Abstract
Surface lipoproteins of Borrelia spirochetes are important virulence determinants in the transmission and pathogenesis of Lyme disease and relapsing fever. To further define the conformational secretion requirements and to identify potential lipoprotein translocation intermediates associated with the bacterial outer membrane (OM), we generated constructs in which Borrelia burgdorferi outer surface lipoprotein A (OspA) was fused to calmodulin (CaM), a conserved eukaryotic protein undergoing calcium-dependent folding. Protein localization assays showed that constructs in which CaM was fused to full-length wild-type (wt) OspA or to an intact OspA N-terminal “tether” peptide retained their competence for OM translocation even in the presence of calcium. In contrast, constructs in which CaM was fused to truncated or mutant OspA N-terminal tether peptides were targeted to the periplasmic leaflet of the OM in the presence of calcium but could be flipped to the bacterial surface upon calcium chelation. This indicated that in the absence of an intact tether peptide, unfolding of the CaM moiety was required in order to facilitate OM traversal. Together, these data further support a periplasmic tether peptide-mediated mechanism to prevent premature folding of B. burgdorferi surface lipoproteins. The specific shift in the OM topology of sequence-identical lipopeptides due to a single-variable change in environmental conditions also indicates that surface-bound Borrelia lipoproteins can localize transiently to the periplasmic leaflet of the OM.
INTRODUCTION
Bacterial lipoproteins are a class of peripherally membrane associated proteins that play significant roles in various cellular and pathogenic processes (29). They are particularly important protagonists in the development of two arthropod-borne infectious diseases, Lyme borreliosis and relapsing fever. Abundantly displayed on the surfaces of the causative Borrelia spirochetes, they consequently dominate the spirochetes' interface with both the vector and the host. It is therefore not entirely surprising that a multitude of Borrelia lipoproteins have been shown to contribute significantly to transmission, infection, persistence, and disease (4, 7, 10, 31, 41).
Due to the required crossing of the outer membrane (OM), the sorting of major lipoproteins is inherently more complex in Borrelia than in other diderm bacteria. In the Gram-negative model organism Escherichia coli, the major lipoprotein Lpp has a known periplasmic function in tethering the OM to the peptidoglycan cell wall (11) but can also assume a transmembrane, partially surface exposed topology in its unbound form (19). In previous studies with two functionally and structurally divergent outer surface lipoproteins, Borrelia burgdorferi OspA and OspC, as well as the OspC-related protein Borrelia turicatae Vsp1, we showed that surface lipoprotein secretion pathways are likely distinct from those in proteobacteria but conserved within the genus (62). We found that established eubacterial lipoprotein-sorting rules governed by N-terminal residues proximal to the triacyl-modified cysteine did not apply (22, 37, 40, 52, 53, 58). Yet lipoprotein-targeting instructions remained restricted to disordered N-terminal peptides that function as “tethers,” i.e., that link the folded domains of the protein to the triacyl membrane anchor (32, 33, 50, 51) (Fig. 1). Our previous data also suggested that OspA, OspC, and Vsp1 lipoprotein tether mutants that were blocked from translocation through the spirochetal OM folded prematurely within the periplasm (50). It remained unclear, however, whether the identified lipoprotein tether mutants localizing to the periplasmic leaflet of the OM represented the products of an aborted translocation event or were equivalent to translocation intermediates. Therefore, we set out to develop an experimental system that would allow us to “catch and release” borrelial lipopeptides on their way to the bacterial surface. Here we present data obtained with constructs in which B. burgdorferi surface lipoproteins are fused to calmodulin (CaM), a eukaryotic protein that undergoes reversible calcium-dependent folding (30, 59). Using a reformulated cation-controlled Borrelia growth medium and radiolabeling experiments, we show that calcium chelation-induced unfolding of CaM specifically permits the translocation of mutant OspA tether-CaM fusion proteins from the periplasmic leaflet of the OM to the bacterial surface. The requirement for chelation-mediated unfolding of these model lipoproteins can be bypassed by providing a wild-type (wt) OspA tether peptide in cis.
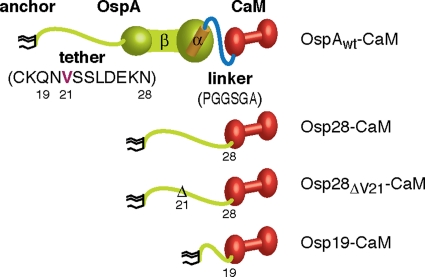
Fig. 1.
OspA-CaM fusion constructs. Cartoons of OspA-CaM fusion constructs generated and used in this study show triacyl anchor lipids in black, OspA peptides in green (N-terminal tether and β-strands) and orange (C-terminal α-helix), linker peptides in blue, and CaM peptides in red. Tether and linker peptide sequences are given in one-letter amino acid code. Mutant nomenclature follows that of earlier publications (50, 51). Briefly, modified residues are numbered according to their prolipoprotein positions; numbers in lipoprotein tether-CaM fusion constructs indicate the C-terminal tether residue present in the fusion.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli strains TOP10 (Invitrogen) and XL10-Gold (Stratagene) were used for recombinant plasmid construction and propagation and were grown in Luria Bertani (LB) broth or on LB agar (Difco). Borrelia burgdorferi B31-e2 or B313, both of which are clones of type strain B31 (ATCC 35210), was used for recombinant expression of OspA-CaM fusion proteins (Table 1). B31-e2 (2) expresses endogenous OspA, while B313 lacks the lp54 plasmid encoding ospA (46, 62, 63). B. burgdorferi was cultured either in BSK-II medium (3, 60) or in BSK-SFcc, a cation-controlled modification of a BSK-II-based serum-free medium (18, 42). BSK-SFcc consisted of basal BSK-II medium (60) supplemented with 26 μM cholesterol, 12 μM palmitic acid, 12 μM oleic acid, 1 mM MgSO4, 0.1 mM MnCl2, and 0.1 mM ZnCl2 (all from Sigma-Aldrich); divalent cations, including Ca2+, were chelated by two treatments with 2.5% (wt/vol) Chelex 100 (Sigma-Aldrich) before the Mn, Mg, and Zn ions, essential for the growth of B. burgdorferi (42), were added. Expression from the regulatable Post promoter (57) was induced by the addition of anhydrotetracycline hydrochloride (ATc; IBA GmbH, Göttingen, Germany) to the culture medium at a final concentration of 1 μg/ml. CaCl2 and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) were used at standard final concentrations of 1 mM and 5 mM, respectively, unless otherwise noted.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Borrelia burgdorferi strains | ||
| B313 | Clone of B31 ATCC 35210 (cp26, cp32-1, cp32-2/7, cp32-3, lp17); OspA− | 46 |
| B31-e2 | Clone of B31 ATCC 35210 (cp26, cp32-1, cp32-3, cp32-4, lp17, lp38, lp54); OspA+ | 2 |
| Plasmids | ||
| pBSXcam | pBS vector containing the Xenopus laevis cam coding sequence (NCBI BC072232) | Gift from G. Carlson |
| pCRW53 | pBSVΦ(ospAp-gfp) derivative with gfp gene under Post promoter control | 14, 57 |
| pCRW56 | pCRW53 lacking Kanr cassette NdeI site and two Tetr cassette NdeI sites, with NdeI site introduced 5′ of gfp gene | This study |
| pRJS1009 | pBSV2::PflaBOspA28-mRFP1 | 51 |
| pRJS1014 | pBSV2::PflaBOspA19-mRFP1 | 51 |
| pRJS1089 | pBSV2::PflaBOspA28ΔV21-mRFPΔ4 | 50 |
| pSC0999 | pBSV2::PflaBospA-His, lacking pBSV2 BamHI/XmaI MCSa | 50 |
| pSC2001 | pBSV2::PflaBOspA-CaM | This study |
| pSC2002 | pBSV2::PflaBOspA28-CaM | This study |
| pSC2003 | pBSV2::PflaBOspA19-CaM | This study |
| pSC2004 | pBSV2::PflaBOspA28ΔV21-CaM | This study |
| pSC2005 | pCRW56::Post OspA19-Xcam | This study |
| pSC2006 | pCRW56::Post OspA28-ΔV21-CaM | This study |
MCS, multiple-cloning site.
OspA-CaM fusion proteins and point mutants.
All plasmids generated and used in this study are derivatives of pBSV2 (56). The expression of OspA-CaM fusion proteins was driven either by the constitutive B. burgdorferi flagellin (flaB) promoter (PflaB) or by the TetR-regulated hybrid Post promoter (57). Gene fusion constructs were generated by sequence overlap extension PCR (SOE-PCR) (26) using Phusion (New England BioLabs/Finnzymes) high-fidelity DNA polymerase. Mutations were introduced using the QuikChange-II XL site-directed mutagenesis kit (Stratagene). Plasmids and custom oligonucleotides (Integrated DNA Technologies [IDT] DNA) are listed in Tables 1 and 2, respectively.
Table 2.
Oligonucleotides used in this study
| Name | Sequence (5′ to 3′) | Description |
|---|---|---|
| PflaBNdeospA-fwd | CATGGAGGAATGACATATGAAAAAATATTTATTGGGAATAG | ospA gene cloning forward primer |
| KpnPflaB-fwd | CGGTACCCTGTCTGTCGCCTCTTG | ospA gene cloning forward primer |
| OspA-Xcam-fwd | AAACCGGGTGGCTCAGGTGCTGCTGACCAACTGACAGAAGAGCAGATTG | OspA-CaM fusion gene cloning forward primer |
| OspA-Xcam-rev | CTCTTCTGTCAGTTGGTCAGCAGCACCTGAGCCACCCGGTTTTAAAGCG | OspA-CaM fusion gene cloning reverse primer |
| BamPflaB-fwd | CGGGATCCTGTCTGTCGCCTCTTG | flaB promoter cloning forward primer |
| Sph-Xcam-rev | AAGCTTGCATGCTCATCACTTTGCTGTCATCATTTGTACAAACTC | SphI site introduction reverse mutagenic primer |
| OspA28-Xcam-rev | CTGCTCTTCTGTCAGTTGGTCAGCGTTTTTCTCGTCAAGGCTGCTAAC | OspA tether-CaM fusion cloning reverse primer |
| OspA28-Xcam-fwd | GTTAGCAGCCTTGACGAGAAAAACGCTGACCAACTGACAGAAGAGCAG | OspA tether-CaM fusion cloning forward primer |
| OspA19-Xcam-fwd | ATATTAGCCTTAATAGCATGTAAGCAAGCTGACCAACTGACAGAAGAGCAGA TTGCAGAG | OspA tether truncation (to aa 19) forward primer |
| OspA19-Xcam-rev | AATCTGCTCTTCTGTCAGTTGGTCAGCTTGCTTACATGCTATTAAGGCTAATATTAGACC | OspA tether truncation (to aa 19) mutagenic primer |
| OspA28-ΔV21-CaM-fwd | TTAATAGCATGTAAGCAAAATAGCAGCCTTGACGAGAAAAACGCTGACCAACTG | V21 codon deletion forward mutagenic primer |
| OspA28-ΔV21-CaM-rev | AGTTGGTCAGCGTTTTTCTCGTCAAGGCTGCTATTTTGCTTACATGCTATTAAGG | V21 codon deletion reverse mutagenic primer |
| Xcam-XmaI-rev | ATCAGCCCGGGTCACTTTGCTGTCATCATTTGTACAAACTC | XmaI site introduction reverse mutagenic primer |
OspAwt-CaM, a construct in which full-length wt OspA is fused to CaM via a 6-amino-acid linker peptide (Fig. 1), is encoded by pSC2001. The ospA gene was amplified from pSC0999 (50) using primer pair BamPflaB-fwd and OspA-Xcam-rev. The Xenopus laevis calmodulin gene (cam) lacking the N-formylmethionine codon was amplified from pBSXcam (a gift from Gerald Carlson, University of Kansas Medical Center) using the primer pair OspA-Xcam-fwd and Sph-Xcam-rev. The overlapping ospA and cam amplicons were fused using the flanking primer pair BamPflaB-fwd and Sph-Xcam-rev. In OspA28-CaM (Fig. 1) (pSC2002), CaM is fused to the 12-amino-acid OspA tether (51). Fragments containing the ospA tether peptide sequence, amplified from pRJS1009 (51) with BamPflaB-fwd and OspA28-Xcam-rev, and cam, amplified from pBSXcam with OspA28-Xcam-fwd and Sph-Xcam-rev, were fused by use of BamPflaB-fwd and Sph-Xcam-rev. The amplicons were then digested with BamHI and SphI and were ligated with an identically cut pRJS1009 backbone.
Constructs in which CaM was fused to the truncated OspA tether peptide OspA19 or OspA28ΔV21 (50, 51) were put under Post control. First, pCRW53 (57) was modified as follows. An NdeI site was introduced into the gfp gene start codon, and one NdeI site in the kanR gene cassette and two NdeI sites in the tetR gene cassette were removed, yielding pCRW56. The 3′ end of the OspA tether sequence was removed from pSC2002 using OspA19-Xcam-fwd and OspA19-Xcam-rev in a QuikChange reaction, resulting in pSC2003, encoding OspA19-CaM (Fig. 1). The OspA Val21 codon was deleted from pSC2002 with primers OspA28-ΔV21-CaM-fwd and OspA28-ΔV21-CaM-rev, resulting in pSC2004, encoding OspA28ΔV21-CaM (Fig. 1). PCR amplicons obtained with PflaBNdeospA-fwd and Xcam-XmaI-rev on pSC2003 and pSC2004 templates were digested with NdeI and XmaI and were ligated with an NdeI-BspEI-cut pCRW56 backbone, generating pSC2005 (OspA19-CaM) and pSC2006 (OspA28ΔV21-CaM), respectively. All plasmids were verified by DNA sequencing (Center for Genetic Medicine, Genomics Core Facility, Northwestern University Medical Center, Chicago, IL, and ACGT, Wheeling, IL).
B. burgdorferi cells were transformed with 5 to 40 μg of plasmid DNA by electroporation using established protocols (47, 56). Transformants were selected in solid BSK-II medium containing 200 μg/ml kanamycin, and three independent clones were expanded in selective liquid BSK-II (60). Plasmid profiles were determined by PCR using plasmid-specific oligonucleotide primers (34, 44).
Gel electrophoresis and immunoblot analysis.
Proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) and were visualized by Coomassie blue staining. For immunoblotting, proteins were electrophoretically transferred to an Immobilon-NC nitrocellulose membrane (Millipore) using a Transblot semidry transfer cell (Bio-Rad) as described previously (62). Membranes were blocked and incubated with antibodies in 5% dry milk, 20 mM Tris–500 mM NaCl, and 0.05% Tween 20. The antibodies used were a rabbit polyclonal antiserum against monomeric red fluorescent protein 1 (mRFP1) (dilution, 1:1,000) (16), a rabbit polyclonal antiserum against OppAIV (dilution, 1:100) (8), or mouse monoclonal antibodies (MAbs) against Lp7.5/6.6 (MAb240.7) (dilution, 1:500) (28), OspA (H5332) (dilution, 1:100) (6), OspC (dilution, 1:50) (38), and FlaB (H9724) (dilution, 1:25) (5). CaM was detected by a rabbit monoclonal antibody (EP799Y; dilution, 1:1,500; Abcam). Secondary antibodies were alkaline phosphatase-conjugated goat-anti-rabbit IgG(H+L) or goat-anti-mouse IgG(H+L) (Sigma). Alkaline phosphatase substrates were 1-Step nitroblue tetrazolium (NBT)–5-bromo-4-chloro-3-indolylphosphate (BCIP) (Pierce) for colorimetric detection and CDP-Star (Amersham Biosciences) for chemiluminescent detection.
Protease and antibody accessibility assays.
To assess the surface exposure of a protein by its accessibility to proteinase K, intact B. burgdorferi cells were harvested, washed, and treated in situ with 200 μg/ml proteinase K (Invitrogen) as described previously (12). Whole-cell protein preparations were analyzed by SDS-PAGE and Western immunoblotting.
Accessibility to antibodies was assessed by indirect fluorescent antibody assay (IFA) microscopy as described previously (63). Spirochetes were resuspended in phosphate-buffered saline (PBS) containing 5 mM MgCl2 (PBS+Mg), 2% bovine serum albumin (BSA), with or without 0.06% (vol/vol) Triton X-100 (20), and were incubated with the primary antibodies described above against CaM (dilution, 1:300) or OppAIV (1:100). Fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (whole molecule; dilution, 1:30; Sigma-Aldrich) was used as a secondary antibody. Cells were analyzed by epifluorescence microscopy using a Nikon Eclipse E600 microscope fitted with an FITC HYQ filter block. Digital images were acquired with a QImaging MicroPublisher digital charge-coupled device (CCD) color camera (epifluorescence micrographs), an Epson Perfection 2450 photo scanner, or a Fuji LAS-4000 luminescent image analyzer (immunoblots) and were processed using Adobe Photoshop CS4 for Macintosh.
Subcellular protein fractionation.
Unless otherwise noted, B. burgdorferi membrane fractions were obtained using a hypotonic citrate buffer as described previously (51, 54). Briefly, B. burgdorferi cells were washed in 1× PBS containing 0.1% BSA, resuspended, and incubated under vigorous shaking for 2 h in 25 mM citrate buffer, pH 3.2, containing 0.1% BSA. Outer membrane vesicle (OMV) and protoplasmic cylinder (PC) fractions were separated by ultracentrifugation in a single discontinuous sucrose gradient in citrate buffer, washed, and resuspended in 1× PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF). An alternative, Ca2+ chelator-free (i.e., citrate-free) membrane fractionation approach was modified from reference 45 and used hypotonic sucrose buffer. Briefly, harvested B. burgdorferi cells were suspended in 20% (wt/vol) sucrose in 10 mM HEPES buffer, 150 mM NaCl, and 1 mM MgCl2 (pH 7.4). After a 1-h incubation, PC and OMV fractions were obtained by ultracentrifugation of the samples in an equal-volume stepped gradient of 20, 25, 30, 35, 40, 45, 50, 55, and 60% sucrose in a buffer consisting of 10 mM HEPES, 150 mM NaCl, and 1 mM MgCl2 (pH 7.4). Fractions were washed and resuspended in HEPES buffer containing 1 mM PMSF. Both approaches used a Beckman L8-80M centrifuge with an SW28 rotor and 25- by 89-mm Ultra-Clear ultracentrifuge tubes.
Soluble and membrane-associated B. burgdorferi proteins were fractionated by Triton X-114 phase partitioning using a protocol modified from the work of Brandt et al. (10) and Nally et al. (39). Briefly, harvested cells were resuspended in a mixture of cold PBS and Mg (PBS+Mg) containing 2% (vol/vol) Triton X-114 (PBS+Mg+TX) and were incubated with end-over-end mixing at 4°C for 1 h. Samples were then heated to 37°C for 15 min and were centrifuged at room temperature for 15 min at 15,000 rpm. The detergent phase and the aqueous phase were separated into different tubes and were washed three times with 1 ml PBS+Mg or PBS+Mg+TX, respectively. Proteins in the detergent and aqueous phases were precipitated overnight in 4 sample volumes of acetone or 15% (vol/vol) trichloroacetic acid (TCA; Sigma), respectively, pelleted by centrifugation at 13,000 rpm for 15 min, washed with ice-cold acetone, and subjected to SDS-PAGE and Western blot analysis.
IP.
Proteins were isolated from whole-cell lysates by immunoprecipitation (IP) using protein-specific antibodies and protein A Sepharose 4 Fast Flow beads (Amersham Biosciences) as described previously (51). Briefly, 5 × 109 spirochetes were harvested and washed twice in ice-cold PBS+Mg. Cells were resuspended in 1 ml of ice-cold lysis buffer (50 mM Tris [pH 7.4], 1% dodecyl-β-d-maltoside [DDM], 1 mM PMSF) and were lysed using a Branson Sonifier cell disruptor. Cellular debris was pelleted by centrifugation for 10 min at 15,000 × g and 4°C. After preclearing with protein A beads for 1 h at 4°C, the lysate was incubated with an anti-Ca2+-CaM (ACC-1; dilution, 1:250; Millipore) (25) or an anti-CaM (EP799Y; dilution, 1:50; Abcam) antibody for 1 h at 4°C with end-over-end mixing. Next, 50 μl of washed protein A beads was added, and the incubation was continued for 1 h, after which the beads were washed three times with lysis buffer and once with 50 mM Tris, pH 7.4. Immunoprecipitated proteins were released from the beads by boiling for 5 min in 40 μl of SDS-PAGE sample buffer, collected in 30 μl of supernatant, and analyzed by SDS-PAGE and immunoblotting with an anti-CaM antibody.
Hydrophobic interaction chromatography (HIC).
A total of 2.5 × 109 late-log-phase spirochetes were harvested, washed three times with PBS+Mg, resuspended in 1.5 ml lysis buffer (50 mM Tris [pH 7.4], 1 M NaCl, 1% DDM, 1 mM PMSF), and lysed by sonication. The lysate was cleared by centrifugation for 10 min at 15,000 × g and 4°C. A 50-μl volume of phenyl-Sepharose CL-4B (Pharmacia LKB Biotechnology) preequilibrated in lysis buffer was added, and the mixture was mixed end-over-end for 2 h at 4°C. Subsequently, the supernatant was removed, and the resin was washed three times with lysis buffer without 1% DDM and once with wash buffer (50 mM Tris-HCl buffer [pH 7.4], 1 mM PMSF). Proteins were eluted by incubating the resin in 0.6 ml wash buffer containing 1 mM EGTA for 10 min at 4°C with end-over-end rotation. The eluted proteins were concentrated by precipitation with TCA and were analyzed by SDS-PAGE and Western immunoblotting with the anti-CaM antibody.
Protein radiolabeling.
A total of 5 × 108 early-log-phase spirochetes were harvested, washed once with PBS+Mg, and resuspended in 0.5 ml protein-free RPMI 1640 medium (pH 7.5; Sigma-Aldrich) containing a cysteine- and methionine-free amino acid mixture and appropriate antibiotics. Final concentrations of 1 μg/ml ATc and 100 μCi/ml [35S]Cys-Met (EasyTag Express 35S protein labeling mix; Perkin-Elmer) were added to simultaneously induce Post promoter-driven expression of OspA-CaM fusion proteins and metabolic radiolabeling of cellular proteins. After incubation for 2 h at 34°C, the cells were pelleted, resuspended in RPMI 1640 medium containing 1.5 mg/ml cold Cys and Met, and incubated for 10 min at 34°C. The spirochetes were then pelleted and resuspended in BSK-SFcc medium containing either calcium or BAPTA (final concentrations, 5 mM). After incubation for 3 h at 34°C, the cells were harvested, washed twice with PBS+Mg, and subjected to in situ proteolysis. SDS-PAGE gels containing whole-cell proteins were stained with 0.05% Coomassie brilliant blue R-250 (Sigma) in 40% methanol and 10% acetic acid, destained with 20% methanol and 10% acetic acid, and immersed in Amplify fluorographic reagent (Amersham Biosciences) for 30 min. After drying under a vacuum for 1 h at 60°C, the gels were exposed overnight to Kodak (Rochester, NY) XAR-5 film.
RESULTS
Development of OspA-CaM fusion proteins and a cation-controlled Borrelia culture medium.
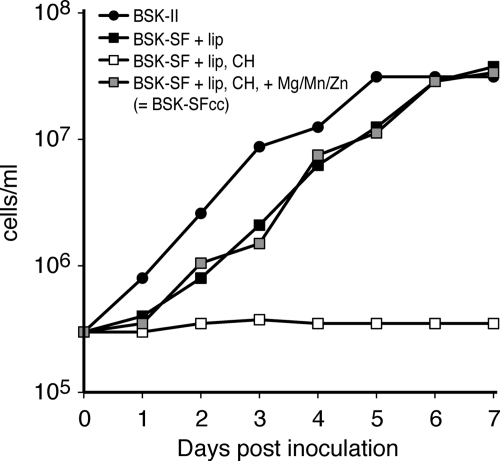
CaM undergoes a significant conformational change upon the binding of calcium cations (Ca2+). The Ca2+-free apo-form is largely unfolded, whereas the Ca2+-loaded protein forms a stable dumbbell structure (30). Hence, the folding state of CaM can be controlled by experimentally manipulating the Ca2+ level (1, 24, 27, 59). To test whether the addition of a tightly folded Ca2+-CaM domain could block the secretion of a Borrelia surface lipoprotein, we generated OspA-CaM fusion proteins (Fig. 1). Because the standard BSK-II medium contains rich sources of calcium, such as rabbit serum, we modified a serum-free Borrelia “minimal medium” developed by Posey and Gherardini (42) and by Cluss et al. (18) to allow for more-precise control of Ca2+ levels. As expected, the growth characteristics of B. burgdorferi in the standard culture medium BSK-II and the calcium-free medium BSK-SFcc were comparable (Fig. 2). Mean log-phase generation times were 13.9 h in BSK-II medium and 16.9 h in BSK-SFcc. Addition of Mg, Mn, and Zn ions was sufficient to support growth in a previously chelated medium, as shown for the similarly formulated medium used by Posey and Gherardini (42). These preliminary experiments confirmed that calcium-limiting conditions had no significant effects on cell growth that would introduce additional undesired variables into the experimental setup.
Fig. 2.
Growth of B. burgdorferi in serum- and calcium-free minimal medium. B. burgdorferi was cultured in complete BSK-II medium and in various modifications of serum-free (SF) medium. Serum-free incomplete or basal BSK medium (BSK-SF) (60) was supplemented with lipids (+ lip), treated with Chelex-100 (CH), or supplemented with essential cations (+Mg/Mn/Zn). Cells were counted under phase-contrast microscopy using a Petroff-Hausser counting chamber. Representative growth curves are shown. Note that the 1-day lag phase for cells grown in BSK-SFcc persisted even when bacteria had been passed in BSK-SFcc previously (data not shown).
Tolerance of the B. burgdorferi lipoprotein secretion machinery for wild-type OspA-CaM fusion constructs.
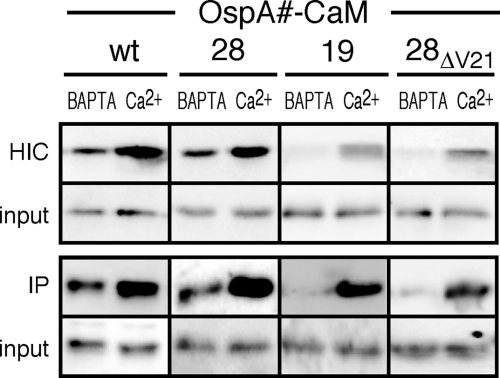
The first constructs to be tested had CaM fused either to full-length wild-type OspA (OspAwt-CaM) or to the OspAwt tether peptide (OspA28-CaM) (Fig. 1; Table 1). B. burgdorferi transformants expressing the two fusion proteins were grown in the selective medium BSK-SFcc supplemented with either 1 mM calcium chloride or 5 mM BAPTA, a calcium-specific chelator. Based on accessibility to proteolytic shaving with proteinase K, both OspAwt-CaM and OspA28-CaM fusion proteins were displayed on the bacterial surface, irrespective of the presence of calcium ions or the chelator (Fig. 3A). The folding statuses of the fusion proteins under both experimental conditions were analyzed by two independent approaches: (i) IP with a Ca2+-CaM fold-specific antibody (25) and (ii) HIC, which takes advantage of the specific hydrophobic interaction of the Ca2+-bound CaM with phenyl Sepharose (23). The CaM contents of the samples obtained by IP and HIC were then assayed by Western immunoblotting with a fold-independent anti-CaM antibody. Figure 4 shows that the availability of Ca2+ in the culture medium clearly enriched for interaction of the CaM fusion proteins with both the Ca2+-CaM-specific antibody and phenyl Sepharose, while the BAPTA chelator suppressed such enrichment. Together, these experiments demonstrated that the folding state of CaM within an OspA fusion construct could indeed be modulated by supplementation with, or chelation of, calcium ions. They also indicated that the B. burgdorferi lipoprotein secretion machinery properly secreted CaM-containing lipopeptides even in the presence of Ca2+, provided that the fusion proteins contained at least a wild-type OspA tether.
Fig. 3.
Chelation-dependent surface exposure of OspA-CaM fusion proteins at steady state. (A) Accessibility of full-length OspA-CaM or OspA tether-CaM fusion proteins to proteinase K. Cells expressing the fusion constructs were grown in BSK-SFcc containing either Ca2+ or BAPTA. Lipoprotein surface exposure was assessed by incubating intact cells with proteinase K (pK+) or a control buffer (pK−), followed by Western immunoblotting with antibodies against CaM, OspC (used as an OspA-independent surface control, due to the coexpression of endogenous OspAwt and OspAwt-CaM), and FlaB (periplasmic control) (αCaM, αOspC, and αFlaB, respectively). (B) Total-protein fractionation by Triton X-114 of B. burgdorferi cells expressing the OspA28ΔV21-CaM or OspA19-CaM fusion protein in the presence of Ca2+. A Coomassie-stained SDS-PAGE gel (Coom) and an immunoblot with αCaM are shown. D, detergent phase; Aq, aqueous phase. The positions of OspA and OspB are indicated on the right. Asterisks indicate the weakly Coomassie stained OspA-CaM fusion bands present in the respective detergent fractions. (C) Membrane fractionation immunoblots of the subsurface OspAΔV21-CaM and OspA19-CaM fusion proteins expressed by B. burgdorferi in the presence of Ca2+. OppAIV served as an inner membrane control, and OspA served as an OM control. PC, protoplasmic cylinder fraction; OMV, outer membrane vesicle fraction enriched for OM proteins. Note that the PC fraction also contains OM proteins, because OMVs were only partially separated from protoplasmic cylinders by treatment of Borrelia cells with a hypotonic citrate buffer; therefore, the PC fraction is similar to a whole-cell protein preparation (54). (D) Accessibility to proteinase K of partial or mutant OspA tether-CaM fusion proteins expressed by B. burgdorferi. The experimental procedures and labels described for panel A were used, except that OspA was used as the standard surface control. (E) Accessibility to proteinase K of previously described periplasmic OspA-mRFP fusion proteins (50, 51) under calcium-containing (Ca2+) or chelating (BAPTA) conditions. The experimental procedures and labels were the same as those for panel D.
Fig. 4.
Assessment of the folding states of the OspA-CaM fusion proteins by hydrophobic interaction chromatography (HIC) and immunoprecipitation (IP). Western blot analysis was performed on OspA-CaM fusion proteins purified by HIC or IP from whole-cell lysates of recombinant B. burgdorferi cultured in the presence of Ca2+ or BAPTA. Phenyl Sepharose was used to enrich for the folded CaM fusion proteins by HIC. For IP experiments, CaM fusion proteins were immunoprecipitated with a Ca2+-CaM complex-specific monoclonal antibody. For both the HIC and IP approaches, equal ratios of whole-cell lysates (input) were loaded for comparison. OspA-CaM fusion proteins in both the input and eluted pulldown samples were detected by immunoblotting with a non-conformation-specific antibody against CaM.
Calcium chelation-dependent OM translocation of “periplasmic” OspA-CaM fusion proteins.
We next fused CaM to the shortened OspA tether peptides OspA28ΔV21 and OspA19. In previous studies, these tether peptides had targeted the monomeric red fluorescent protein (mRFP) reporter protein mRFP1 or its derivative mRFPΔ4 to the periplasm (50, 51). B. burgdorferi transformants expressing the OspA28ΔV21-CaM or OspA19-CaM fusion protein were grown in the selective medium BSK-SFcc supplemented with either calcium chloride or BAPTA as described above. IP and HIC experiments, as described above for the OspAwt-CaM and OspA28-CaM fusion proteins, confirmed that both the OspA28ΔV21-CaM and OspA19-CaM fusion proteins were able to undergo Ca2+-dependent folding as well (Fig. 4).
Western immunoblotting of Triton X-114 detergent fractions showed that the OspA19-CaM and OspA28ΔV21-CaM fusion proteins were associated with the membrane (Fig. 3B). Outer membrane vesicle (OMV) fractions obtained by treatment of cells cultured in BSK-II medium with a hypotonic citrate buffer (51, 54) showed that both CaM fusion proteins localized to the OM (Fig. 3C, left). Because citrate has Ca2+-chelating activity, which might influence the folding and localization of the CaM fusion proteins during the procedure, we repeated the assay with an alternative membrane fractionation protocol using a chelator-free hypotonic sucrose buffer (45) (Fig. 3C, right). The two fractionation protocols yielded identical results. It should be noted that the “protoplasmic cylinder” (PC) fractions obtained by both protocols also contain OM proteins; this is due to the inefficient release of OMVs and therefore the presence of intact cells in the PC fraction (54). Therefore, the PC fractions presented here and elsewhere should be considered similar to whole-cell protein preparations.
In proteinase K accessibility assays, the presence of Ca2+ protected both OspA28ΔV21-CaM and OspA19-CaM fusion proteins from cleavage, while chelation with BAPTA rendered both fusion proteins accessible to proteolysis (Fig. 3D). In addition to the standard periplasmic FlaB control, we assayed the previously described constructs in which the identical OspA tether peptides were fused either to mRFP1 (OspA19-mRFP1) (51) or to its derivative mRFPΔ4 (OspA28ΔV21-mRFPΔ4) (50). As shown previously for proteins harvested from cultures grown in standard BSK-II broth (47, 48), both OspA-mRFP fusion proteins remained localized to the periplasm under both Ca2+-containing and Ca2+-free conditions (Fig. 3E). These steady-state differences suggested that calcium chelation specifically stimulated the unfolding and translocation of lipopeptide-CaM fusion proteins through the borrelial OM.
Calcium chelation-induced rescue of translocationally blocked OspA-CaM fusion proteins.
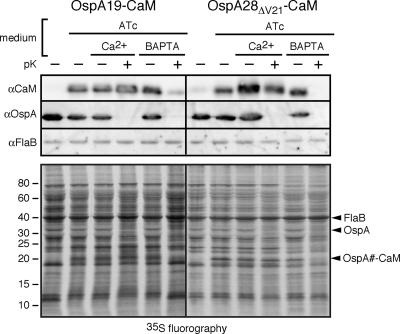
The dynamics of lipoprotein secretion in B. burgdorferi have not been determined precisely, but the time appears to be in the range of several hours (20). It therefore remained possible that the chelation-dependent steady-state surface localization of OspA28ΔV21-CaM and OspA19-CaM proteins described above was due to the proper secretion of fusion proteins newly expressed under Ca2+-free conditions. In an attempt to exclude this possibility and to concurrently gain further insight into lipoprotein secretion kinetics, we used a radiolabeling approach in conjunction with our recently described tetracycline-responsive Post promoter system (57). This combination allowed us first to express and label cohorts of OspA19-CaM or OspA28ΔV21-CaM molecules under conditions that blocked their translocation through the OM and then to observe the protein cohorts' fate when that restriction was lifted. Recombinant B. burgdorferi cells grown in Ca2+-containing BSK-SFcc were resuspended in RPMI 1640 medium, where recombinant lipoprotein expression and 35S metabolic protein labeling were initiated concurrently for 2 h. After a 10-min incubation in RPMI 1640 medium with cold amino acids, cells were incubated in BSK-SFcc containing either Ca2+ or BAPTA for 2 h, harvested, and subjected to in situ proteolysis and fluorography analysis. As shown in the lower panel of Fig. 5, the 19- to 20-kDa bands corresponding to either OspA19-CaM or OspAΔV21-CaM appeared only upon induction of the Post promoter with ATc and remained protected from proteolysis in the presence of Ca2+. BAPTA treatment, however, rendered the CaM fusion proteins susceptible to in situ proteinase K treatment on the spirochetal surface. In agreement with the OspA-mRFP data shown in Fig. 3E, there were no discernible pleiotropic effects on other borrelial proteins. Western immunoblotting for the lipidated CaM proteins and the OspA and FlaB controls confirmed these findings (Fig. 5, upper panel).
Fig. 5.
Chelation-dependent surface exposure of radiolabeled, conditionally expressed OspA-CaM fusion proteins. (Top) Western immunoblot analysis; (bottom) fluorography analysis. Prior to analysis, B. burgdorferi cells were cultured to early-log phase in BSK-SFcc containing Ca2+. Radiolabeling with [35S]Cys-Met amino acids and anhydrotetracycline (ATc)-mediated induction of Post promoter-driven expression were initiated simultaneously in RPMI 1640 medium. Subsequently, cells were incubated in BSK-SFcc containing either Ca2+ or BAPTA, followed by in situ surface proteolysis with proteinase K (+pK). Total fusion proteins expressed upon ATc induction were detected by Western immunoblotting with antibodies against CaM, OspA (surface control), and FlaB (subsurface control), while fluorography detected proteins expressed during the pulse. The proteins bands detected by Western immunoblotting are indicated on the right.
In a complementary approach, we checked for release of periplasmically trapped CaM lipopeptides by immunofluorescence microscopy as described previously (51). To visualize total or subsurface proteins, cells were permeabilized with Triton X-100 prior to incubation with antibodies (20). Immunofluorescence due to the interaction of specific antibodies with the CaM fusion proteins was observed only in the presence of BAPTA or with detergent-permeabilized cells. At the same time, only detergent permeabilization, not BAPTA treatment, led to accessibility of the inner membrane lipoprotein OppAIV (8, 51; also data not shown). Together, these assays confirmed that Ca2+ chelation led to the specific and rapid release of lipopeptide-CaM fusion proteins from the periplasm to the B. burgdorferi surface.
DISCUSSION
The deployment of bacterial virulence factors to their proper compartments is essential to microbial pathogenesis. For the surface lipoproteins involved in the colonization, persistence, and pathogenesis of Borrelia spirochetes, this involves the crossing of two lipid bilayers as well as the intervening aqueous periplasm. In this study, we focused on the molecular events at the OM by fusing N-terminal tether peptides of the B. burgdorferi surface lipoprotein OspA to the calcium-binding eukaryotic protein CaM. Thus, we were able to demonstrate that the same OspA-CaM fusion proteins that were restricted to the periplasmic leaflet of the OM under experimental conditions that favored folding regained their capacity to be translocated through the OM under experimental conditions that induced unfolding. These findings are in accordance with our earlier interpretations of individual sets of OspA and OspC lipoprotein mutant phenotypes, where periplasmic OspA and OspC tether mutants could be redirected to the bacterial surface by mutational destabilization of a C-terminal α-helix or by the addition of short disordered C-terminal epitope/affinity tags, respectively (32, 50). Interestingly, the present study reiterates that C-terminal structural destabilization, achieved this time by chelating Ca2+ from an admittedly larger calmodulin “tag,” is sufficient to promote secretion through the OM. Thus, the current findings corroborate the requirement for the delivery to the OM of a translocation-competent surface lipoprotein molecule that is at least partially unfolded. They also remain compatible with the potential C-to-N vectoriality of surface lipoprotein translocation proposed previously (50) (Fig. 6).
Fig. 6.
Proposed molecular events at the B. burgdorferi OM during surface lipoprotein secretion. Where possible, the color scheme follows that of Fig. 1. Hypothetical components are drawn in gray. Tether mutants of surface lipoproteins such as OspA (light green, with an X marking the tether mutation) fold prematurely in the periplasm, and their secretion through the OM is thereby prevented (17, 18). Periplasmic folding or interactions with periplasmic envelope components may be responsible for subsurface retention of wt periplasmic lipoproteins such as Lp6.6 (blue). Translocation of periplasmic mutant OspA tether-CaM fusion proteins (red) is dependent on chelation-mediated structural changes (this study). Intact surface lipoprotein tether peptides prevent premature folding of proteins—and thereby allow for OM translocation—via interaction with a hypothetical “holding” chaperone (gray). As shown for wt OspA (but also valid for the wt OspA-CaM fusion protein), translocation can initiate at the lipoprotein's C terminus if an unfolded peptide is provided (50; also this study). The peptide portion travels via the hydrophilic lumen of a hypothetical OM lipoprotein “flippase” pore (gray), while the lipid anchor (black) flips from the periplasmic leaflet to the surface leaflet of the OM bilayer; this ultimate anchor topology was recently shown for OspA and B. turicatae Vsp1 (17). In the absence of ATP, the directionality of the process is likely driven by the folding and assembly of the protein on the bacterial surface. This figure updates the Borrelia envelope biogenesis model shown in the work of Bergström and Zückert (7).
How wt borrelial surface lipoproteins are maintained in such a translocation-competent conformation remains to be revealed. Yet it is becoming increasingly clear that lipoprotein tether peptides play a central role in this process. As demonstrated here, the secretion requirement for chelation-mediated unfolding of CaM can be circumvented if an intact surface lipoprotein N terminus is provided in cis, either as part of a full-length OspA protein or by a full-length OspA28 tether peptide (Fig. 1 and 6). This finding again closely correlates with our earlier observations: wt tether peptides of OspA and OspC were able to properly direct mRFP reporter proteins to the spirochetal surface, whereas mutant tether peptides (mis)localizing OspA, OspC, and mRFP to the periplasm did not prevent protein folding and, in the case of mRFP, periplasmic activity, observed as red fluorescence (32, 50, 51). Our previous studies indicated the absence of any minimal tether length requirement for lipoprotein surface localization (50, 51). Also, an OspC tether peptide had no intrinsic structure-destabilizing properties (32). One interpretation that remains consistent with all currently available data is that surface lipoprotein tethers are involved indirectly in proper targeting by enabling interaction with a periplasmic “holding” chaperone that prevents its cargo from premature periplasmic folding (Fig. 6). If this were indeed the case, sequestration of surface lipoprotein tether mutants, and possibly wt periplasmic OM lipoproteins, such as Lp6.6 (43), to the periplasm could be explained by their failure to interact with this lipoprotein secretion pathway component (Fig. 6). Such a chaperone-mediated mechanism may be similar to the interaction of the pilin subunits of Gram-negative bacteria with their cognate periplasmic chaperones, which prevents untimely periplasmic pilus assembly and guides the complex to the OM assembly platform (the usher) (49). Yet in light of the tether peptide divergence among the numerous surface lipoproteins encoded by the Borrelia genome, with only 1.6 Mbp (15, 21, 50), a likely single chaperone would have to be quite promiscuous in its interactions, i.e., it would have to function similarly to the Skp and/or SurA chaperones, which are involved in shepherding integral OM protein peptides through the periplasm (9, 55).
The data presented here also raise the potential for transient anchoring of surface B. burgdorferi surface lipoproteins within the periplasmic leaflet of the OM. One of our recent studies already hinted at that possibility, but our preliminary conclusions at the time were based on steady-state OM topologies of a combinatorial series of different OspA mutants under identical experimental conditions (50). Here, the use of a model lipoprotein system undergoing conditional conformational change allows us to substantiate these interpretations by identifying the topologies of identical lipopeptides under different environmental conditions. The apparent tolerance of the B. burgdorferi lipoprotein secretion machinery for CaM domains and the stability of the periplasmic OspA-CaM mutants is intriguing in the context of two findings for Gram-negative diderm bacteria. In one study, replacement of an internal passenger subdomain of the E. coli type V “autotransporter” secretion protein Hbp with CaM blocked secretion, leading to envelope stress and degradation of the fusion protein by the periplasmic protease DegP (27). A similar CaM-dependent secretory block was seen with E. coli intimin, an intimin/invasin-type protein (1). Based on the absence of a secretion block phenotype with the OspAwt-CaM fusion proteins, one might therefore argue that the association of the mutant OspA-CaM fusion proteins with the periplasmic OM leaflet is “off-pathway.” Our current data cannot fully exclude such a conclusion. However, the differing effects of introducing a CaM moiety into the three experimental systems may be due simply to the significant variability in their structures and secretion mechanisms. Also, the surface phenotype of the wt OspA-CaM fusion protein could be explained by the prevention of folding via a tether-mediated association with a periplasmic protein, mentioned above. Most importantly, an “off-pathway” periplasmic surface lipoprotein mutant would be unlikely to retain its ability to be flipped to the surface, i.e., it would be incompatible with the conditional topologies of two separate OspA tether-CaM fusion proteins demonstrated here. We recently demonstrated that OspA and Borrelia turicatae Vsp1, a structural homolog of B. burgdorferi OspC (35, 61), are anchored in the surface leaflet of the spirochetal OM (17), i.e., they ultimately adopt an “outside anchor-outside protein,” or “out-out,” topology. This finding corroborated prior indirect evidence obtained with recombinant lipidated OspA and OspA-mRFP1 fusion proteins (13, 50, 51). The transbilayer crossing of the OM by borrelial surface lipoproteins may therefore be mechanistically similar to that of other lipidated bacterial cell envelope components. Grounded in the reductionist observations of polar lipid behavior in bilayers, several mechanistic models have been proposed; all involve “flipping” of the lipid moiety sequestered to the hydrophobic environment, while hydrophilic moieties are translocated through a protein channel such as a “flippase” or at least through interaction with a transmembrane protein (48) (Fig. 6). While the existence, identity, and role of a similar pathway component remain to be established, B. burgdorferi surface lipoprotein secretion appears to be at least indirectly dependent on a homolog of the OM β-barrel assembly machinery protein BamA (36).
Together, these findings encourage us to further elucidate the precise molecular mechanism of OM crossing by spirochetal surface lipoproteins. To that end, we are currently using our regulatable Post promoter system (57) to generate conditional knockouts of several pathway candidates. At the same time, we are also further deploying CaM as well as protein-protein interaction approaches to advance our understanding of the individual steps involved in the secretion of this important class of Borrelia virulence factors.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (grant R01 AI063261 to W.R.Z.).
We thank Christine Whetstine and Eszter Adany for experimental support, Frank Gherardini and Bob Cluss for advice on culture medium composition, and Gerald Carlson and Owen Nadeau for the recombinant calmodulin-encoding plasmid. We are also grateful to Joe Lutkenhaus, Ryan Schulze, and Brian Stevenson for inspiring discussions and helpful comments on the manuscript.
Footnotes
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Adams T. M., Wentzel A., Kolmar H. 2005. Intimin-mediated export of passenger proteins requires maintenance of a translocation-competent conformation. J. Bacteriol. 187:522–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babb K., McAlister J. D., Miller J. C., Stevenson B. 2004. Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J. Bacteriol. 186:2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbour A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521–525 [PMC free article] [PubMed] [Google Scholar]
- 4. Barbour A. G., Guo B. P. 2010. Pathogenesis of relapsing fever, p. 333–357 In Samuels D. S., Radolf J. D. (ed.), Borrelia: molecular biology, host interaction, and pathogenesis. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 5. Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect. Immun. 52:549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbour A. G., Tessier S. L., Todd W. J. 1983. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect. Immun. 41:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergström S., Zückert W. R. 2010. Structure, function and biogenesis of the Borrelia cell envelope, p. 139–166 In Samuels D. S., Radolf J. D. (ed.), Borrelia: molecular biology, host interaction, and pathogenesis. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 8. Bono J. L., Tilly K., Stevenson B., Hogan D., Rosa P. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144:1033–1044 [DOI] [PubMed] [Google Scholar]
- 9. Bos M. P., Robert V., Tommassen J. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61:191–214 [DOI] [PubMed] [Google Scholar]
- 10. Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. 1990. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect. Immun. 58:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braun V., Rehn K. 1969. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur. J. Biochem. 10:426–438 [DOI] [PubMed] [Google Scholar]
- 12. Bunikis J., Barbour A. G. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 67:2874–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunikis J., Mirian H., Bunikiene E., Barbour A. G. 2001. Non-heritable change of a spirochaete's phenotype by decoration of the cell surface with exogenous lipoproteins. Mol. Microbiol. 40:387–396 [DOI] [PubMed] [Google Scholar]
- 14. Carroll J. A., Stewart P. E., Rosa P., Elias A. F., Garon C. F. 2003. An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi. Microbiology 149:1819–1828 [DOI] [PubMed] [Google Scholar]
- 15. Casjens S., et al. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 16. Chen J. C., Viollier P. H., Shapiro L. 2005. A membrane metalloprotease participates in the sequential degradation of a Caulobacter polarity determinant. Mol. Microbiol. 55:1085–1103 [DOI] [PubMed] [Google Scholar]
- 17. Chen S., Kumru O. S., Zückert W. R. 9 September 2011. Determination of Borrelia surface lipoprotein anchor topology by surface proteolysis. J. Bacteriol. doi:10.1128/JB.05849-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cluss R. G., Silverman D. A., Stafford T. R. 2004. Extracellular secretion of the Borrelia burgdorferi Oms28 porin and Bgp, a glycosaminoglycan binding protein. Infect. Immun. 72:6279–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cowles C. E., Li Y., Semmelhack M. F., Cristea I. M., Silhavy T. J. 2011. The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Mol. Microbiol. 79:1168–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox D. L., et al. 1996. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 93:7973–7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraser C. M., et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586 [DOI] [PubMed] [Google Scholar]
- 22. Gennity J. M., Inouye M. 1991. The protein sequence responsible for lipoprotein membrane localization in Escherichia coli exhibits remarkable specificity. J. Biol. Chem. 266:16458–16464 [PubMed] [Google Scholar]
- 23. Gopalakrishna R., Anderson W. B. 1982. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem. Biophys. Res. Commun. 104:830–836 [DOI] [PubMed] [Google Scholar]
- 24. Guerini D., Krebs J. 1983. Influence of temperature and denaturing agents on the structural stability of calmodulin. A 1H-nuclear magnetic resonance study. FEBS Lett. 164:105–110 [DOI] [PubMed] [Google Scholar]
- 25. Hansen R. S., Beavo J. A. 1982. Purification of two calcium/calmodulin-dependent forms of cyclic nucleotide phosphodiesterase by using conformation-specific monoclonal antibody chromatography. Proc. Natl. Acad. Sci. U. S. A. 79:2788–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59 [DOI] [PubMed] [Google Scholar]
- 27. Jong W. S., et al. 2007. Limited tolerance towards folded elements during secretion of the autotransporter Hbp. Mol. Microbiol. 63:1524–1536 [DOI] [PubMed] [Google Scholar]
- 28. Katona L. I., Beck G., Habicht G. S. 1992. Purification and immunological characterization of a major low-molecular-weight lipoprotein from Borrelia burgdorferi. Infect. Immun. 60:4995–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovacs-Simon A., Titball R. W., Michell S. L. 2011. Lipoproteins of bacterial pathogens. Infect. Immun. 79:548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kretsinger R. H., Rudnick S. E., Weissman L. J. 1986. Crystal structure of calmodulin. J. Inorg Biochem. 28:289–302 [DOI] [PubMed] [Google Scholar]
- 31. Kudryashev M., et al. 2009. Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Mol. Microbiol. 71:1415–1434 [DOI] [PubMed] [Google Scholar]
- 32. Kumru O. S., Schulze R. J., Rodnin M. V., Ladokhin A. S., Zückert W. R. 2011. Surface localization determinants of Borrelia OspC/Vsp family lipoproteins. J. Bacteriol. 193:2814–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumru O. S., Schulze R. J., Slusser J. G., Zückert W. R. 2010. Development and validation of a FACS-based lipoprotein localization screen in the Lyme disease spirochete Borrelia burgdorferi. BMC Microbiol. 10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Labandeira-Rey M., Skare J. T. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawson C. L., Yung B. H., Barbour A. G., Zückert W. R. 2006. Crystal structure of neurotropism-associated variable surface protein 1 (Vsp1) of Borrelia turicatae. J. Bacteriol. 188:4522–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lenhart T. R., Akins D. R. 2010. Borrelia burgdorferi locus BB0795 encodes a BamA orthologue required for growth and efficient localization of outer membrane proteins. Mol. Microbiol. 75:692–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewenza S., Vidal-Ingigliardi D., Pugsley A. P. 2006. Direct visualization of red fluorescent lipoproteins indicates conservation of the membrane sorting rules in the family Enterobacteriaceae. J. Bacteriol. 188:3516–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mbow M. L., Gilmore R. D. J., Titus R. G. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 67:5470–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nally J. E., Timoney J. F., Stevenson B. 2001. Temperature-regulated protein synthesis by Leptospira interrogans. Infect. Immun. 69:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Narita S., Tokuda H. 2007. Amino acids at positions 3 and 4 determine the membrane specificity of Pseudomonas aeruginosa lipoproteins. J. Biol. Chem. 282:13372–13378 [DOI] [PubMed] [Google Scholar]
- 41. Norris S. J., Coburn J., Leong J. M., Hu L. T., Höök M. 2010. Pathobiology of Lyme disease Borrelia, p. 299–332 In Samuels D. S., Radolf J. D. (ed.), Borrelia: molecular biology, host interaction, and pathogenesis. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 42. Posey J. E., Gherardini F. C. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651–1653 [DOI] [PubMed] [Google Scholar]
- 43. Promnares K., et al. 2009. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol. Microbiol. 74:112–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Purser J. E., Norris S. J. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 97:13865–13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radolf J. D., et al. 1995. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 63:2154–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sadziene A., Thomas D. D., Barbour A. G. 1995. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect. Immun. 63:1573–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samuels D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanyal S., Menon A. K. 2009. Flipping lipids: why an' what's the reason for? ACS Chem. Biol. 4:895–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sauer F. G., Remaut H., Hultgren S. J., Waksman G. 2004. Fiber assembly by the chaperone-usher pathway. Biochim. Biophys. Acta 1694:259–267 [DOI] [PubMed] [Google Scholar]
- 50. Schulze R. J., Chen S., Kumru O. S., Zückert W. R. 2010. Translocation of Borrelia burgdorferi surface lipoprotein OspA through the outer membrane requires an unfolded conformation and can initiate at the C-terminus. Mol. Microbiol. 76:1266–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schulze R. J., Zückert W. R. 2006. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol. Microbiol. 59:1473–1484 [DOI] [PubMed] [Google Scholar]
- 52. Seydel A., Gounon P., Pugsley A. P. 1999. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol. 34:810–821 [DOI] [PubMed] [Google Scholar]
- 53. Silva-Herzog E., Ferracci F., Jackson M. W., Joseph S. S., Plano G. V. 2008. Membrane localization and topology of the Yersinia pestis YscJ lipoprotein. Microbiology 154:593–607 [DOI] [PubMed] [Google Scholar]
- 54. Skare J. T., et al. 1995. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J. Clin. Invest. 96:2380–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sklar J. G., Wu T., Kahne D., Silhavy T. J. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 21:2473–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stewart P. E., Thalken R., Bono J. L., Rosa P. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714–721 [DOI] [PubMed] [Google Scholar]
- 57. Whetstine C. R., Slusser J. G., Zückert W. R. 2009. Development of a single-plasmid-based regulatable gene expression system for Borrelia burgdorferi. Appl. Environ. Microbiol. 75:6553–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamaguchi K., Yu F., Inouye M. 1988. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53:423–432 [DOI] [PubMed] [Google Scholar]
- 59. Zhang M., Tanaka T., Ikura M. 1995. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 2:758–767 [DOI] [PubMed] [Google Scholar]
- 60. Zückert W. R. 2007. Laboratory maintenance of Borrelia burgdorferi. Curr. Protoc. Microbiol. 2007:Chapter 12, Unit 12C.1 [DOI] [PubMed] [Google Scholar]
- 61. Zückert W. R., Kerentseva T. A., Lawson C. L., Barbour A. G. 2001. Structural conservation of neurotropism-associated VspA within the variable Borrelia Vsp-OspC lipoprotein family. J. Biol. Chem. 276:457–463 [DOI] [PubMed] [Google Scholar]
- 62. Zückert W. R., Lloyd J. E., Stewart P. E., Rosa P. A., Barbour A. G. 2004. Cross-species surface display of functional spirochetal lipoproteins by recombinant Borrelia burgdorferi. Infect. Immun. 72:1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zückert W. R., Meyer J., Barbour A. G. 1999. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect. Immun. 67:3257–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]