) in patients weighing above 65 kg.
The autoinduction previously reported for the artemesinins, including artemether (4, 32, 37, 38), was consistent with our study finding concentrations after the last artemether dose (60 to 68 h) significantly lower than after the first dose (0 to 8 h). Decreased overall artemether and dihydroartemisinin exposure may increase the risk of delayed parasite clearance in malaria patients on nevirapine-based ARVs, particularly given recent evidence of resistance to the artemisinins emerging in Southeast Asia (11, 24). However, malaria illness could attenuate the autoinduction seen in our clinically well HIV-infected patients and in previously described studies on healthy volunteers. Artemether and dihydroartemisinin exposure in our ART-naïve group is similar to previously published results in healthy volunteer studies (15, 21), suggesting that there is not a marked HIV disease effect.
Unexpectedly, co-trimoxazole prophylaxis was also associated with a doubling in lumefantrine exposure and a slight decrease in artemether exposure but not with the more clinically important dihydroartemisinin exposure. Adjusting for co-trimoxazole did not decrease the significance or alter the direction of the group effect on lumefantrine. The interaction of hepatically metabolized artemether-lumefantrine with co-trimoxazole is poorly delineated and may be due to inhibition of renal tubular secretion or liver enzyme inhibition (22, 31).
Our study has several limitations. As we expected nevirapine to cause subtherapeutic artemether-lumefantrine exposure, our study excluded patients with malaria, and the results may not be the same in patients acutely ill with malaria. Dosing was planned with a minimum of 1.2 g of fat; however, we subsequently learned that 40 ml of local soya milk contains only 0.8 g of fat (2). Patients received a meal containing a minimum of 6 g of fat with 5 of their 6 doses, but dose 2 was given with 40 ml of soya milk (0.8 g of fat) only. As both groups were treated uniformly, the fat allocation could not have accounted for the observed difference between groups. Lumefantrine is chemically similar to halofantrine, which is known to cause a concentration-dependent QT interval prolongation (34). Our ECGs were performed at 68 h, the presumed lumefantrine Tmax based on data from prior studies. However, the median Tmax for lumefantrine in our study was 42 and 48 h in the ART-naïve and nevirapine groups, respectively. Mixed-effect regression, taking repeated measures and group effect into account, showed lumefantrine concentrations at 42 to 48 and 68 h to be similar (P = 0.19). The participants in our study were predominantly female, reflecting the use of nevirapine use as current first-line treatment for women of child-bearing age in South Africa. This may affect the external validity of the study and supports the need for further study in a broader population. Differences in baseline characteristics (albumin, mean corpuscular volume, alkaline phosphatase, and age) between treatment groups may reflect an HIV disease effect or direct antiretroviral therapy effect. However, the nevirapine effect on artemether-lumefantrine exposure and the difference in exposure seen between dosing periods in artemether and dihydroartemisinin remained significant after correcting for these potential confounders.
Conclusions.
Although nevirapine is widely regarded as an enzyme inducer, our data indicate that it may also inhibit the metabolism of some drugs. The mechanism for this effect remains to be elucidated. Despite the elevated lumefantrine concentrations, artemether-lumefantrine has a favorable adverse effect profile. The high prevalence of subtherapeutic lumefantrine concentrations in our ART-naïve patients and the decreased artemether and dihydroartemisinin concentrations in our nevirapine group may be cause for concern when using artemether-lumefantrine for treating malaria in patients coinfected with HIV. In addition to the effect of drug interactions, HIV has been shown to increase P. falciparum biomass, with the largest relative increases seen in southern Africa (39). The strong correlation of weight-adjusted dose with combined artemether/dihydroartemisinin exposure shown suggests that large adults may be underdosed with current dosing recommendations. White and colleagues have cautioned that current dosing recommendations provide a resistance selection opportunity in those patients with low drug levels and high parasite burdens (40). This could not be evaluated in our small study of HIV-infected patients who did not have malaria, particularly since therapeutic levels of exposure to artemether/dihydroartemisinin have yet to be defined. There is an urgent need to study artemether-lumefantrine exposure in patients with comorbid HIV/AIDS and malaria and to evaluate its interactions with nevirapine and other widely used antiretrovirals in this important target population.
ACKNOWLEDGMENTS
This investigator-initiated study was funded by the Haughton Institute, which is funded through the Global Health Research Board from the Irish Department of Foreign Affairs and by the ACT Consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine.
We gratefully acknowledge the contributions of the study patients for their time and patience, the SEACAT evaluation team, in particular Rae Thomas, Liz Allen, and Ludwig Heiberg, and the invaluable guidance provided by the Data Safety Monitoring Board (Anton Pozniak, Marta Boffito, Piero Olliaro, Bill Burman, and Bonnie Cundill).
G.M. has received a grant from Novartis to support academic doctoral training. The remaining authors declare that they have no potential conflicts of interest that may have unduly influenced the results of this study.
Footnotes
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Aarnoutse R. E. 2003. Combination of nelfinavir/ritonavir and nevirapine in a once daily antiretroviral regimen for healthy volunteers. Ph.D. thesis. University Medical Centre Nijmegen, Nijmegen, Netherlands [Google Scholar]
- 2. Ashley E. A., et al. 2007. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop. Med. Int. Health 12: 195–200 [DOI] [PubMed] [Google Scholar]
- 3. Ashley E. A., et al. 2007. Pharmacokinetic study of artemether-lumefantrine given once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop. Med. Int. Health 12: 201–208 [DOI] [PubMed] [Google Scholar]
- 4. Asimus S., Gordi T. 2007. Retrospective analysis of artemisinin pharmacokinetics: application of a semiphysiological autoinduction model. Br. J. Clin. Pharmacol. 63: 758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Back D., Gibbons S., Khoo S. 2003. Pharmacokinetic drug interactions with nevirapine. J. Acquir. Immune Defic. Syndr. 34(Suppl. 1): S8–S14 [DOI] [PubMed] [Google Scholar]
- 6. Barnes K. I., Watkins W. M., White N. J. 2008. Antimalarial dosing regimens and drug resistance. Trends Parasitol. 24: 127–134 [DOI] [PubMed] [Google Scholar]
- 7. Checchi F., et al. 2006. Supervised versus unsupervised antimalarial treatment with six-dose artemether-lumefantrine: pharmacokinetic and dosage-related findings from a clinical trial in Uganda. Malar. J. 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen K., et al. 2008. Effect of rifampicin-based antitubercular therapy on nevirapine plasma concentrations in South African adults with HIV-associated tuberculosis. J. Antimicrob. Chemother. 61: 389–393 [DOI] [PubMed] [Google Scholar]
- 9. Denis M. B., et al. 2006. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop. Med. Int. Health 11: 1800–1807 [DOI] [PubMed] [Google Scholar]
- 10. de Vries P. J., Dien T. K. 1996. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52: 818–836 [DOI] [PubMed] [Google Scholar]
- 11. Dondorp A. M., et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361: 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ezzet F., Mull R., Karbwang J. 1998. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br. J. Clin. Pharmacol. 46: 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ezzet F., van Vugt M., Nosten F., Looareesuwan S., White N. J. 2000. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob. Agents Chemother. 44: 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15. German P., et al. 2009. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J. Acquir. Immune Defic. Syndr. 51: 424–429 [DOI] [PubMed] [Google Scholar]
- 16. Giao P. T., de Vries P. J. 2001. Pharmacokinetic interactions of antimalarial agents. Clin. Pharmacokinet. 40: 343–373 [DOI] [PubMed] [Google Scholar]
- 17. Goldenberg I., Moss A. J., Zareba W. 2006. QT interval: how to measure it and what is “normal”. J. Cardiovasc. Electrophysiol. 17: 333–336 [DOI] [PubMed] [Google Scholar]
- 18. Hatz C., et al. 2008. Treatment of acute uncomplicated falciparum malaria with artemether-lumefantrine in nonimmune populations: a safety, efficacy, and pharmacokinetic study. Am. J. Trop. Med. Hyg. 78: 241–247 [PubMed] [Google Scholar]
- 19. Khoo S., Back D., Winstanley P. 2005. The potential for interactions between antimalarial and antiretroviral drugs. AIDS 19: 995–1005 [DOI] [PubMed] [Google Scholar]
- 20. Lefèvre G., et al. 2000. Pharmacokinetic interaction trial between co-artemether and mefloquine. Eur. J. Pharm. Sci. 10: 141–151 [DOI] [PubMed] [Google Scholar]
- 21. Lefèvre G., et al. 2002. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet) with concomitant administration of ketoconazole in healthy subjects. Br. J. Clin. Pharmacol. 54: 485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masters P. A., O'Bryan T. A., Zurlo J., Miller D. Q., Joshi N. 2003. Trimethoprim-sulfamethoxazole revisited. Arch. Intern. Med. 163: 402–410 [DOI] [PubMed] [Google Scholar]
- 23. McGready R., et al. 2008. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated plasmodium falciparum treatment in pregnancy. PLoS Med. 5: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noedl H., et al. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359: 2619–2620 [DOI] [PubMed] [Google Scholar]
- 25. Piola P., et al. 2005. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet 365: 1467–1473 [DOI] [PubMed] [Google Scholar]
- 26. Pozniak A. L., Boffito M., Russell D., Ridgway C. E., Muirhead G. J. 2008. A novel probe drug interaction study to investigate the effect of selected antiretroviral combinations on the pharmacokinetics of a single oral dose of maraviroc in HIV-positive subjects. Br. J. Clin. Pharmacol. 65(Suppl. 1): 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Price R. N., et al. 2006. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin. Infect. Dis. 42: 1570–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman M. M., et al. 2008. Adherence and efficacy of supervised versus non-supervised treatment with artemether/lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Bangladesh: a randomised controlled trial. Trans. R. Soc. Trop. Med. Hyg. 102: 861–867 [DOI] [PubMed] [Google Scholar]
- 29. Sekar V., et al. 2009. Pharmacokinetic interaction between nevirapine and darunavir with low-dose ritonavir in HIV-1-infected patients. Br. J. Clin. Pharmacol. 68: 116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. South African National Department of Health 2004. National antiretroviral treatment guidelines, 1st ed. Jacana, Pretoria, South Africa [Google Scholar]
- 31. Stockley I. H. (ed.). 2002. Stockley's drug interactions, 6th ed. Pharmaceutical Press, Nottingham, United Kingdom [Google Scholar]
- 32. Svensson U. S., Alin H., Karlsson M. O., Bergqvist Y., Ashton M. 2002. Population pharmacokinetic and pharmacodynamic modelling of artemisinin and mefloquine enantiomers in patients with falciparum malaria. Eur. J. Clin. Pharmacol. 58: 339–351 [DOI] [PubMed] [Google Scholar]
- 33. Tang C., Lin J. H., Lu A. Y. 2005. Metabolism-based drug-drug interactions: what determines individual variability in cytochrome P450 induction? Drug Metab. Dispos. 33: 603–613 [DOI] [PubMed] [Google Scholar]
- 34. Tie H., et al. 2000. Inhibition of HERG potassium channels by the antimalarial agent halofantrine. Br. J. Pharmacol. 130: 1967–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. United States National Library of Medicine. Artemether, ChemIDplus Advanced. National Institutes of Health, Bethesda, MD: http://chem.sis.nlm.nih.gov/chemidplus/. Accessed 16 September 2011 [Google Scholar]
- 36. United States National Library of Medicine Dihydroartemisinin, ChemIDplus Advanced. National Institutes of Health, Bethesda, MD: http://chem.sis.nlm.nih.gov/chemidplus/. Accessed 16 September 2011 [Google Scholar]
- 37. van Agtmael M. A., Cheng-Qi S., Qing J. X., Mull R., van Boxtel C. J. 1999. Multiple dose pharmacokinetics of artemether in Chinese patients with uncomplicated falciparum malaria. Int. J. Antimicrob. Agents 12: 151–158 [DOI] [PubMed] [Google Scholar]
- 38. van Agtmael M. A., Gupta V., van der Graaf C. A. A., van Boxtel C. J. 1999. The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin. Pharmacol. Ther. 66(4): 408–414 [DOI] [PubMed] [Google Scholar]
- 39. Van Geertruyden J. P., Menten J., Colebunders R., Korenromp E., D'Alessandro U. 2008. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar. J. 7: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White N. J., et al. 2009. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar. J. 8: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White N. J., van Vugt M., Ezzet F. 1999. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin. Pharmacokinet. 37: 105–125 [DOI] [PubMed] [Google Scholar]
- 42. Wilkinson G. R. 2008. Membrane transporters and drug response, p. 26–42 In Parker K., Brunton L. L., Blumenthal D., Buxton I. (ed.), Goodman and Gilman's manual of pharmacology and therapeutics. McGraw-Hill, New York, NY [Google Scholar]
- 43. World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 44. World Health Organization 2004. Technical consultation on malaria and HIV interactions and public health policy implications. World Health Organization, Geneva, Switzerland [Google Scholar]