Abstract
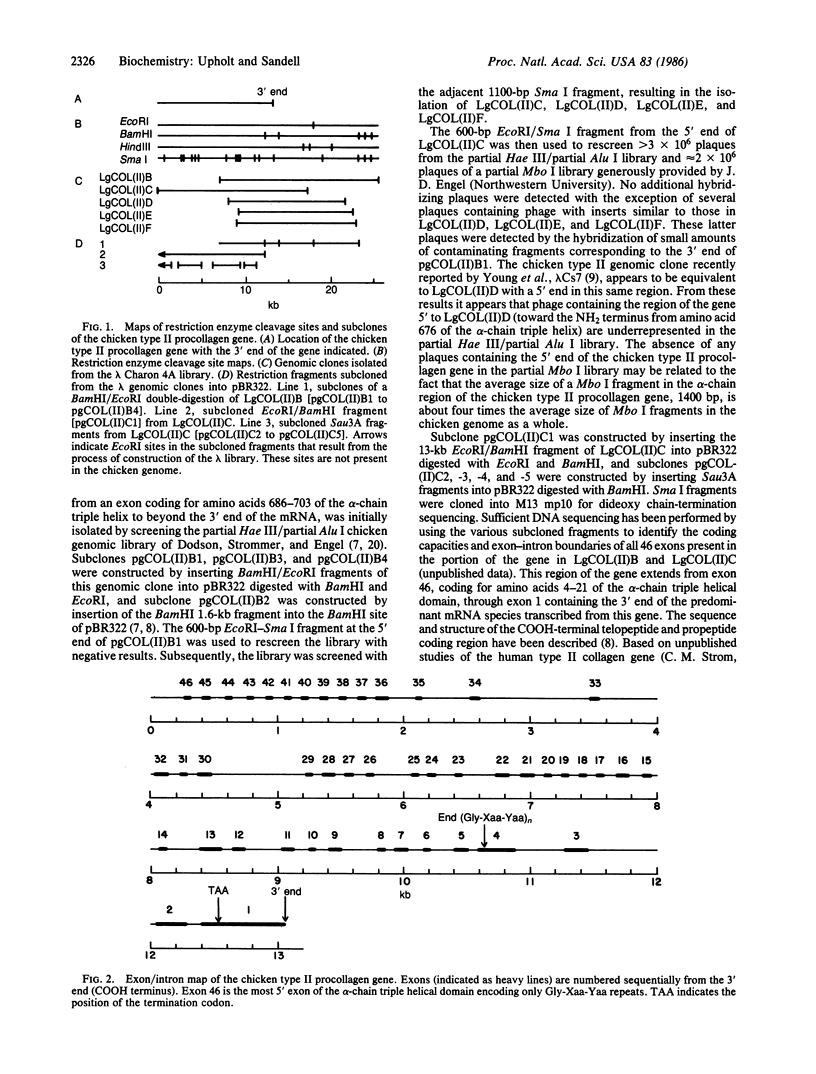
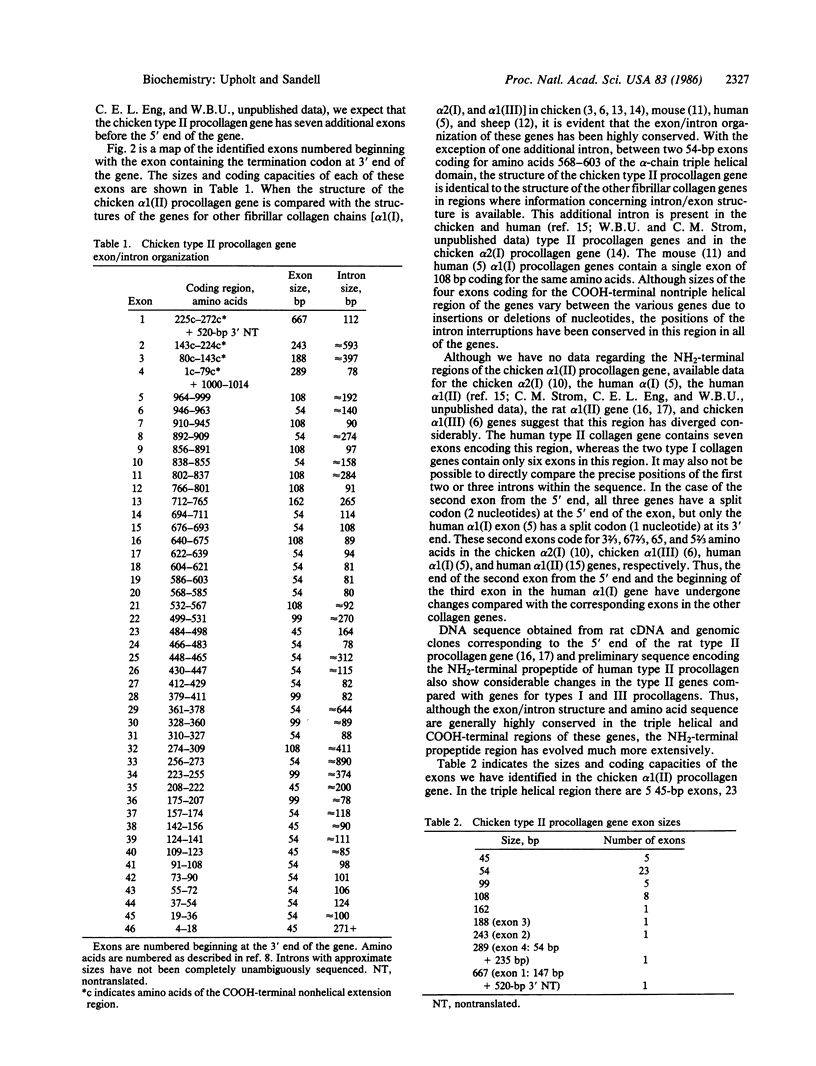
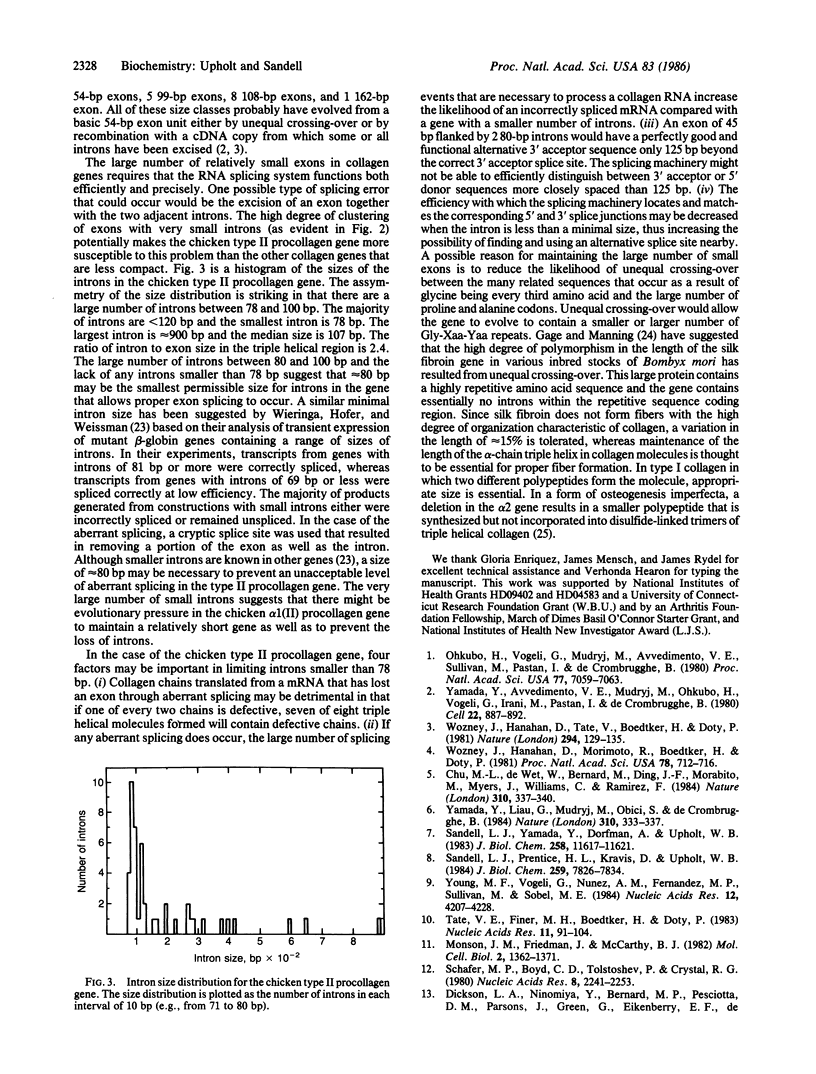
Overlapping genomic clones have been isolated that contain the alpha chain and COOH-terminal propeptide coding regions of the chicken type II procollagen gene. All type II procollagen exon sequences present in these clones have been identified and mapped by DNA sequencing. These include 43 exons coding for the alpha-chain triple helix, 1 exon coding for the junction between the COOH-terminal propeptide and the alpha-chain region, and 3 exons coding for the COOH-terminal propeptide and 3' noncoding sequences. With the exception of one additional intron between 2 exons coding for amino acids 568-585 and 586-603, exon-intron boundaries have been conserved when compared with genes for all other characterized genes for fibrillar collagens. The chicken type II procollagen gene differs from most other collagen genes in having introns of considerably smaller average size. The size distribution of the introns suggests that approximately equal to 80 base pairs may be a minimal functional size for introns in this gene. This size of intron may be necessary in a gene with a very large number of small exons to prevent aberrant splicing from removing exon sequence together with intron sequence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boedtker H., Aho S. Collagen gene structure: the paradox may be resolved. Biochem Soc Symp. 1984;49:67–84. [PubMed] [Google Scholar]
- Cheah K. S., Stoker N. G., Griffin J. R., Grosveld F. G., Solomon E. Identification and characterization of the human type II collagen gene (COL2A1). Proc Natl Acad Sci U S A. 1985 May;82(9):2555–2559. doi: 10.1073/pnas.82.9.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ding J. F., Morabito M., Myers J., Williams C., Ramirez F. Human pro alpha 1(I) collagen gene structure reveals evolutionary conservation of a pattern of introns and exons. 1984 Jul 26-Aug 1Nature. 310(5975):337–340. doi: 10.1038/310337a0. [DOI] [PubMed] [Google Scholar]
- Deak S. B., Nicholls A., Pope F. M., Prockop D. J. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem. 1983 Dec 25;258(24):15192–15197. [PubMed] [Google Scholar]
- Dickson L. A., Ninomiya Y., Bernard M. P., Pesciotta D. M., Parsons J., Green G., Eikenberry E. F., de Crombrugghe B., Vogeli G., Pastan I. The exon/intron structure of the 3'-region of the pro alpha 2(I) collagen gene. J Biol Chem. 1981 Aug 25;256(16):8407–8415. [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Manning R. F. Internal structure of the silk fibroin gene of Bombyx mori. I The fibroin gene consists of a homogeneous alternating array of repetitious crystalline and amorphous coding sequences. J Biol Chem. 1980 Oct 10;255(19):9444–9450. [PubMed] [Google Scholar]
- Kohno K., Martin G. R., Yamada Y. Isolation and characterization of a cDNA clone for the amino-terminal portion of the pro-alpha 1(II) chain of cartilage collagen. J Biol Chem. 1984 Nov 25;259(22):13668–13673. [PubMed] [Google Scholar]
- Kohno K., Sullivan M., Yamada Y. Structure of the promoter of the rat type II procollagen gene. J Biol Chem. 1985 Apr 10;260(7):4441–4447. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monson J. M., Friedman J., McCarthy B. J. DNA sequence analysis of a mouse pro alpha 1 (I) procollagen gene: evidence for a mouse B1 element within the gene. Mol Cell Biol. 1982 Nov;2(11):1362–1371. doi: 10.1128/mcb.2.11.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo H., Vogeli G., Mudryj M., Avvedimento V. E., Sullivan M., Pastan I., de Crombrugghe B. Isolation and characterization of overlapping genomic clones covering the chicken alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7059–7063. doi: 10.1073/pnas.77.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. J., Prentice H. L., Kravis D., Upholt W. B. Structure and sequence of the chicken type II procollagen gene. Characterization of the region encoding the carboxyl-terminal telopeptide and propeptide. J Biol Chem. 1984 Jun 25;259(12):7826–7834. [PubMed] [Google Scholar]
- Sandell L. J., Yamada Y., Dorfman A., Upholt W. B. Identification of genomic DNA coding for chicken type II procollagen. J Biol Chem. 1983 Oct 10;258(19):11617–11621. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Ramirez F. Analysis of cDNA and genomic clones coding for the pro alpha 1 chain of calf type II collagen. Nucleic Acids Res. 1985 Apr 25;13(8):2815–2826. doi: 10.1093/nar/13.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Tsipouras P., Ramirez F. Isolation and partial characterization of the entire human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1985 Apr 11;13(7):2207–2225. doi: 10.1093/nar/13.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M. P., Boyd C. D., Tolstoshev P., Crystal R. G. Structural organization of a 17 KB segment of the alpha 2 collagen gene: evaluation by R loop mapping. Nucleic Acids Res. 1980 May 24;8(10):2241–2253. doi: 10.1093/nar/8.10.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate V. E., Finer M. H., Boedtker H., Doty P. Chick pro alpha 2 (I) collagen gene: exon location and coding potential for the prepropeptide. Nucleic Acids Res. 1983 Jan 11;11(1):91–104. doi: 10.1093/nar/11.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Morimoto R., Boedtker H., Doty P. Fine structural analysis of the chicken pro alpha 2 collagen gene. Proc Natl Acad Sci U S A. 1981 Feb;78(2):712–716. doi: 10.1073/pnas.78.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Tate V., Boedtker H., Doty P. Structure of the pro alpha 2 (I) collagen gene. Nature. 1981 Nov 12;294(5837):129–135. doi: 10.1038/294129a0. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Avvedimento V. E., Mudryj M., Ohkubo H., Vogeli G., Irani M., Pastan I., de Crombrugghe B. The collagen gene: evidence for its evolutinary assembly by amplification of a DNA segment containing an exon of 54 bp. Cell. 1980 Dec;22(3):887–892. doi: 10.1016/0092-8674(80)90565-6. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Liau G., Mudryj M., Obici S., de Crombrugghe B. Conservation of the sizes for one but not another class of exons in two chick collagen genes. 1984 Jul 26-Aug 1Nature. 310(5975):333–337. doi: 10.1038/310333a0. [DOI] [PubMed] [Google Scholar]
- Young M. F., Vogeli G., Nunez A. M., Fernandez M. P., Sullivan M., Sobel M. E. Isolation of cDNA and genomic DNA clones encoding type II collagen. Nucleic Acids Res. 1984 May 25;12(10):4207–4228. doi: 10.1093/nar/12.10.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]



