Abstract
The opportunistic pathogen Pseudomonas aeruginosa can utilize a variety of carbon sources and produces many secondary metabolites to help survive harsh environments. P. aeruginosa is part of a small group of bacteria that use the kynurenine pathway to catabolize tryptophan. Through the kynurenine pathway, tryptophan is broken down into anthranilate, which is further degraded into tricarboxylic acid cycle intermediates or utilized to make numerous aromatic compounds, including the Pseudomonas quinolone signal (PQS). We have previously shown that the kynurenine pathway is a critical source of anthranilate for PQS synthesis and that the kynurenine pathway genes (kynA and kynBU) are upregulated in the presence of kynurenine. A putative Lrp/AsnC-type transcriptional regulator (gene PA2082, here called kynR), is divergently transcribed from the kynBU operon and is highly conserved in Gram-negative bacteria that harbor the kynurenine pathway. We show that a mutation in kynR renders P. aeruginosa unable to utilize l-tryptophan as a sole carbon source and decreases PQS production. In addition, we found that the increase of kynA and kynB transcriptional activity in response to kynurenine was completely abolished in a kynR mutant, further indicating that KynR mediates the kynurenine-dependent expression of the kynurenine pathway genes. Finally, we found that purified KynR specifically bound the kynA promoter in the presence of kynurenine and bound the kynB promoter in the absence or presence of kynurenine. Taken together, our data show that KynR directly regulates the kynurenine pathway genes.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative bacillus that is ubiquitous throughout nature and infects a variety of hosts. It is a common nosocomial pathogen known to cause serious opportunistic infections in immunocompromised individuals (25). P. aeruginosa also causes a chronic infection in cystic fibrosis (CF) patients that is difficult, if not impossible, to eradicate and ultimately leads to increased morbidity and mortality in this population (4, 37). In order to survive during such infections and in many other harsh environments, P. aeruginosa utilizes numerous different carbon sources and produces a wide range of secondary metabolites (21, 24, 28, 35, 38). One potential nutrient, tryptophan, can be used by some bacteria to provide building blocks for many secondary metabolites, some of which function as siderophores, signaling molecules, and protective compounds (1, 11, 28, 44). Similar to eukaryotes, P. aeruginosa catabolizes tryptophan through the kynurenine pathway (11, 22). Kurnasov et al. utilized comparative genetics to identify several bacteria with putative kynurenine pathways, including Bacillus anthracis, P. aeruginosa, and Bordetella pertussis (22). The kynurenine pathway contrasts significantly from the major tryptophan catabolic pathway of Escherichia coli (and many other species of bacteria), which catabolizes l-tryptophan anaerobically into indole, pyruvate, and ammonia via a pyridoxal phosphate-dependent tryptophanase (41, 50). Nevertheless, with either catabolic pathway the ability to utilize both tryptophan and tryptophan breakdown products as a carbon and nitrogen source, and as precursors for many secondary metabolites, provides a unique advantage for survival within nutrient-limited and harsh environments.
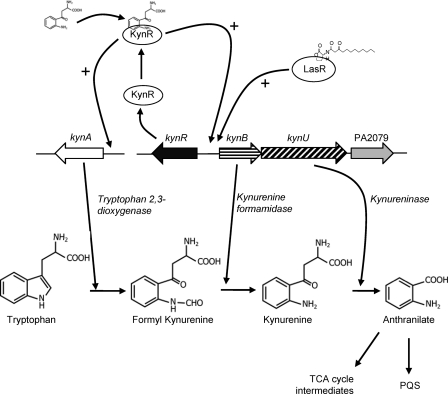
The conversion of l-tryptophan into quinolinate via the kynurenine pathway has been demonstrated in multiple bacterial species (22). However, P. aeruginosa only encodes the genes for the anthranilate branch of the kynurenine pathway (23). This branch catabolizes l-tryptophan into anthranilate via a three-step enzymatic pathway (22). The three enzymes are encoded by kynA (which encodes a tryptophan-2,3-dioxygenase), kynB (kynurenine formamidase), and kynU (kynureninase) (see Fig. 7 for pathways) (11, 23). The kynA gene is located separately on the P. aeruginosa chromosome, while kynB and kynU are encoded in a putative operon. The kynurenine pathway was linked to P. aeruginosa virulence when it was shown that radiolabeled tryptophan was incorporated into the Pseudomonas quinolone signal (PQS) (11), which is important for virulence in multiple models of infection (10, 13, 27, 34, 49). These data also showed that despite the presence of two alternative pathways for anthranilate production, the kynurenine pathway is the main source of anthranilate for PQS production when P. aeruginosa is grown in the presence of tryptophan or tryptophan breakdown metabolites (11, 31).
Fig. 7.
Proposed model for KynR regulation and the kynurenine biosynthetic pathway. In this model, tryptophan is degraded via three separate enzymes and made into anthranilate. Anthranilate can either be used for the production of 4-quinolones, such as PQS, or is broken down into TCA cycle intermediates. KynR interacts with kynurenine to become a transcriptional activator for both kynA and kynBU.
Due to both the importance of the kynurenine pathway in the production of PQS and because tryptophan is a costly amino acid to synthesize (11, 52), it would be advantageous for P. aeruginosa to strictly regulate the catabolism of tryptophan. Multiple transcriptome arrays have shown that quorum sensing may regulate the kynurenine pathway (36, 43), and the quorum-sensing regulator LasR was predicted through chromatin immunoprecipitation-chip analysis to bind to the promoter region of kynB (15). Our laboratory has also shown that the transcription of both kynA and kynB was significantly increased in the presence of kynurenine (11). With these data in mind, we began to search for a transcriptional regulator that could specifically regulate kynA and kynB in the presence of kynurenine. We identified a putative transcriptional regulator encoded by gene PA2082, which is divergently transcribed from kynB and is homologous to the Lrp/AsnC family of transcriptional regulators. Lrp/AsnC-type regulators are known to control amino acid metabolism, and though Lrp has a global regulatory role in E. coli, many other regulators within the family have more specific regulons (3, 51). We demonstrate here that the protein encoded by gene PA2082 directly binds to and regulates the kynurenine pathway genes, and we therefore propose that this protein be named the kynurenine pathway regulator (KynR).
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
E. coli and P. aeruginosa strains were maintained in 30% glycerol and 10% skim milk (Difco), respectively, at −80°C and were freshly plated to begin each experiment. All strains and plasmids used are listed in Table 1. Bacteria were cultured at 37°C in Luria-Bertani medium (LB), Vogel Bonner minimal medium supplemented with 0.5% glycerol (VBG) (42), or sole carbon source medium (SCM); containing 73.4 mM K2HPO4, 16.76 mM NaNH5PO4 · 4H2O, 0.8 mM MgSO4, and with either 10 mM l-tryptophan (TSCM) or 10 mM l-alanine (ASCM) (pH 7.0). Cultures were supplemented with the kynurenine pathway metabolites as indicated below. Growth was monitored spectrophotometrically based on the optical density at 600 nm (OD600) for E. coli or OD660 for P. aeruginosa. To maintain plasmids, 30 μg/ml chloramphenicol, 100 μg/ml ampicillin, 200 μg/ml carbenicillin, or 15 μg/ml (E. coli) or 30 μg/ml (P. aeruginosa) gentamicin was added when appropriate.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or phenotypea | Reference |
|---|---|---|
| E. coli DH5α | F′ endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 (lacZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15recA1] | 48 |
| P. aeruginosa strains | ||
| PAO1 | Wild type | 19 |
| PAO-R1 | lasR::Tc deletion mutant derived from PAO1 | 14 |
| PJF-KA1 | kynA deletion mutant derived from PAO1 | 11 |
| PJF-KB1 | kynB deletion mutant derived from PAO1 | 11 |
| PJF-KU1 | kynU deletion mutant derived from PAO1 | 11 |
| PAΔKynR | kynR deletion mutant derived from PAO1 | This study |
| PAO-R1ΔKynR | kynR lasR deletion mutant derived from PAO-R1 | This study |
| Plasmids | ||
| pHERD30T | PBAD-based shuttle vector, Gmr | 33 |
| pKynRex | KynR expression vector, Gmr | This study |
| pACYC184 | General purpose cloning vector, Tetr Chlr | 5 |
| pKynRsubex | KynR expression vector derived from pKynRex, Chlr | This study |
| pEX18Ap | Suicide vector, Ampr | 17 |
| pkynRdel | kynR deletion suicide vector, Ampr | This study |
| pLP170 | lacZ transcriptional fusion vector, Ampr | 32 |
| pJF01 | kynB′-lacZ transcriptional fusion, Ampr | 11 |
| pJF03 | kynA′-lacZ transcriptional fusion, Ampr | 11 |
Abbreviations: Gm, gentamicin; Tet, tetracycline; Chl, chloramphenicol; Amp, ampicillin.
In order to generate an expression plasmid for KynR, a 568-bp DNA fragment, which began at the kynR start codon (ATG) and ended 46 bp downstream from the stop codon, was amplified by PCR using strain PAO1 chromosomal DNA as the template. The oligonucleotide primers used for this amplification were engineered to contain a single HindIII site downstream from the stop codon (Table 2). Vector plasmid pHERD30T, which contains an araBAD (PBAD) promoter to control gene expression, was digested sequentially with SmaI and HindIII. The digested plasmid DNA was ligated with the kynR PCR fragment, which had also been digested with HindIII, to create the plasmid pKynRex. This plasmid was used to amplify kynR and regulatory elements for use in a two-plasmid system in E. coli. PCR was used to amplify both the kynR and divergently transcribed araC to ensure all elements necessary for controlled expression of KynR were included in the subcloned plasmid. Primers were located 52 bp downstream from araC and 46 bp downstream from kynR. The resulting PCR fragment, as well as pACYC184, were sequentially digested with SalI and HindIII and ligated together, generating plasmid pKynRsubex.
Table 2.
Primers used in this study
| Primer use and name | Sequence (5′–3′)a |
|---|---|
| Primers for mutagenesis | |
| 2082-F1-remake | AAAAAAGAATTCGAGGTAGATCACGCCGTC |
| PA2082Rev1 | CCGGCCTTGATCTCCTGCAGTCCTGGTTGCTCATCCGCCC |
| PA2082Fwd2 | GGGCGGATGAGCAACCAGGACTGCAGGAGATCAAGGCCGG |
| PA2082Rev2 | AAAAAAGAATTCGAACTTGGCGGCGATGG |
| Primers for KynR expression | |
| 2082atgfwd | ATGTCCCTGGACGCCATCGA |
| 2082 Rev Herd | AAAAAAGCTTCGTCCCTCTCATTGCACT |
| Herd2082exsubRev | AAAAGTCGACCGAAGCAGGGTTATGCAGC |
| EMSA | |
| kynApromoterfwd | GAGTGAGGGCAAGGACACAT |
| kynApromoterrev | CGCGAGTGATCCGAAATTCG |
| kynBpromoterfwd | GACTGATGTCCCAGTAGCGG |
| kynBpromoterrev | GACGGAGAATGCGCAGATCG |
| FwdKynU-EMSA | GGTACTTGTAGGTGCAGCCG |
| RevKynU-EMSA | CAAGACCGGCTACCTGCACG |
Restriction sites are indicated by bold type.
Generation of kynR mutants.
A splicing-by-overlap extension protocol was used to generate mutant alleles (47). Alleles were constructed to contain in-frame deletions in the coding DNA sequences corresponding to amino acids 25 to 140 for KynR (73% of the protein sequence). Primers were designed to contain approximately 1 kb of DNA both upstream and downstream from the splice junction, and each primer added an EcoRI restriction site to both ends of the PCR product. Both the kynRdel PCR product and pEX18Ap (suicide vector) were digested with EcoRI and ligated together. The resulting plasmid, pkynRdel, was transformed into strains PAO1 and PAO-R1 by electroporation (6). Mutants were selected by plating transformants on medium containing carbenicillin and then on medium containing 6% sucrose to remove the vector sequence (17). PCR was used to screen colonies, and DNA sequencing of PCR products was used to confirm mutants.
PQS production.
Washed cells from overnight cultures were used to inoculate 10-ml cultures of LB or VBG supplemented with either water, 1 mM l-tryptophan, 1 mM l-kynurenine, or 1 mM anthranilate (final concentrations) to an OD660 of 0.05. After 6 and 24 h of growth, 300-μl samples of each culture were extracted with 900 μl of acidified ethyl acetate as previously described (8). One-half of the resulting organic phase was evaporated to dryness at 37°C, and 50 μl of 1:1 acidified ethyl acetate-acetonitrile was used to reconstitute the extract. Samples were analyzed by thin-layer chromatography (TLC), visualized by long-wave UV light, and photographed (8).
β-Gal assays for P. aeruginosa.
Cells from overnight cultures of P. aeruginosa grown in VBG were washed and resuspended in fresh medium to an OD660 of 0.05 and incubated at 37°C with shaking at ≥180 rpm for 6 h. At that time, either water or 1 mM l-kynurenine was added, and cultures continued to grow at 37°C with shaking at ≥180 rpm for an additional 18 h. The cells were collected by centrifugation at 12,000 × g for 2 min, resuspended in fresh VBG, and assayed for β-Galactosidase (β-Gal) activity in duplicate. Data are presented in Miller units as the mean ± standard deviation [σ(n − 1)] of at least 3 separate experiments.
β-Gal assays for E. coli.
Overnight cultures of E. coli carrying the appropriate plasmids were grown in LB and used to inoculate 10-ml cultures of fresh medium to an OD600 of 0.08. The subcultures were incubated at 37°C with shaking at ≥180 rpm until an OD600 of 0.3 was reached. Then, 2-ml aliquots were transferred to tubes that contained 0.1% l-arabinose to induce kynR expression, and either water, 1 mM l-kynurenine, 1 mM l-tryptophan, or 1 mM anthranilic acid (final concentrations) was added. The cells were incubated for 2 h at 37°C with shaking at ≥180 rpm. After incubation, the β-Gal activity produced by each culture was assayed, and data are reported as the mean ± σ(n − 1) of at least 3 independent experiments.
Purification of KynR.
The method used for purification of KynR is a modification of those described by Madhusudhan et al. for the purification of BkdR from Pseudomonas putida (26). A 10-ml overnight culture of E. coli strain DH5α(pKynRex) was used to inoculate 250 ml of LB and was incubated at 37°C with shaking at ≥180 rpm until an OD600 of 0.7 was reached. At this point, 1% l-arabinose (Sigma-Aldrich) was added to induce kynR expression, and the culture was grown for an additional 3 h at 37°C with shaking at ≥180 rpm. Cells were harvested by centrifugation at 6,000 × g for 10 min and then resuspended in 20 mM Tris-HCl (pH 7.4) and 1 mM dithiothreitol (DTT) (buffer A). The cells were lysed by sonication, and the lysate was cleared by centrifugation at 37,000 × g for 1 h at 4°C (Beckman Coulter Optima L-100 XP ultracentrifuge). KynR purification was performed at room temperature, and all samples were kept on ice or at 4°C until purification was complete. The soluble fraction (90 mg of total protein) was applied to a HiTrap DEAE Sepharose Fast Flow column (1 ml; GE Healthcare) in buffer A at a flow rate of 0.5 ml/min. The column was washed with buffer A until the A280 returned to 0 (∼50 ml). Bound proteins were eluted with a 30-ml linear gradient of 0 to 0.3 M NaCl at a flow rate of 1 ml/min, and 3-ml fractions were collected. Samples from the collected fractions were precipitated using deoxycholic acid and tricholoracetate acid (DCA/TCA) and then separated by 15% SDS-PAGE to identify fractions containing KynR. These fractions were collected and dialyzed against a buffer containing 20 mM Tris-HCl (pH 7.4), 100 mM NaCl, 5 mM MgCl2, and 1 mM DTT (buffer B) overnight at 4°C. The dialyzed fractions were then applied to a HiTrap heparin column (5-ml; GE Healthcare) equilibrated with buffer B. The column was washed with buffer B until the A280 returned to 0 (∼24 ml). Bound proteins were eluted with a 50-ml linear gradient of 0.1 to 0.4 M NaCl in buffer B at a flow rate of 1 ml/min, and 3-ml fractions were collected. Again, proteins from the collected fractions were precipitated with DCA/TCA and were separated on a 15% SDS-polyacrylamide gel to identify the fractions containing KynR. All fractions containing KynR were pooled and dialyzed against 20 mM Tris-HCl (pH 7.8) and 1 mM DTT (buffer C) overnight at 4°C. The dialyzed fraction was then added to a HiTrap DEAE Sepharose Fast Flow column (1 ml; GE Healthcare) equilibrated with buffer C. The column was washed with buffer C until the A280 returned to 0 (∼12 ml), bound proteins were eluted with a 30-ml linear gradient of 0 to 0.3 M NaCl at a flow rate of 1 ml/min, and 3-ml fractions were collected. Samples from collected fractions were precipitated with DCA/TCA and separated on a 15% SDS-PAGE to identify the fractions that contained KynR. The fractions containing KynR resulted in 95% purity of KynR as judged by SDS-PAGE (data not shown). Glycerol was added to the pooled KynR fractions to a final concentration of 15%, and the protein was stored at −80°C.
Column fractions during purification were monitored by absorbance measurements at 280 and 260 nm with a NanoDrop ND-1000 spectrophotometer. After purification, the final protein concentration was 4.34 mg/ml (244 mol) of protein, as determined by a Bradford assay using Bio-Rad reagents. In addition, protein concentrations for electrophoretic mobility shift assays (EMSA) were determined by the Bradford assay using Bio-Rad reagents.
EMSA.
PCR was used to generate DNA fragments containing the kynA (228 bp, +208 bp to −20 bp relative to ATG) and kynB (200 bp, +169 bp to −31 bp relative to the ATG) promoter regions. An internal fragment of kynU (157 bp, +527 bp to +685 bp relative to the ATG) was also generated by PCR as a negative control. DNA fragments were labeled with [γ-32P]ATP (Perkin-Elmer, Wellesley, MA) by using T4 polynucleotide kinase (Invitrogen, Carlsbad, CA). The binding assays were carried out in buffer containing 20 mM Tris-HCl (pH 7.4), 50 mM NaCl2, 5 mM MgCl2, 1 mM dithiothreitol, and 10% glycerol. Each reaction mixture contained 0.3 μg of salmon sperm DNA, approximately 1.5 × 104 cpm radiolabeled DNA, and 0 to 488 pmol of protein. Binding reactions were performed in both the presence and absence of 0.1 mM l-kynurenine or 0.1 mM l-tryptophan. Reaction mixtures were incubated at room temperature for 20 min and separated by electrophoresis at 4°C on a native 10% polyacrylamide gel (29:1 acrylamide–bis-acrylamide, 1× Tris-borate buffer [89 mM Tris and 89 mM borate, pH 8.0], and 2.5% glycerol) for 5 min at 200 V and then for 4 h at 100 V. After electrophoresis, the gels were dried and visualized by autoradiography.
RESULTS
Identification of a putative regulator of the kynurenine pathway.
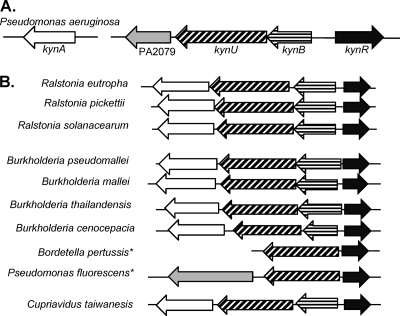
To begin our search for potential regulators of the kynurenine pathway, we first analyzed the predicted function of annotated genes located near the kynurenine pathway operons in P. aeruginosa (The Pseudomonas Genome Database). The kynurenine pathway genes are located separately on the P. aeruginosa chromosome, with kynB and kynU located in a putative polycistronic operon and kynA located on a distant putative monocistronic operon (Fig. 1A). A potential transcriptional regulator encoded by gene PA2082 was divergently transcribed from kynB in P. aeruginosa and appeared to be a member of the Lrp/AsnC family of transcriptional regulators (Fig. 1B). These regulators are known to be important for controlling amino acid metabolism and are often encoded adjacent to pathway genes that they regulate (3). We used a comparative genomics approach and began to examine other bacteria that harbor the kynurenine pathway genes to determine if they also possessed similar regulator proteins. Both the Burkholderiaceae and Pseudomonadaceae families encode a PA2082 homolog divergently oriented from either kynB or kynU in the Burkholderia, Ralstonia, Cupavarius, Bordetella, and Pseudomonas genera (Fig. 1B). P. aeruginosa only contains the anthranilate branch of the kynurenine pathway, while some of the Burkholderia species and Pseudomonas fluorescens apparently contain the pathway to catabolize tryptophan into quinolinate or the siderophore quinolobactin (22, 28). Also, Bacillus cereus and Bacillus anthracis contain the kynurenine pathway genes (23), but these species have a TetR-like regulator divergently oriented from kynU that may be responsible for an alternative regulatory scheme. Overall, these findings suggest that the putative transcriptional regulator encoded by PA2082, which we hereby designate KynR (kynurenine pathway regulator), might play a role in regulating tryptophan catabolism in P. aeruginosa.
Fig. 1.
The kynR gene is conserved in other Gram-negative bacteria that have the kynurenine pathway genes. (A) The two loci of the kynurenine pathway genes in P. aeruginosa. (B) A schematic to show the conservation of kynR. The shading on each gene corresponds to the homologous genes in panel A. *, kynB and kynA are located separately on the chromosome in a putative operon.
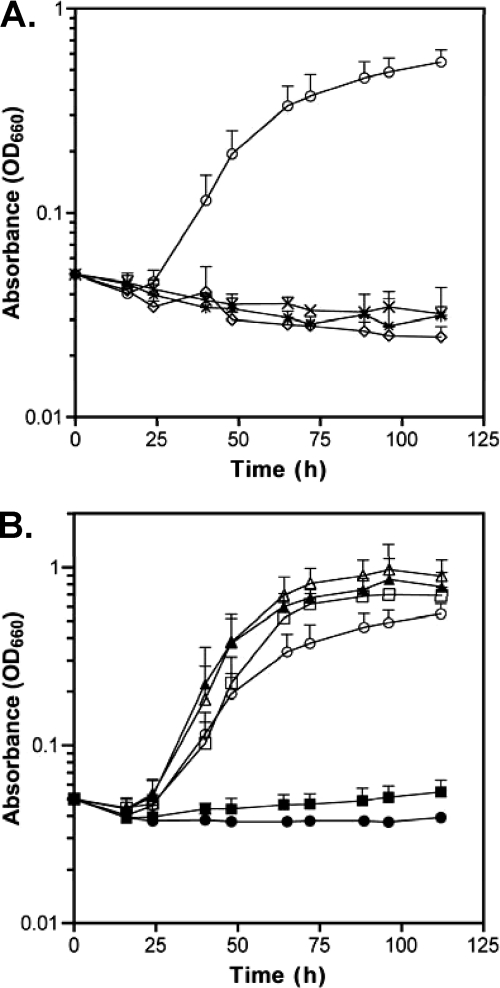
In order to examine the role of KynR in tryptophan catabolism, we assessed the impact of KynR on kynurenine pathway-dependent phenotypes. The kynurenine pathway is the only enzymatic pathway identified in P. aeruginosa capable of degrading tryptophan (22). To confirm this, we grew mutants containing deletions of the kynurenine pathway genes kynA (PJF-KA1), kynB (PJF-KB1), and kynU (PJF-KU1) in a minimal salts medium with tryptophan as the only carbon source (TSCM) (Fig. 2A). The wild-type strain PAO1 utilized tryptophan as a sole carbon source and grew to an OD660 of over 0.5 (Fig. 2A). However, the kynurenine pathway mutants were unable to grow with only tryptophan as a carbon source (Fig. 2A). As a control, the strains were also grown on alanine as a sole carbon source (ASCM), and the kynurenine pathways mutants were able to grow to wild-type levels (data not shown). These results confirmed that the kynurenine pathway is required for tryptophan catabolism in P. aeruginosa. Next, we tested whether the kynR mutant strain PAΔKynR was able to grow on tryptophan as a sole carbon source (Fig. 2B). The results indicated that, like the kynurenine pathway mutants, PAΔKynR was unable to grow on tryptophan alone. In order to determine if the inhibition of growth was due to the loss of kynR, strains PAΔKynR and PAO1 were transformed with a plasmid harboring wild-type kynR under an arabinose-inducible promoter (pKynRex). Expression of KynR in strain PAΔKynR caused growth on tryptophan to be similar to that of the parent strain PAO1 (Fig. 2B). Since P. aeruginosa can utilize arabinose as a carbon source, we also included control cultures to ensure that the arabinose that was added for KynR expression was not affecting the growth phenotypes, and we observed that the effect was negligible (Fig. 2B). Overall, these data indicated that a kynR mutant was unable to grow on tryptophan as a sole carbon source and further supported the notion that KynR has a role in the regulation of the kynurenine pathway.
Fig. 2.
Utilization of l-tryptophan as a sole carbon source. (A) Bacterial strains PAO1 (circles), PJF-KA1 (X), PJF-KB1 (diamonds), and PJF-KU1 (asterisks) were grown in TSCM, and the OD660 was measured. (B) Bacterial strains PAO1 (open circles), PAO1 with 0.1% arabinose (open squares), PAO1(pKynRex) with 0.1% arabinose (open triangles), PAΔKynR (closed circles), PAΔKynR with 0.1% arabinose (closed squares), and PAΔKynR(pKynRex) with 0.1% arabinose (closed triangles) were grown in TSCM, and the OD660 was measured. Values depict the mean ± σ(n − 1) from at least three separate experiments.
PQS production is affected in a kynR mutant.
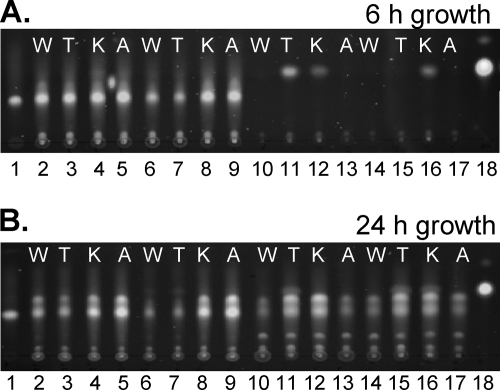
Previous studies showed that the kynurenine pathway mutants produced little to no detectable PQS and suggested that the kynurenine pathway is the main source of anthranilate for PQS production when tryptophan or its breakdown metabolites are present (11). Therefore, we were interested in testing whether a mutation in kynR would also have an effect on PQS production. Strains PAO1 and PAΔKynR were grown in LB and VBG supplemented with tryptophan, kynurenine, anthranilic acid, or water (as a control). The cultures were grown for either 6 or 24 h and then were extracted with acidified ethyl acetate. The extracts were analyzed by TLC to assay both PQS and anthranilate production. After 6 and 24 h of growth in LB or LB supplemented with tryptophan, strain PAΔKynR produced less PQS (Fig. 3A and B, lanes 6 and 7) than the wild-type strain PAO1 (Fig. 3A and B, lanes 2 and 3). This suggested that kynR affects the supply of anthranilate for PQS production by positively controlling an element of the kynurenine pathway.
Fig. 3.
The production of anthranilate and PQS is decreased in the kynR mutant. Ethyl acetate extracts from cultures grown for 6 (A) and 24 h (B) were analyzed by TLC. Equal volumes of extracts were resolved in each lane, and the addition of water (W), 1 mM l-tryptophan (T), 1 mM l-kynurenine (K), or 1 mM anthranilic acid (A) to the respective culture is indicated at the top of each lane. Lane 1 contains 75 ng of PQS, and lane 18 contains 1.25 ng of anthranilic acid (controls). Lanes 2 to 5 contain extracts from strain PAO1 grown in LB; lanes 6 to 9 contain extracts from strain PAΔKynR grown in LB; lanes 10 to 13 contain extracts from strain PAO1 grown in VBG; lanes 14 to 17 contain extracts from strain PAΔKynR grown in VBG.
We then saw something unexpected, when the addition of kynurenine to LB resulted in wild-type amounts of PQS being produced by strain PAΔKynR after both 6 and 24 h (Fig. 3A and B, lane 8). This suggested that KynR was not the only factor that controls kynBU, which must be induced for the conversion of kynurenine to anthranilate to be used for PQS synthesis (see Fig. 7 for pathway). PQS was also extracted from both strains grown in a minimal medium to assess the effects of supplementing with individual kynurenine pathway metabolites. The production of PQS by strains PAO1 and PAΔKynR grown in VBG differed somewhat from those in LB but followed a similar trend. PQS was not detectable from either strain after 6 h of growth in VBG, but the addition of tryptophan or kynurenine to the medium induced the production of anthranilate by the wild-type strain PAO1 (Fig. 3A, lanes 11 and 12). Unlike the wild-type strain PAO1, strain PAΔKynR only produced anthranilate with the addition of kynurenine and not when tryptophan was added (Fig. 3A, lanes 15 and 16). After 24 h of growth in VBG, PQS was detectable from both strains under all conditions, and anthranilate was no longer detectable after presumably being used by the cells (Fig. 3B, lanes 10 to 17). Taken together, the data suggest that KynR indirectly regulates PQS production, presumably through the kynurenine pathway, but that it is not absolutely required for PQS to be produced.
KynR is required for kynurenine-dependent induction of kynurenine pathway genes.
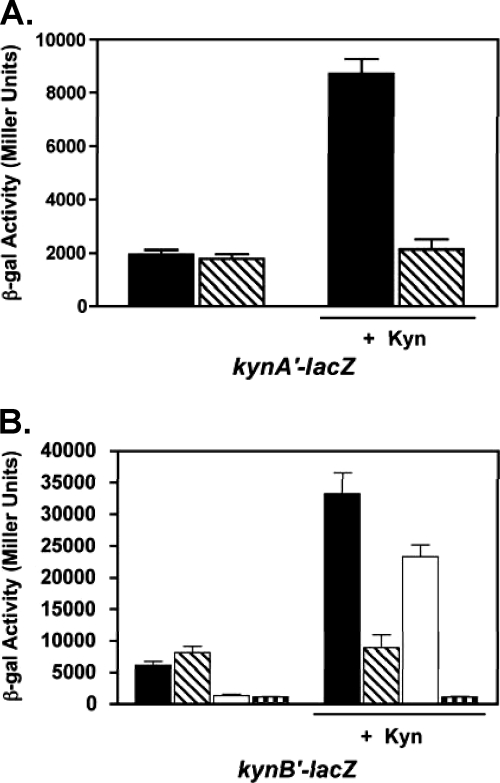
Thus far, the phenotypes of the kynR mutant have implied that KynR plays a role in the kynurenine pathway. In addition, our previous work had shown that the transcription of both kynA and kynB was induced in the presence of kynurenine (11). Therefore, we decided to determine if KynR affected the expression of kynA and kynB in a defined medium (VBG) where we could control the concentration of kynurenine. To study this, we performed β-Gal assays in strains PAO1 and PAΔKynR harboring the respective kynA′-lacZ and kynB′-lacZ transcriptional fusion plasmids pJF03 and pJF01. Our data showed that after 24 h of growth in VBG medium in the absence of kynurenine, the expression levels of both kynA and kynB were similar in both the parental and kynR mutant strains (Fig. 4A and B). However, the addition of 1 mM kynurenine resulted in a large induction of both kynA and kynB in the wild-type strain PAO1, but this induction did not occur in the kynR mutant (Fig. 4A and B). These data led us to conclude that in the presence of kynurenine, KynR positively regulates kynA and kynB transcription.
Fig. 4.
The kynurenine pathway genes are not induced by kynurenine in a kynR mutant. β-Gal activity of kynA′-lacZ fusion (pJF03) (A) and (B) kynB′-lacZ fusion (pJF01) (B) in strains PAO1 (solid bars), PAΔKynR (striped bars), PAO-R1 (open bars), and PAO-R1ΔKynR (stippled bars) were assayed in cultures grown in VBG for 6 h and then supplemented with either water (as a control) or 1 mM l-kynurenine for 18 h. β-Gal activity is presented in Miller units as the mean ± σ(n − 1) of results from duplicate assays from at least three separate experiments.
It has been suggested from microarray experiments that kynB is directly regulated by the quorum-sensing regulator LasR (15). Since our data in Fig. 3 also suggested that a factor in addition to KynR was regulating kynBU, it seemed logical that we should examine whether LasR affected kynBU transcription. The data of Fig. 4B showed that kynB transcription was greatly decreased in a lasR mutant when kynurenine was not present but that the addition of kynurenine would override the lasR mutation. In addition, a lasR kynR double mutant exhibited no transcription of kynB in the presence or absence of kynurenine (Fig. 4B). Taken together, the data of Fig. 4 lead us to conclude that kynBU is controlled by LasR when kynurenine is absent and by KynR when kynurenine is available.
KynR directly regulates the kynurenine pathway.
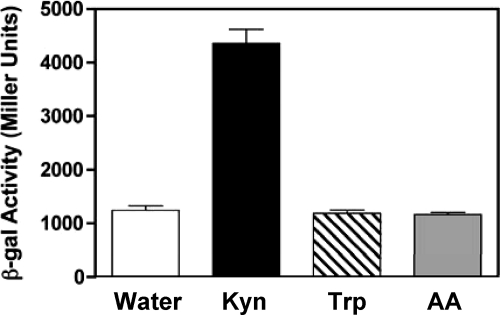
Our data so far showed that KynR is required for the kynurenine-dependent increase of both kynA and kynB transcription (Fig. 4A and B), but whether this regulation was direct or indirect was unknown. To eliminate any possibility of endogenous P. aeruginosa factors altering the results of our kynA and kynB transcriptional fusion assays, we utilized an E. coli two-plasmid system to try to establish a more direct link between KynR and the induction of kynA and kynB. (It is important to point out here that E. coli does not contain the genes for the kynurenine pathway.) E. coli strain DH5α harboring either a kynA′-lacZ (pJF03) or a kynB′-lacZ (pJF01) transcriptional fusion and the KynR overexpression plasmid (pKynRsubex) was grown in LB in the presence of tryptophan, kynurenine, or anthranilate, and the expression of KynR was induced with the addition of 0.1% arabinose. The results presented in Fig. 5 show that the expression of kynB′-lacZ was only induced in the presence of KynR and kynurenine and not when tryptophan or anthranilate was present. Unfortunately, the similar assays performed with the kynA′-lacZ fusion showed that it was constitutively active in E. coli, and direct regulation by KynR could not be demonstrated in this experiment (data not shown). Nevertheless, the results of Fig. 5 suggest that KynR can directly activate the expression of kynB and that kynurenine acts as a coinducer for KynR.
Fig. 5.
KynR can induce expression of kynB′-lacZ in the presence of l-kynurenine in E. coli. E. coli strain DH5α(pJF01)(pKynRex) was grown in LB medium in the presence of water as a control water (white bar), 1 mM l-kynurenine (black bar), 1 mM l-tryptophan (striped bar), or 1 mM anthranilate (gray bar). After 3 h of growth the cultures were induced with 0.1% arabinose and grown for an additional 3 h. The cultures were then assayed for β-Gal activity, which is presented in Miller units as the mean ± σ(n − 1) of results from at least three experiments.
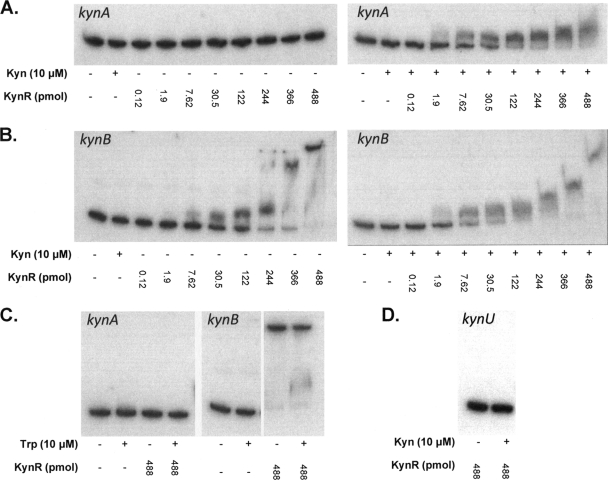
To determine if KynR directly binds to the kynA and kynB promoter regions in response to kynurenine, we purified KynR and performed EMSA. KynR was purified from E. coli strain DH5α(pKynRex) and incubated with either the kynA or kynB promoter region in the presence or absence of kynurenine. We also added tryptophan in separate binding reaction mixtures to determine if it had an effect on the ability of KynR to bind DNA. As a nonspecific binding control, an internal kynU fragment was radiolabeled and utilized in binding reactions with KynR and kynurenine. The results of all EMSA are shown in Fig. 6. These data indicate that KynR only bound to the kynA promoter in the presence of kynurenine (Fig. 6A, right panel), and KynR was unable to bind kynA in the absence of a coinducer (Fig. 6A, left panel). Additionally, tryptophan had no effect on the ability of KynR to bind to the kynA promoter (Fig. 6C, left panel). In contrast to kynA, the kynB promoter was bound by KynR in both the presence and absence of kynurenine (Fig. 6B). This binding was not affected by tryptophan (Fig. 6C, right panel) and indicated that the kynA and kynB promoters are recognized by KynR in different manners. It should also be noted that in the presence of kynurenine, an observable shift with kynB occurred with 1.9 pmol of KynR (Fig. 6B, right panel), while 7.62 pmol of KynR was needed for a kynB shift to occur in the absence of kynurenine (Fig. 6B, left panel). This showed that KynR has a greater affinity for the kynB promoter in the presence of kynurenine. Furthermore, compared to the interaction with kynA, the binding of KynR to kynB exhibited a more complex pattern of DNA migration retardation as the protein concentration increased. These results suggest that the binding of KynR-kynurenine to the kynA promoter represented the binding of a single complex to the DNA and that multiple KynR complexes, with or without a kynurenine coinducer, most likely interact with the kynB promoter. Taken together, these data indicated that KynR binds to the kynA promoter in a kynurenine-dependent manner, while the interaction of KynR with the kynB promoter occurs in both the presence and absence of kynurenine in a less constricted manner.
Fig. 6.
KynR binds to the kynA and kynB promoter regions. (A) KynR was added to [γ-32P]kynA in both the presence and absence of 10 μM kynurenine (kyn). (B) KynR was added to [γ-32P]kynB in both the presence and absence of 10 μM kynurenine. (C) KynR was added to [γ-32P]kynA and [γ-32P]kynB in both the presence and absence of 10 μM tryptophan (Trp). (D) KynR was added to a [γ-32P]kynU DNA fragment in both the presence and absence of 10 μM kynurenine. The total amounts of KynR are indicated below each lane. Total binding reaction mixtures were electrophoresed on nondenaturing 6% polyacrylamide gels. Gels were dried, and overlaid X-ray film was exposed for 2 days before being developed.
DISCUSSION
We previously showed that the transcription of both kynA and kynB was induced in the presence of kynurenine (11). This led us to believe that a transcriptional regulator was responsible for the induction of the kynurenine pathway genes in the presence of kynurenine. With this in mind, we identified KynR as the transcriptional regulator for the kynurenine pathway. The kynR gene is divergently transcribed from kynB and is found in the same genomic context in other Gram-negative bacteria with kynurenine pathway genes (Fig. 1). To begin to characterize the role of KynR in the expression of the kynurenine pathway genes, we first determined that P. aeruginosa required kynA, kynB, and kynU to grow on tryptophan as a sole carbon source (Fig. 2). This phenotype provided a testable method to determine if KynR was important for expression of the kynurenine pathway. When the kynR mutant was grown in TSCM, it was unable to utilize tryptophan as a sole carbon source, thereby indicating that KynR was involved in tryptophan degradation and leading us to explore its role as the regulator of the kynurenine pathway genes.
Due to the similar phenotypes of the kynurenine pathway mutants and kynR mutants when grown on tryptophan as a sole carbon source, we felt that this regulator would probably affect PQS production. We previously showed that the kynurenine pathway is the main source of anthranilate for PQS production in rich media and that kynA and kynU mutants produce no detectable PQS while kynB mutants produce trace amounts of PQS (11). To our surprise, the data indicated that a mutation in kynR caused only a partial decrease in PQS production (Fig. 3). This was probably due to the fact that we had mutated a pathway regulator as opposed to a structural gene (i.e., kynA, -B, or -U) that directly acts to break down tryptophan. We also found that the addition of kynurenine to the kynR mutant resulted in a wild-type level of PQS production, which means that kynBU was at least partly induced in the absence of KynR. This was explained by showing that kynB is positively regulated by LasR (Fig. 4), indicating that multiple regulators control this gene.
To determine how KynR affects the kynurenine pathway genes, we analyzed the transcriptional activity of kynA and kynB in our kynR mutant. Our data showed that both kynA and kynB were induced only in the presence of both KynR and kynurenine (Fig. 4), thereby implying that kynurenine was acting as a coinducer for KynR. These studies were then taken a step further when we showed that KynR and kynurenine were required and sufficient for the activation of kynB in E. coli (Fig. 5). This suggested that the regulation of kynB by KynR was direct and made it clear that the regulation of the kynurenine pathway in P. aeruginosa differs from tryptophan degradation pathways in other bacteria, in which the expression of the pyridoxal phosphate-dependent tryptophanase is posttranscriptionally regulated through attenuation (50). Taken together, the data we accumulated suggested that the kynurenine pathway is directly regulated in a positive way by KynR in a kynurenine-dependent manner.
To explore this potential direct regulation, we utilized purified KynR in DNA binding assays to study its interaction with the kynA and kynB promoters (Fig. 6). These studies showed that KynR bound to the kynA promoter only in the presence of kynurenine and that it would bind to the kynB promoter in the presence or absence of kynurenine. The addition of kynurenine did cause KynR to have a higher affinity for the kynB promoter, but the ability to bind kynB in the absence of kynurenine was interesting and not entirely unexpected. Ligand-independent binding by Lrp/AsnC-type regulators is typically observed when the regulator and target gene are divergently transcribed and adjacent to each other, as is the case with kynB and kynR (3). Such genetic organization often results in decreased transcription of either the regulator or both the regulator and target gene (3), but we have not demonstrated this for kynB or kynR.
Another interesting observation from the EMSA experiments was the differences that were observed between the binding complexes that KynR formed with the kynA and kynB promoter regions. The kynA-KynR-kynurenine complexes migrated the same regardless of the KynR concentration, while the kynB-KynR complexes had different migration rates with increasing concentrations of KynR (with and without kynurenine present). This type of migration pattern could be the result of KynR binding to multiple sites within the promoter region. Multiple binding sites, as well as DNA bending, have been reported in several Lrp/AsnC-type regulators in E. coli and other bacteria (9, 12, 20, 45, 46). In addition, Lrp/AsnC-type transcriptional regulators can bind as multimers both in the presence and absence of a ligand and are capable of acting as both activators and repressors (3, 51), so this seems to be the most likely explanation for the results seen with the kynB promoter binding assays.
Overall, our findings show that tryptophan degradation in P. aeruginosa is upregulated by a positive feedback mechanism and suggest that the pathway would be activated in tryptophan-rich environments. To help understand this, we have included a proposed model of tryptophan degradation by P. aeruginosa (Fig. 7). This model incorporates our previous results, which showed that the kynurenine pathway is a major source of anthranilate for PQS production in the presence of tryptophan and tryptophan-breakdown products (11). Similarly, Chugani and Greenberg (7) demonstrated that the kynurenine pathway was necessary for the expression of catB, a gene encoding an enzyme involved in anthranilate catabolism. These findings suggest that the kynurenine pathway could be active in amino acid-rich environments, such as in the CF lung (1, 30, 39). While one study found only 10 μM free tryptophan in CF sputum (30), it is well known that CF sputum also contains increased amounts of protein (2, 18, 29, 40). P. aeruginosa is well known to utilize proteases during infections within the CF lung (16, 39), and these enzymes could readily liberate tryptophan from host or bacterial proteins found in sputum. The ability of P. aeruginosa to catabolize tryptophan via the kynurenine pathway would provide a good source of both carbon for growth and anthranilate for the production of 4-quinolones (and many other secondary metabolites) during human infections (11). Whether the kynurenine pathway provides anthranilate for the production of PQS or for nutrient acquisition through anthranilate catabolism, this pathway provides a unique tool for P. aeruginosa to regulate virulence through PQS or survival through carbon and nitrogen source acquisition.
ACKNOWLEDGMENTS
We thank C. Caswell, M. Ellison, and K. Tipton for helpful discussions and thoughtful insight.
This work was supported by a research grant from the National Institute of Allergy and Infectious Diseases (grant R01-AI076272).
Footnotes
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Balibar C. J., Walsh C. T. 2006. In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum. Biochemistry 45:15444–15457 [DOI] [PubMed] [Google Scholar]
- 2. Barth A. L., Pitt T. L. 1996. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J. Med. Microbiol. 45:110–119 [DOI] [PubMed] [Google Scholar]
- 3. Brinkman A. B., Ettema T. J., de Vos W. M., van der Oost J. 2003. The Lrp family of transcriptional regulators. Mol. Microbiol. 48:287–294 [DOI] [PubMed] [Google Scholar]
- 4. Burns J. L., et al. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444–452 [DOI] [PubMed] [Google Scholar]
- 5. Chang A. C., Cohen S. N. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi K. H., Kumar A., Schweizer H. P. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397 [DOI] [PubMed] [Google Scholar]
- 7. Chugani S., Greenberg E. P. 2010. LuxR homolog-independent gene regulation by acyl-homoserine lactones in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 107:10673–10678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collier D. N., et al. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41–46 [DOI] [PubMed] [Google Scholar]
- 9. Cui Y., Wang Q., Stormo G. D., Calvo J. M. 1995. A consensus sequence for binding of Lrp to DNA. J. Bacteriol. 177:4872–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deziel E., et al. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad.Sci. U. S. A. 101:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farrow J. M., III, Pesci E. C. 2007. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J. Bacteriol. 189:3425–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedberg D., Midkiff M., Calvo J. M. 2001. Global versus local regulatory roles for Lrp-related proteins: Haemophilus influenzae as a case study. J. Bacteriol. 183:4004–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallagher L. A., Manoil C. 2001. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J. Bacteriol. 183:6207–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gambello M. J., Iglewski B. H. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert K. B., Kim T. H., Gupta R., Greenberg E. P., Schuster M. 2009. Global position analysis of the Pseudomonas aeruginosa quorum-sensing transcription factor LasR. Mol. Microbiol. 73:1072–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henke M. O., et al. 2011. Serine proteases degrade airway mucins in cystic fibrosis. Infect. Immun. 79:3438–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 18. Hoiby N. 1998. Pseudomonas in cystic fibrosis: past, present, and future. Cystic Fibrosis Trust, London, England [Google Scholar]
- 19. Holloway B. W., Krishnapillai V., Morgan A. F. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jafri S., Chen S., Calvo J. M. 2002. ilvIH operon expression in Escherichia coli requires Lrp binding to two distinct regions of DNA. J. Bacteriol. 184:5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kersten R. D., Dorrestein P. C. 2009. Secondary metabolomics: natural products mass spectrometry goes global. ACS Chem. Biol. 4:599–601 [DOI] [PubMed] [Google Scholar]
- 22. Kurnasov O., et al. 2003. NAD biosynthesis: identification of the tryptophan to quinolinate pathway in bacteria. Chem. Biol. 10:1195–1204 [DOI] [PubMed] [Google Scholar]
- 23. Kurnasov O., et al. 2003. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiol. Lett. 227:219–227 [DOI] [PubMed] [Google Scholar]
- 24. Lepine F., Milot S., Deziel E., He J., Rahme L. G. 2004. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J. Am. Soc. Mass Spec. 15:862–869 [DOI] [PubMed] [Google Scholar]
- 25. Lyczak J. B., Cannon C. L., Pier G. B. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051–1060 [DOI] [PubMed] [Google Scholar]
- 26. Madhusudhan K. T., Huang N., Sokatch J. R. 1995. Characterization of BkdR-DNA binding in the expression of the bkd operon of Pseudomonas putida. J. Bacteriol. 177:636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahajan-Miklos S., Tan M. W., Rahme L. G., Ausubel F. M. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47–56 [DOI] [PubMed] [Google Scholar]
- 28. Matthijs S., et al. 2004. The Pseudomonas siderophore quinolobactin is synthesized from xanthurenic acid, an intermediate of the kynurenine pathway. Mol. Microbiol. 52:371–384 [DOI] [PubMed] [Google Scholar]
- 29. Ohman D. E., Chakrabarty A. M. 1982. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect. Immun. 37:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer K. L., Aye L. M., Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189:8079–8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer K. L., Mashburn L. M., Singh P. K., Whiteley M. 2005. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 187:5267–5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Preston M. J., et al. 1997. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun. 65:3086–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiu D., Damron F. H., Mima T., Schweizer H. P., Yu H. D. 2008. PBAD-based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Appl. Environ. Microbiol. 74:7422–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahme L. G., et al. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl. Acad. Sci. U. S. A. 94:13245–13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramos J. L. (ed.). 2004. Pseudomonas. Kluwer Academic/Plenum Publishers, New York, NY [Google Scholar]
- 36. Schuster M., Lostroh C. P., Ogi T., Greenberg E. P. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stuart B., Lin J. H., Mogayzel P. J., Jr 2010. Early eradication of Pseudomonas aeruginosa in patients with cystic fibrosis. Paediatr. Respir. Rev. 11:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sullivan N. L., Tzeranis D., Wang Y., So P. T., Newman D. 2011. Quantifying the dynamics of bacterial secondary metabolites by spectral multiphoton microscopy. ACS Chem. Biol. 6:893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suter S. 1994. The role of bacterial proteases in the pathogenesis of cystic fibrosis. Am. J. Respir. Crit. Care Med. 150:S118–S122 [DOI] [PubMed] [Google Scholar]
- 40. Thomas S. R., Ray A., Hodson M. E., Pitt T. L. 2000. Increased sputum amino acid concentrations and auxotrophy of Pseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax 55:795–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vederas J. C., Schleicher E., Tsai M. D., Floss H. G. 1978. Stereochemistry and mechanism of reactions catalyzed by tryptophanase Escherichia coli. J. Biol. Chem. 253:5350–5354 [PubMed] [Google Scholar]
- 42. Vogel H. J., Bonner D. M. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97–106 [PubMed] [Google Scholar]
- 43. Wagner V. E., Bushnell D., Passador L., Brooks A. I., Iglewski B. H. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walters M., Sperandio V. 2006. Quorum sensing in Escherichia coli and Salmonella. Int. J. Med. Microbiol. 296:125–131 [DOI] [PubMed] [Google Scholar]
- 45. Wang Q., Calvo J. M. 1993. Lrp, a global regulatory protein of Escherichia coli, binds co-operatively to multiple sites and activates transcription of ilvIH. J. Mol. Biol. 229:306–318 [DOI] [PubMed] [Google Scholar]
- 46. Wang Q., Calvo J. M. 1993. Lrp, a major regulatory protein in Escherichia coli, bends DNA and can organize the assembly of a higher-order nucleoprotein structure. EMBO J. 12:2495–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Warrens A. N., Jones M. D., Lechler R. I. 1997. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene 186:29–35 [DOI] [PubMed] [Google Scholar]
- 48. Woodcock D. M., et al. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiao G., et al. 2006. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 62:1689–1699 [DOI] [PubMed] [Google Scholar]
- 50. Yanofsky C. 2007. RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA 13:1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yokoyama K., et al. 2006. Feast/famine regulatory proteins (FFRPs): Escherichia coli Lrp, AsnC and related archaeal transcription factors. FEMS Microbiol. Rev. 30:89–108 [DOI] [PubMed] [Google Scholar]
- 52. Zegarra-Moran O., et al. 2004. Double mechanism for apical tryptophan depletion in polarized human bronchial epithelium. J. Immunol. 173:542–549 [DOI] [PubMed] [Google Scholar]