Abstract
Malaria is one of the deadliest infectious diseases in the world, with the eukaryotic parasite Plasmodium falciparum causing the most severe form of the disease. Discovery of new classes of antimalarial drugs has become an urgent task to counteract the increasing problem of drug resistance. Screening directly for compounds able to inhibit parasite growth in vitro is one of the main approaches the malaria research community is now pursuing for the identification of novel antimalarial drug leads. Very recently, thousands of compounds with potent activity against the parasite P. falciparum have been identified and information about their molecular descriptors, antiplasmodial potency, and cytotoxicity is publicly available. Now the challenges are how to identify the most promising chemotypes for further development and how best to progress these compounds through a lead optimization program to generate antimalarial drug candidates. We report here the first chemical series to be characterized from one of those screenings, a completely novel chemical class with the generic name cyclopropyl carboxamides that has never before been described as having antimalarial or other pharmacological activities. Cyclopropyl carboxamides are potent inhibitors of drug-sensitive and -resistant strains of P. falciparum in vitro and show in vivo oral efficacy in malaria mouse models. In the present work, we describe the biological characterization of this chemical family, showing that inhibition of their still unknown target has very favorable pharmacological consequences but the compounds themselves seem to select for resistance at a high frequency.
INTRODUCTION
Malaria is one of the most severe infectious diseases, affecting mainly countries in the developing world. With more than 220 million cases and 781,000 deaths reported in 2009 (27), the heavy malaria burden demands urgent solutions. While malaria vaccines complete their clinical development (1, 9, 18, 21) and integrated approaches to malaria eradication start to make an impact (2), effective treatment of symptomatic malaria is still the cornerstone of malaria control. As the parasite evolves continuously to develop resistance, even to the newest front-line antimalarials, the spread of that resistance can soon compromise current treatments (26). The efficacy of artemisinin-based combination therapies (ACTs) is starting to be curtailed by the emergence of resistance to the endoperoxide component of the combination (2, 8, 14, 24). This fact has added renewed urgency to the search for new effective antimalarial drugs useful in public health settings.
Antimicrobial-target-based lead discovery has produced disappointing results over recent years, one of the main reasons being a lack of whole-cell activity of the compounds identified as target inhibitors. This is well documented in the case of antibacterials (16). To ensure this property early in the identification of new leads, the antimalarial community is pursuing whole-cell screening as a valuable source of new chemical starting points. In recent years, thousands of new molecules with potent activity against Plasmodium falciparum have been identified and information about them publicly released (10, 11, 17) (http://www.ebi.ac.uk/chemblntd). Molecules identified through this approach will not only have some desirable physicochemical properties from the beginning (such as cell penetration), but they also will be active against the relevant target in its intracellular context, a much more physiological situation than when using purified recombinant enzymes for screening.
A chemical series from a whole-cell screening has already reached clinical development (20). The present work describes the first series to be characterized from one of the latter screenings (10). It represents a new chemical class of inhibitors, designated cyclopropyl carboxamides, not previously described to have antimalarial or other pharmacological activities. Cyclopropyl carboxamides have been considered for lead optimization because they are drug-like, chemically developable molecules with potent inhibitory activity against drug-sensitive and -resistant P. falciparum strains and oral efficacy in malaria mouse models. An unexpected challenge which has arisen in the characterization of these molecules is their propensity to select for resistant parasites, which, if not solved, will hamper further progression of the series.
MATERIALS AND METHODS
Culture of P. falciparum strains.
P. falciparum strain 3D7A and the rest of the strains used in this study (Dd2, HB3, K1, TM90C2B, and FCR3) were obtained from the Malaria Research and Reference Reagent Resource Center (MR4). Accurate descriptions can be obtained at http://www.mr4.org. Parasites were grown in human erythrocytes (from a transfusion blood bank in Madrid, Spain) with Albumax II medium as previously described (23). RPMI 1640 medium (Gibco) was supplemented with 0.5% Albumax II (Invitrogen), 2% d-sucrose (Sigma-Aldrich), 0.3% glutamine (Sigma-Aldrich), and 150 mM hypoxanthine (Sigma-Aldrich). Cultures were maintained at 37°C at an atmosphere of 5% O2, 5% CO2, and 90% N2.
In vitro antiplasmodial activity.
Parasite growth inhibition assays and 50% inhibitory concentration (IC50) determinations were carried out by following standard methods using the [3H]hypoxanthine incorporation assay (7). Briefly, cultures with 0.5% parasitemia and 2% hematocrit were incubated with the drug as described for 48 h. After this period, parasites were harvested and the amount of incorporated radiolabeled precursor was determined. Radioactivity was added 24 h before harvesting of the parasites.
In vitro cytotoxicity.
Cytotoxicity was measured using a panel of six different mammalian cell lines, i.e., human hepatoma (HepG2), mouse lymphoid (L1210), human neural (Neuro2A), dog kidney (MDCK1), and human cardiac (H92c and HC-04) cell lines. Cells were grown and maintained in Eagle's minimum essential medium (Sigma-Aldrich) supplemented with 2 mM l-glutamine and 10% fetal calf serum. Cultures were maintained at 37°C in a humidified incubator containing 5% CO2 and 95% air and routinely passaged upon reaching 80 to 90% confluence. For experiments, cells were seeded into 96-well clear-bottom black plates coated with type I collagen (Biocoat, reference 354649; Becton Dickinson) at a cell density of 104 cells/well.
To measure cytotoxicity, cells were exposed to serial dilutions of test compounds for 48 h at 37°C. The culture medium was as described above but supplemented with 5% fetal calf serum. Following the 48-h exposure period, a 0.004% resazurin solution was prepared by adding 60 ml Dulbecco's phosphate-buffered saline to each tablet of resazurin (reference 330884Y; VWR International). The tablet was allowed to dissolve by placing the container in a bath set at 37°C, protected from light, for approximately 30 min.
Medium was removed, and 200 μl of fresh culture medium and 50 μl of resazurin solution were added to each well. Plates were incubated for 90 min. The fluorescence was allowed to stabilize at room temperature for 15 min protected from light.
Fluorescence was measured using a fluorescence plate reader (Victor V; Perkin-Elmer) at an excitation wavelength of 515 nm and an emission wavelength of 590 nm. Percent inhibition relative to the control wells was calculated.
In vivo efficacy studies, P. falciparum mouse model.
Cohorts of age-matched female immunodeficient NOD-scid IL-2Rγnull mice (Charles River, Gannat, France, under license from The Jackson Laboratory, Bar Harbor, ME) were engrafted with human erythrocytes (generously provided by a transfusion blood bank in Madrid, Spain) by daily intraperitoneal injection of 1 ml of a 50% hematocrit erythrocyte suspension throughout the experiment using RPMI 1640 medium–25% (vol/vol) decomplemented human serum–3.1 mM hypoxanthine (Sigma) for suspension as described previously (3, 13). When mice reached 40% human erythrocyte engraftment, they were intravenously infected with 2 × 107 P. falciparum Pf3D70087/N9-infected erythrocytes on day 0. On day 3 after infection, mice were randomly distributed into groups of three and treated orally once a day for 4 consecutive days with the vehicle or increasing doses of the compounds formulated in water–20% captisol. Parasitemia was measured by flow cytometry in samples of peripheral blood stained with the fluorescent nucleic acid dye SYTO-16 and anti-murine erythrocyte TER119 monoclonal antibody (Pharmingen, San Diego, CA) as previously described (12). Efficacy was expressed as the dose in mg/kg that reduced parasitemia at day 7 after infection in compound-treated mice by 50% or 90% with respect to vehicle-treated groups (50 and 90% effective doses [ED50 and ED90], respectively). All experiments were approved by the DDW Ethical Committee on Animal Research, performed at the DDW Laboratory Animal Science facilities accredited by AAALAC, and conducted according to European Union legislation and GlaxoSmithKline policy on the care and use of animals.
In vivo tolerability/plasma exposure.
In vivo experiments were conducted to estimate the generic toxicity of the drug family when administered by the oral route. These studies were also used to calculate drug exposures by measuring blood drug levels at the doses used. Compounds were administered orally as a suspension in 1% methyl cellulose at 20 ml/kg, starting with 1,000 mg/kg. If signs of toxicity were observed, the dose without signs was identified following an up-and-down procedure based on a functional observational battery. Compounds were administered as a single dose to groups of 6 female CD-1 mice of 8 weeks of age at the corresponding selected doses. Animals were observed for signs of overt toxicity/poor tolerability every 15 min for the first hour postdosing and then hourly for 4 h after dosing. Clinical chemistry and hematological analyses were performed 24 h after administration. Blood samples (25 μl) drawn from the tail vein were collected 1, 3, 6, 24, and 48 h after oral administration following a sparse sampling schedule, with 3 mice per sampling time point and a maximum of 2 samples per mouse for the first 24 h. Samples were diluted with 25 μl of an aqueous solution of 1% (wt/vol) saponin to lyse erythrocytes, processed under standard liquid-liquid extraction conditions, and stored at −80°C until analysis. Concentrations of both compounds in blood were quantified using high-pressure liquid chromatography coupled with tandem mass spectrometry. The maximum drug concentration in serum (Cmax) and the area under the concentration-time curve (AUC) were calculated using noncompartmental analysis (WinNonlin 5.2).
Studies of compound action throughout the intraerythrocytic life cycle.
To assess the effects of cyclopropyl carboxamides on the individual intraerythrocytcytic stages, parasitized erythrocytes (pRBC) were subjected to sorbitol synchronization. Ring-enriched cultures were allowed to complete a full 48-h cycle prior to drug treatment to ensure that results were not affected by the synchronization procedure.
Drug treatments were carried out at 10 times the IC50 for each of the drugs used. Parasites were cultured in the presence and absence of each drug at 1% parasitemia and 2% hematocrit using P6 sterile well plates incubated under standard conditions at 37°C. Treatments were maintained for 72 h. The morphology of treated parasites was compared to that of untreated controls every 24 h by examining Giemsa-stained thin blood smears. Images of the more representative phenotypes in the smears were captured by an Olympus BX41 microscope using a 100× oil immersion lens.
Selection of resistant parasites clones by limiting dilution.
A P. falciparum 3D7A inoculum of 108 parasites at 2% parasitemia and 5% hematocrit was exposed to a concentration of GSK2645947A corresponding to 10 times the IC50. Medium supplemented with the drug was changed every other day. Fresh erythrocytes were added once a week. Cultures were observed daily by Giemsa staining of thin blood smears. Exposure to inhibitor was maintained until cultures reached a detectable parasitemia and robust growth. Drug pressure was then removed, and cultures were maintained for two additional weeks to ensure the stability of the resistant phenotype. Resistant parasites underwent a cloning process by limiting dilution to obtain genetically homogeneous parasites for further study as follows. Drug-resistant parasites were cultured with gentle shaking at 37°C for 24 h to avoid multiple infections. Accurate parasitemia was determined by counting from Giemsa-stained thin blood smears (more than 1,000 pRBC). Cultures were sequentially diluted with complete RPMI medium with red cells at 5% hematocrit down to an average of 2 parasites/ml of culture. Then, 0.1-ml aliquots of the diluted cultures were dispensed in 96-well microtiter plates. Plates were incubated under standard conditions for 3 weeks, and parasite growth in wells was checked by Giemsa staining. The number of wells with growth matched expectations. Well cultures from the most diluted inocula were expanded as single clones in independent flasks.
RESULTS
Identification of lead compounds.
GSK1057714 (Fig. 1) was identified as an antimalarial hit in the phenotypic screening of GlaxoSmithKline's corporate compound collection (10). This chemical family had not previously been part of drug discovery projects in the company and hence it had no associated biological information. It was progressed to hit-to-lead studies for its antiplasmodial potency, chemical novelty, and apparently developable structure-activity relationships (SAR). GSK2645947 (Fig. 1) is a trifluoromethyl derivative of the original hit resulting from the exploratory chemistry program (unpublished data).
Fig. 1.
Structures of the cyclopropyl carboxamides used in this study.
Cyclopropyl carboxamides are potent inhibitors of P. falciparum drug-sensitive and -resistant strains in vitro.
GSK1057714 was tested against six different P. falciparum strains in comparison with chloroquine, atovaquone, and artemisinin. As shown in Table 1, the compound potently inhibited all of the strains tested, multidrug-resistant parasites included. IC50s ranged from 76 to 164 nM. The trifluoromethyl derivative GSK2645947, which had shown a 30-fold higher potency than the original hit against 3D7A parasites, displayed the same advantage against the other P. falciparum strains (IC50s of 2 to 7 nM) (Table 1). These results suggested an antimalarial mode of action through a novel target unchanged in the strains tested.
Table 1.
In vitro antimalarial activities of cyclopropyl carboxamides against different P. falciparum strains
| Compound | IC50 (μM)a for P. falciparum strain: |
|||||
|---|---|---|---|---|---|---|
| 3D7A | K1 | DD2 | HB3 | FCR3 | TM90C2B | |
| GSK1057714A | 0.103 | 0.129 | 0.102 | 0.164 | 0.142 | 0.076 |
| GSK2645947A | 0.003 | NTb | 0.002 | 0.005 | 0.007 | 0.004 |
| Atovaquone | 0.002 | 0.002 | 0.003 | 0.001 | 1.372 | >5 |
| Artemisinin | 0.029 | 0.019 | 0.031 | 0.028 | 0.014 | 0.030 |
| Chloroquine | 0.024 | 0.796 | 0.558 | 0.027 | 0.341 | 0.564 |
All results are the means of at least three independent experiments.
NT, not tested.
Cytotoxicity of cyclopropyl carboxamides.
The series did not reveal itself as cytotoxic to human hepatoma cells in the original screening (10). A deeper analysis of its cytotoxicity profile was carried out to further investigate its safety in vitro. Compounds were assayed in standard assays against a panel of mammalian cell lines representing different tissues, as described in Materials and Methods. As shown in Table 2, compounds did not affect the viability of most of the cell lines tested up to the maximum concentration assayed (limited in the case of GSK2645947 to 6.25 μM, its maximal solubility in the assay medium). Only the mouse lymphoid line L1210 was affected by GSK1057714 but at a concentration 400-fold higher than that required to inhibit P. falciparum. These observations are consistent with a specific inhibitory effect on the parasite, as opposed to nonspecific toxicity against eukaryotic cells.
Table 2.
Determination of cyclopropyl carboxamide cytotoxicity
| Compound | Cell line Tox50 (μM)a |
|||||
|---|---|---|---|---|---|---|
| HepG2 | L1210 | Neuro2A | H9c2 | MDCK1 | HC-04 | |
| GSK1057714A | >50 | 40.5 | >50 | >50 | >50 | NTb |
| GSK2645947A | >6.25 | NT | NT | NT | NT | >6.25 |
| Doxorubicin | 0.03 | 0.02 | 0.6 | 0.28 | >1 | 0.013 |
Tox50 is the 50% cytotoxic concentration. Results are the means of three independent experiments.
NT, not tested.
Tolerability studies.
GSK1057714 and GSK2645947 were administered to mice by oral gavage as a single dose of 1,000 mg/kg as described in Materials and Methods. None of the treated animals died, but those treated with GSK2645947 presented signs of acute toxicity such as disorientation, passivity, and convulsions, probably related to alterations in the central nervous system. These alterations were also observed at a dose of 207 mg/kg but were not noticeable at 94 mg/kg. In contrast, only mild symptoms were observed in animals treated with GSK1057714 at 1,000 mg/kg, even though overall systemic exposure in whole blood (determined as the AUC) (Table 3) was higher for GSK1057714 at 1,000 mg/kg (AUC0-t, 179 μg·h/ml) than for GSK2645947 at both 94 and 207 mg/kg (AUC0-t of 100 and 116 μg·h/ml, respectively). These results suggested that the toxic effects of GSK2645947 were compound dependent and not intrinsically linked to the family.
Table 3.
Pharmacokinetic parameters of cyclopropyl carboxamides in CD1 micea
| Compound | Dose (mg/kg) | Cmax (μg/ml)b | tmax (h) | AUC0-t (μg·h/ml)b |
|---|---|---|---|---|
| GSK1057714 | 1,000 | 10.7 ± 1.07 | 6 | 179 ± 16.5 |
| GSK2645947 | 94 | 2.90 ± 0.527 | 1 | 100 ± 13.2 |
| GSK2645947 | 207 | 4.25 ± 1.10 | 6 | 116 ± 6.20 |
Concentrations were measured in whole blood.
Mean values ± standard errors are shown.
In vivo efficacy studies in a P. falciparum mouse model.
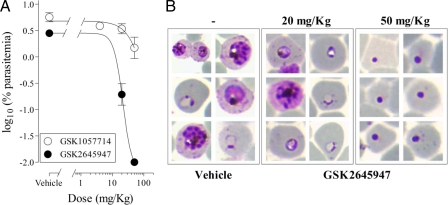
A proof of concept study was performed to assess if the cyclopropyl carboxamide class held potential for antimalarial oral efficacy in vivo. Both GSK1057714 and GSK2645947 were tested against P. falciparum Pf3D70087/N9 growing in NOD-scid IL-2Rγnull mice engrafted with human erythrocytes (3). Both compounds significantly reduced parasitemia in mice when administered orally at or above 20 mg/kg once a day (ED50s: GSK1057714, 24 mg/kg; GSK2645947, 20 mg/kg) (Table 4). Of additional value was the observation that GSK2645947 reached exposure levels sufficient to achieve the death of P. falciparum in peripheral blood within two cycles of parasite growth, as evidenced by the appearance of pycnotic forms (Fig. 2). Overall, results show that orally administered cyclopropyl carboxamides are absorbed by mice and can kill the parasite when it is growing in vivo under the conditions of the model used in this study.
Table 4.
In vivo efficacy of cyclopropyl carboxamides
| Compound | Efficacy (mg/kg/day) |
|
|---|---|---|
| ED50 | ED90 | |
| GSK1057714A | 24 | >50 |
| GSK2645947A | 12 | 20 |
Fig. 2.
Efficacy of cyclopropyl carboxamides in the P. falciparum mouse model. (A) Dose-response relationship. Data are presented as the mean of three mice ± the standard error of the mean for log10 [percentage of parasitemias at day 7 after infection]. (B) Giemsa-stained blood smears from vehicle- and GSK2645947-treated mice. Blood samples were taken at day 7 after infection (2 cycles of parasite growth with drug exposure).
Cyclopropyl carboxamides act on the early trophozoite stages of the erythrocytic Plasmodium life cycle.
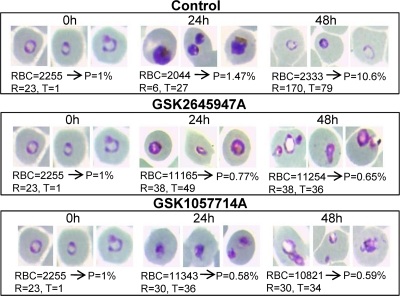
We studied the stage specificity of the inhibitory actions of GSK1057714 and GSK2645947 in comparison to those of chloroquine on the erythrocytic life cycle of P. falciparum 3D7A (Fig. 3). Synchronous cultures enriched in ring stages were treated with the drugs at concentrations 10 times the IC50s. As illustrated in Fig. 3, rings can initially develop in the presence of cyclopropyl carboxamides but further parasite growth is arrested in the early trophozoite stage. This effect is clearly different from those observed in chloroquine-treated cultures, in which ring maturation is prevented and parasite progression to the trophozoite stage is blocked, as previously described (29).
Fig. 3.
In vitro antimalarial activity of cyclopropyl carboxamides in the different intraerythrocytic stages of P. falciparum. Ring-enriched cultures of strain 3D7 were treated as described in Materials and Methods. Treated parasites were followed during an entire life cycle, and effects were checked by microscopy. Numbers of observed red blood cells (RBC), rings (R), and trophozoites (T) are indicated. P is parasitemia expressed as a percentage. Cyclopropyl carboxamides stop further progression of trophozoites through the life cycle.
Cyclopropyl carboxamides show a high propensity to select for resistance.
One of the greatest challenges in working with compounds identified through growth inhibition screening is the complete lack of knowledge of the molecular target responsible for antimalarial activity. This ignorance impedes early investigation of possible target-specific issues. One which may be associated with the target and can be investigated is the frequency of spontaneous resistance to inhibitors, which is crucially linked to the drug's useful life in the field. This issue was explored by culturing 108 parasitized red blood cells in the presence of a concentration of GSK2645947 corresponding to 10 times the IC50. Although at this drug concentration parasites seemed to disappear from the culture in only a few days, growth was observed again after 3 weeks under drug pressure, suggesting selection for drug-resistant parasites.
This was confirmed by culturing the parasites in the absence of the drug for 2 weeks and measuring the IC50 of GSK2645947 in the resulting culture. Results showed that the drug-selected cultures were 2 orders of magnitude less sensitive to GSK2645947 than the parent culture was (results not shown). Individual resistant parasite lines were cloned for further analysis using the limiting-dilution technique as described in Materials in Methods.
Four different clones were selected for analysis, and IC50s of structurally different cyclopropyl carboxamides were determined against the individual clones. The IC50s for the clones selected were 2 orders of magnitude higher than those for the 3D7A parent strain (Table 5). The sensitivities of the wild type and the resistant clones to the standard antimalarials atovaquone, chloroquine, and artemisinin were similar. The mechanism of resistance to cyclopropyl carboxamides therefore seems to be class specific and not extensible to other drug types. A second resistance selection experiment performed using identical conditions yielded similar results, with parasites growing after just 3 weeks in the presence of the drug, confirming that cyclopropyl carboxamides have a facility to select for mutants with a high level of resistance.
Table 5.
In vitro antimalarial activities of cyclopropyl carboxamides against selected resistant clones
| Compound | IC50 (μM)a for P. falciparum: |
||||
|---|---|---|---|---|---|
| 3D7Ab | Clone E2 | Clone E3 | Clone C3 | Clone C7 | |
| GSK1057714A | 0.047 | >5 | >5 | >5 | 1.640 |
| GSK2645947A | 0.002 | 0.385 | 0.436 | 0.369 | 0.147 |
| GSK2611622A | 0.020 | 2.241 | 2.366 | 2.486 | 1.236 |
| GSK2661685A | 0.052 | >5 | >5 | >5 | 2.766 |
| GSK2678892A | 0.146 | 4.862 | ND | ND | 4.533 |
| Atovaquone | 0.003 | 0.005 | 0.004 | 0.005 | 0.004 |
| Artemisinin | 0.027 | 0.026 | 0.024 | 0.027 | 0.022 |
| Chloroquine | 0.027 | 0.031 | 0.031 | 0.033 | 0.029 |
| Pyrimethamine | 0.072 | 0.050 | 0.058 | 0.055 | 0.037 |
Results are the means of three independent experiments.
Wild type.
DISCUSSION
There is an urgent need for new therapeutic approaches to malaria because of rapidly evolving resistance to existing drugs and the current gap in the development of new molecules. Whole-cell screening is a possible way to accelerate antimalarial drug discovery and avoid some of the attrition in target-based lead identification, because if successful, it provides hit compounds with activity against the parasite from the start. This approach has produced a large set of starting chemical points for antimalarial lead discovery (10, 11, 17). However, the cost of not having a biochemical assay for antiparasitic potency is that lead optimization becomes more challenging. Whole-cell potency is a composite result of several compound properties, such as target affinity, cell permeability, efflux, or intracellular modifications, and it is more difficult to establish consistent structure-activity relationships. Nevertheless, recent publications support the effectiveness of these approaches in the identification of new chemical classes with antimalarial activity, such as the spiroindolinone class of compounds (20, 28). The key to success is a good chemical starting point and an adequate progression path to identify the potential to become a drug, or lack thereof, as quickly as possible.
In this work, we describe the characterization of one of the a priori most attractive chemical classes identified in a large phenotypic screening for antimalarial compounds (10). They have been named cyclopropyl carboxamides simply for descriptive reasons, owing to a common functional group in the series. The in vitro antimalarial activity of cyclopropyl carboxamides is very attractive, with antimalarial potencies in the nanomolar range for several family members, down to IC50s of 2 nM for compound GSK2645947. Cyclopropyl carboxamides are equally potent against a panel of drug-resistant strains, including chloroquine-resistant parasites. This result is particularly important, as new drugs will be used in regions where chloroquine resistance is now endemic (25). Activity against multidrug-resistant strains without cytotoxicity against a panel of six mammalian cell lines also indicated a specific antimalarial mode of action independent of known resistance mechanism. This is currently the most parsimonious hypothesis, but only identification of the target responsible for the antimalarial activity or a thorough study of the mode of action can confirm it.
A key requirement for antimalarials useful in public health settings is oral efficacy. Cyclopropyl carboxamides showed efficacy in a P. falciparum mouse model when administered orally. The two compounds tested had ED50s under these conditions of 12 and 25 mg/kg (GSK2645947 and GSK1057714, respectively; Table 4). This in vivo antimalarial potency, although still not considered high enough, is not far from that of other molecules close to the preclinical candidate stage in this same malarial model (4). Also importantly, this result validates the relevance of the cyclopropyl carboxamide target under in vivo conditions, not always a given when starting from in vitro growth inhibitors. It also appears to be a good target from a pharmacological point of view, since parasites exposed in vitro to this chemical class died within the first cycle of exposure, right after ring maturation and only pycnotic forms were observed in vivo at the highest dose tested (50 mg/kg; Fig. 2). Consistent with this observation, antimalarial potencies measures at 48 h and 96 h were nearly identical (data not shown). This is contrary to the “delayed death phenotype” observed with other chemical classes from the same screening and some antimalarial drugs, mainly antibiotics (6). This is a very positive feature, as a quick onset of action is considered necessary for first-line treatments. Current data cannot explain the small 2-fold difference in in vivo efficacy between the two compounds characterized in this study, while in vitro potencies against P. falciparum differ by more than 1 order of magnitude. Differences in pharmacokinetic exposure, as measured in the tolerability studies (see Results), or in protein binding are the most likely explanation and should be carefully analyzed in the further progression of these molecules. The type of animal toxicity displayed by GSK2645947 should also be tested in more compounds of the series to confirm that it is not a class effect. GSK2645947 also showed efficacy in a P. berghei mouse model, with an ED90 of <50 mg/kg (data not shown). This result allows the use of this simpler and less expensive animal model to support compound progression and indicates that the cyclopropyl carboxamide target is also relevant to other Plasmodium species, contrary to what has been found for other P. falciparum targets (5, 22).
All of the results discussed so far support the progression of this class of compounds to a full lead optimization program aiming to select a preclinical candidate. However, efforts to better characterize their antimicrobial properties and approach target identification found an unexpectedly high propensity to select for resistance.
We were able to obtain resistant parasites in cultures treated with 10 times the IC50 of GSK2645947 only 3 weeks after culturing in the presence of the compound, using an inoculum of only 108 parasites. Resistance was genetically determined since it persisted for several weeks in the absence of drug pressure and highly resistant parasite lines could be cloned by limiting dilution. This implies a high potential for the selection of spontaneous resistant parasites. Atovaquone, also described to be affected by a high frequency of spontaneous resistance when used alone (19), did not always select for resistant parasites under the same conditions (data not shown). Absolute values for the frequency of spontaneous resistance cannot be calculated from these data, but the results suggest that cyclopropyl carboxamides will select for resistant parasites at least as frequently as atovaquone does. Frequency of spontaneous resistance for the latter antimalarial varies, depending on the strain used (10−5 for the multidrug-resistant W2 strain to 10−8 for the HB3 or D6 strain) (19). In this work, we used a clone derived from the MR4 3D7A strain, which in our studies displayed frequencies of resistance to standard antimalarials similar to those found in the HB3 or D6 strain (not shown). Most patients who are ill with malaria harbor between 108 and 1012 parasites at presentation (15, 25) Unfortunately then, most patients can be expected to have parasites resistant to this chemical class of compounds from the beginning of treatment, making their development as single agents difficult.
Resistance was class dependent, since structurally different members of the cyclopropyl carboxamide family were equally affected by whichever mutations are present in our resistant strains. The level of resistance was also very high, with IC50 increases of 2 orders of magnitude in most clones. Resistant parasites did not show obvious defects in growth rate compared to the parental strain. This issue detracts from the attractiveness of the series as first line antimalarials, even though it is anticipated that new compounds will always be used in combination.
The nature of the mutation(s) selected in these experiments remains unknown, but it appears specific for cyclopropyl carboxamides as described above. Wild-type strains and resistant parasites are equally sensitive to the standard antimalarials tested. The resistant clones will be invaluable tools to approach target identification by genetic means, and their genomes will be fully sequenced. As the target otherwise shows good pharmacological properties, it is important to learn if the high frequency of resistant mutations is target specific or stems from other mechanisms linked to features common to the cyclopropyl carboxamide series (e.g., chemical modification, selective efflux).
Despite the promising antimicrobial and pharmacological properties of these molecules, until the above issues are resolved, cyclopropyl carboxamides will be down-prioritized for development. Resistance studies and other experiments aiming to better characterize the antiparasitic mode of action will now be introduced earlier in the progression of hits from the growth inhibition screening in order to quickly identify relevant liabilities and thus save time and precious resources.
ACKNOWLEDGMENTS
This work was supported by the Medicines for Malaria Venture.
We acknowledge Jose Luis Llergo for his help in the resistance experiments. We also acknowledge D. Shultz (The Jackson Laboratory, Bar Harbor, ME) for the licensing of immunodeficient NOD-scid IL-2Rγnull mice for efficacy experiments.
Footnotes
Published ahead of print on 3 October 2011.
REFERENCES
- 1. Aide P., et al. 2011. Four year immunogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 29:6059–6067 [DOI] [PubMed] [Google Scholar]
- 2. Alonso P. L., et al. 2011. A research agenda to underpin malaria eradication. PLoS Med. 8:e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angulo-Barturen I., et al. 2008. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One 3:e2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booker M. L., et al. 2010. Novel inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with anti-malarial activity in the mouse model. J. Biol. Chem. 285:33054–33064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan C., Goh L. L., Sim T. S. 2005. Differences in biochemical properties of the plasmodial falcipain-2 and berghepain-2 orthologues: implications for in vivo screens of inhibitors. FEMS Microbiol. Lett. 249:315–321 [DOI] [PubMed] [Google Scholar]
- 6. Dahl E. L., Rosenthal P. J. 2007. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob. Agents Chemother. 51:3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dondorp A. M., et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis R. D., et al. 2010. Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naive adults. PLoS One 5:e8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gamo F. J., et al. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310 [DOI] [PubMed] [Google Scholar]
- 11. Guiguemde W. A., et al. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiménez-Díaz M. B., et al. 2009. Quantitative measurement of Plasmodium-infected erythrocytes in murine models of malaria by flow cytometry using bidimensional assessment of SYTO-16 fluorescence. Cytometry A 75:225–235 [DOI] [PubMed] [Google Scholar]
- 13. Jiménez-Díaz M. B., et al. 2009. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rgammanull mice engrafted with human erythrocytes. Antimicrob. Agents Chemother. 53:4533–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin J. T., Juliano J. J., Wongsrichanalai C. 2010. Drug-resistant malaria: the era of ACT. Curr. Infect. Dis. Rep. 12:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paget-McNicol S., Saul A. 2001. Mutation rates in the dihydrofolate reductase gene of Plasmodium falciparum. Parasitology 122:497–505 [DOI] [PubMed] [Google Scholar]
- 16. Payne D. J., Gwynn M. N., Holmes D. J., Pompliano D. L. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6:29–40 [DOI] [PubMed] [Google Scholar]
- 17. Plouffe D., et al. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc. Natl. Acad. Sci. U. S. A. 105:9059–9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porter D. W., et al. 2011. A human phase I/IIa malaria challenge trial of a polyprotein malaria vaccine. Vaccine 29:7514–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rathod P. K., McErlean T., Lee P. C. 1997. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 94:9389–9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rottmann M., et al. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sagara I., et al. 2009. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine 27:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh A., et al. 2002. Critical role of amino acid 23 in mediating activity and specificity of vinckepain-2, a papain-family cysteine protease of rodent malaria parasites. Biochem. J. 368:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trager W., Jensen J. B. 2005. Human malaria parasites in continuous culture. 1976. J. Parasitol. 91:484–486 [DOI] [PubMed] [Google Scholar]
- 24. White N. J. 2008. Qinghaosu (artemisinin): the price of success. Science 320:330–334 [DOI] [PubMed] [Google Scholar]
- 25. White N. J., et al. 1999. Averting a malaria disaster. Lancet 353:1965–1967 [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization 2010. Global report on antimalarial drug efficacy and drug resistance: 2000-2010. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241500470_eng.pdf [Google Scholar]
- 27. World Health Organization 2010. World malaria report. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf [Google Scholar]
- 28. Yeung B. K., et al. 2010. Spirotetrahydro beta-carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J. Med. Chem. 53:5155–5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y., Asante K. S., Jung A. 1986. Stage-dependent inhibition of chloroquine on Plasmodium falciparum in vitro. J. Parasitol. 72:830–836 [PubMed] [Google Scholar]