Abstract
In Salmonella enterica serovar Typhimurium, a biofilm mode of growth known as the rdar morphotype is regulated by several networks which sense multiple environmental signals. The transcriptional regulator CsgD is the major target for these regulatory pathways. In this study, we show that two lytic transglycosylases of family I, MltE and MltC, in combination increase CsgD expression and rdar morphotype. MltE and MltC, which share a highly similar transglycosylase SLT domain, work redundantly to regulate CsgD at the transcriptional and posttranscriptional levels. The effect of MltE and MltC on CsgD levels was independent of the known regulatory pathways that sense cell envelope stress. These findings reveal, for the first time, a specific function of lytic transglycosylases in S. Typhimurium and suggest the existence of a new signaling pathway that links cell wall turnover to biofilm formation.
INTRODUCTION
Biofilm formation is the most common mode of growth of bacteria. The food-borne gastrointestinal pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium) establishes biofilms on a variety of surfaces, such as those of plants, the eukaryotic host, industrial equipment, and medical supplies. Under laboratory conditions, S. Typhimurium biofilms are manifested as a distinct colony morphotype on Congo red agar plates, named rdar, due to the red dry and rough colony appearance. This form of biofilm is characterized by the expression of cellulose and curli fimbriae, two major extracellular matrix components in S. Typhimurium (7, 44, 56, 64). The transcriptional regulator CsgD is the major regulator of the rdar morphotype (11, 48). CsgD positively regulates the transcription of the csgBAC operon, which encodes the structural subunits for curli fimbriae (18, 49), and contributes indirectly to cellulose production by activating the transcription of adrA (48, 63). AdrA is a diguanylate cyclase that synthesizes the second messenger cyclic-di-GMP (c-di-GMP), the effector molecule that binds to and allosterically activates cellulose synthase (55, 64).
CsgD expression integrates many environmental signals. Several diverse proteins bind to the csgD promoter, including the stress sigma factor RpoS and the response regulator OmpR (11, 12, 39, 45). In addition, CsgD is regulated by the second messenger c-di-GMP at the transcriptional and posttranscriptional levels (29). Two redundant small RNAs, OmrA and OmrB, control CsgD expression on a posttranscriptional level, although their effect is not pronounced under growth on petri plates (21). The regulation of the rdar morphotype therefore integrates a variety of complex regulatory systems.
Lytic transglycosylases are a class of ubiquitous bacterial enzymes involved in the turnover of the bacterial cell wall constituent peptidoglycan (PG). Lytic transglycosylases catalyze the cleavage of the β-1,4-glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine with anhydromuropeptide monomers as products (23). Escherichia coli harbors seven lytic transglycosylases, the soluble Slt70 and six outer membrane-anchored lipoproteins, MltA to MltF (52). Lytic transglycosylases have redundant functions. Although Slt is the principal lytic transglycosylase responsible for the turnover of PG (41), the deletion of as many as six lytic transglycosylases is required for an observable effect on cell morphology and the cleavage of the PG septum (19).
Four different families of lytic transglycosylases can be distinguished (4). However, due to functional redundancy, the correlation of function with family assignment is limited. Lytic transglycosylases are involved in the biosynthesis and recycling of the PG sacculus during cell growth and division, as well as in the insertion of macromolecular complexes spanning the cell wall (22, 30, 52). In E. coli, half of the cell wall is broken down in one generation (15). The anhydromuropeptide monomers formed as a result of PG hydrolysis are reused directly or transported into the cytoplasm through the permease AmpG (34). In the cytoplasm, the PG degradation products have a signaling function in the regulatory network sensing cell wall turnover (26, 27). Together with other PG degrading enzymes, lytic transglycosylases separate daughter cells through the cleavage of the septum during cell division (19). Lytic transglycosylases also hydrolyze PG to make space for the insertion of macromolecular complexes, e.g., type III secretion systems, flagella, and pili (40, 62).
In addition to these functions, lytic transglycosylases contribute to bacterium-host interaction, because anhydromuropeptide monomers are recognized by eukaryotic pattern recognition receptors (13, 60). Bacteria as diverse as Bordetella pertussis, Neisseria gonorrhoeae, and Vibrio fischeri release significant amounts of anhydromuropeptides, which are required for both virulence and symbiosis (14, 31, 37).
In this study, we show that two of seven family I lytic transglycosylases from S. Typhimurium, MltE and MltC, are specifically involved in the regulation of biofilm formation by affecting the expression of the master biofilm regulator CsgD.
MATERIALS AND METHODS
Bioinformatic analysis.
A search with the MltE protein sequence using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) at NCBI (http://www.ncbi.nlm.nih.gov/) with standard parameters was done to identify lytic transglycosylases in S. Typhimurium. Sequences were aligned using CLUSTALX 2.0.12 software (57). The phylogenetic tree was constructed using Dendroscope software (25). The domain architecture was analyzed using the Pfam database (10).
Strains, plasmids, and growth conditions.
Strains and plasmids used in this study are listed in Table S1 in the supplemental material. The MAE52 strain differs from the wild-type UMR1 by a single point mutation in the csgD promoter, resulting in semiconstitutive, temperature-independent CsgD and rdar morphotype expression. In this strain, csgD expression is independent of RpoS (49). For rdar morphotype analysis, strains were grown in Luria-Bertani (LB) medium without salt or on LB without salt agar. If required, antibiotics and inducers were added at the following concentrations: chloramphenicol (20 μg ml−1), ampicillin (100 μg ml−1), and 0.1% arabinose. For cloning purposes, Escherichia coli and S. Typhimurium were grown on LB and LB agar plates supplemented with antibiotics.
Construction of strains and plasmids.
The deletion of genes was performed by one-step gene inactivation as described by Datsenko and Wanner (8). A chloramphenicol resistance marker replaced the gene of interest with the exclusion of the first and last 40 bp. Phage transduction was carried out using phage P22 HT105/1 int-201 (53). Mutant alleles were verified by PCR with control primers located up- and downstream of the targeted open reading frame (ORF) (see Table S2 in the supplemental material).
In general, the genes were cloned with a C-terminal 6×His tag in the vector pBAD30. mltE and mltC with a glutamic acid-to-glutamine replacement at positions 64 and 219 were constructed by PCR-based mutagenesis using overlapping primers containing the mutations and subsequently cloned into pBAD30 (17), resulting in pCPM14 and pCPM15. To construct plasmids for protein purification, DNA fragments of the transglycosylase SLT domains of S. Typhimurium MltE and MltC proteins (wild-type and mutants) were cloned into vector pMAL-c2x(NEB) in frame with the maltose-binding protein (MBP). The MltE protein contained residues 34 to 202, and the MltC protein contained residues 189 to 325.
All recombinant plasmids were confirmed by sequencing. The primers used to construct and sequence the plasmids are listed in Table S2 in the supplemental material.
Phenotypic evaluation of rdar morphotype formation.
To detect curli fimbriae and cellulose production, the colony morphology phenotype was assessed on Congo red agar plates using the spot test. Strains were precultured on LB agar plates at 37°C for approximately 16 h. Cells were suspended in LB medium and adjusted to an optical density at 600 nm (OD600) of 3. Five microliters of the suspension was spotted on LB without salt agar plates supplemented with Congo red (40 μg ml−1) and Coomassie brilliant blue G-250 (20 μg ml−1). The phenotype was observed after 24 h, if not otherwise indicated.
Western blot analysis.
Bacteria were grown on LB without salt agar plates at 28°C for 17 h. Five mg (wet weight) cells was harvested, resuspended in 200 μl sample buffer, and incubated at 95°C for 10 min. The total protein content was analyzed by Coomassie blue staining after gel separation. Equal amounts of total protein were separated by SDS-PAGE (12% resolving gel and 4% stacking gel) and transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore).
Detection of CsgD was carried out as described previously (48) using a polyclonal anti-CsgD peptide antibody (1:5,000) and horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Jackson ImmunoResearch Laboratories Inc.).
His tag was detected with a monoclonal penta-His antibody (1:2,000; Qiagen) and horseradish peroxidase-conjugated anti-mouse IgG (1:10,000; Jackson ImmunoResearch Laboratories Inc.).
Chemiluminescence (Lumi-Light WB substrate; Roche) was recorded using the Las-1000 system (Fuji Film) and quantified with ImageJ software (http://rsb.info.nih.gov/ij/).
CsgA detection.
CsgA was detected after an enrichment procedure (46). Bacteria were grown on LB agar plates without salt at 28°C for 17 h. Three mg of bacteria was harvested and resuspended in 1.5 ml TE (10 mM Tris, 1 mM EDTA, pH 7.5) with 2% SDS, followed by an incubation at 95°C for 45 min. Afterwards the sample was centrifuged, and the pellet washed with water and finally resuspended in 30 μl water. The suspension was lyophilized and the pellet dissolved in 20 μl formic acid to depolymerize curli fimbriae. After the removal of the formic acid, the CsgA-enriched pellet was resuspended in 20 μl SDS sample buffer and 7 μl was loaded on the gel (15% separating gel with a 4% stacking gel, with a double concentration of buffer in the separation gel and running buffer). After the run, CsgA was visualized by staining the gel with colloidal Coomassie.
RNA extraction, cDNA synthesis, and quantitative real-time reverse transcription-PCR (RT-PCR).
Bacteria were grown on LB without salt agar plates at 28°C for 17 h and immediately frozen in liquid nitrogen after harvest. The extraction of total RNA was performed using the SV total RNA isolation system (Promega) with minor modifications (16). The quality of RNA samples was assessed by gel electrophoresis, and RNA concentrations were determined using the NanoDrop System (Thermo Scientific). cDNA synthesis from total RNA was performed with the high-capacity cDNA reverse transcription kit (Applied Biosystems) by following the manufacturer's instructions.
The expression of the genes of interest was determined by two-step real-time RT-PCR using the power SYBR green PCR master mix (Applied Biosystems) and the 7500 real-time PCR system (Applied Biosystems). Relative transcript abundance was determined with the 2−ΔΔCT method using 7500 SDS software, v1.3.1 (Applied Biosystems). The rpsV gene was used as an endogenous control for internal normalization. Experiments were performed as biological triplicates using the mean expression from quadruplicates per real-time PCR assay relative to a calibrator value (UMR1).
Zymogram analysis.
The MBP-MltE and MBP-MltC fusions were expressed in E. coli DH5α. The proteins were induced at an optical density at 600 nm (OD600) of 0.6 using 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. The proteins were partially purified from the cell extracts using affinity chromatography on amylose resin by following the manufacturer's protocol (NEB).
Cell wall hydrolytic activities were detected after the separation of the whole-cell protein extracts, or partially purified MBP-MltE and MBP-MltC proteins, using 12% denaturing SDS-PAGE according to the method reported previously (3, 32). Briefly, the samples were boiled for 5 min in SDS sample buffer (Bio-Rad, Hercules, CA) and resolved by 12% SDS-PAGE containing 0.6% (wt/vol) lyophilized cells of Micrococcus lysodeikticus (Sigma, St. Louis, MO). After PAGE, the gel was briefly rinsed with water and renaturation buffer (25 mM sodium phosphate, 10 mM MgCl2, and 0.1% Triton X-100) with gentle agitation at room temperature. The gel was incubated overnight in 500 ml of renaturation buffer at 30°C followed by an incubation for 24 h at 37°C. The cell wall material in the gel was stained with 0.1% methylene blue in 0.01% KOH. The gel subsequently was destained with water until unstained autolytic bands appeared on the blue background (32).
Microscopy.
To perform light and transmission electron microscopy (TEM), strains were grown on LB agar plates without salt at 28°C for 24 h. For TEM, cells were harvested, fixed using 2% glutaraldehyde, and resuspended in phosphate-buffered saline (PBS). TEM was performed by the electron microscopy unit (EMil) of the Karolinska Institutet.
RESULTS
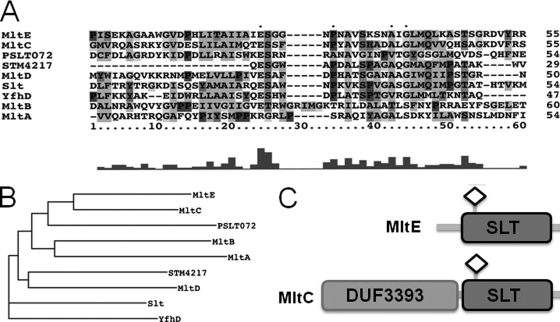
Lytic transglycosylases in S. Typhimurium.
The c-di-GMP-binding protein YcgR is involved in the transition of motile cells to sessility at high intracellular c-di-GMP concentrations (5, 42, 51). In several enterobacterial species, including S. Typhimurium, E. coli, Shigella, Klebsiella, and Erwinia, the ycgR and mltE genes are located next to each other and are transcribed in opposite directions. To investigate a potential link between MltE and c-di-GMP metabolism or c-di-GMP-controlled functions, we investigated the impact of mltE coding for a family I lytic transglycosylase on biofilm formation (rdar morphotype expression) in S. Typhimurium.
The mltE deletion mutant did not show a change in rdar morphotype expression compared to its expression in the S. Typhimurium wild-type UMR1 (Fig. 1A). On the other hand, the overexpression of MltE from a plasmid led to an upregulated rdar morphotype after 17 h, and upon extended incubation it manifested as an extended matrix structure on Congo red agar plates (Fig. 1B). It is important to note that the overexpression of MltE did not result in the retardation of growth or cell lysis (data not shown).
Fig. 1.
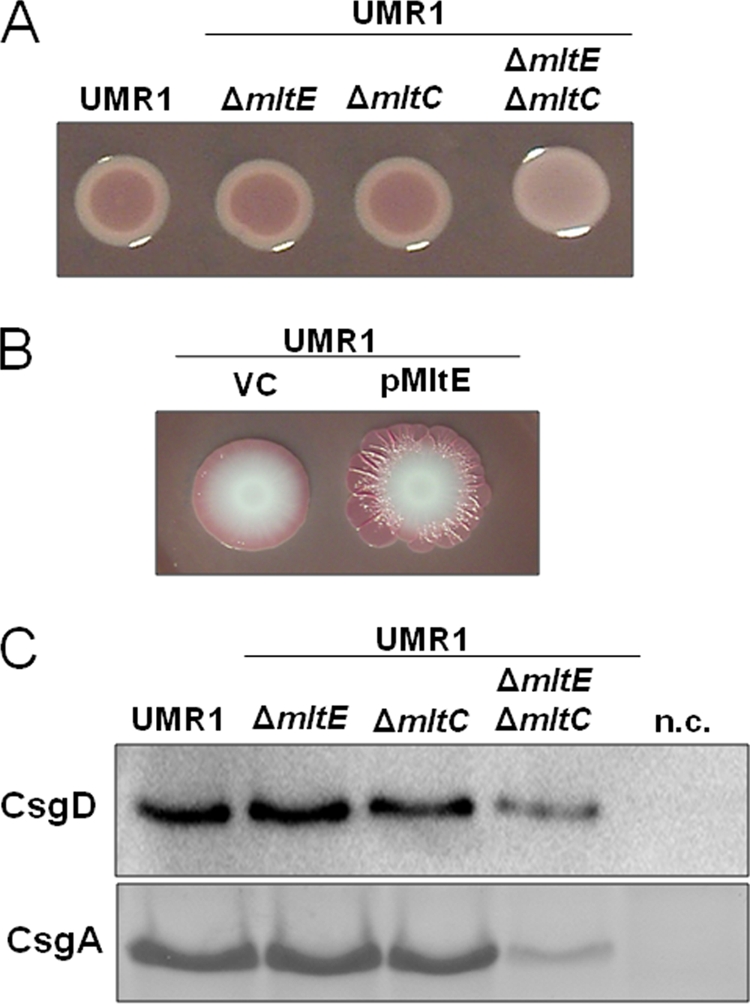
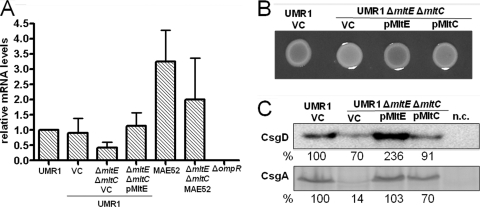
MltE in combination with MltC affects the expression of the rdar morphotype through CsgD. (A) Wild-type S. Typhimurium UMR1 and mutants of lytic transglycosylases MltE and MltC grown on Congo red agar plates at 28°C for 24 h. The mltE and mltC single mutants do not show any difference in rdar morphotype expression compared to that of wild-type UMR1, while the mltE mltC double mutant shows a visible downregulation of rdar morphotype expression. (B) MltE overexpression enhanced the rdar morphotype expression of UMR1 compared to that of the strain containing the vector control. Spot colonies were grown on Congo red agar plates at 28°C for 7 days. VC, vector control pBAD30. (C) CsgD and CsgA expression in S. Typhimurium UMR1 and its mltE and mltC mutants. Compared to wild-type UMR1, a significant reduction of CsgD and CsgA expression is observed in the mltE mltC double mutant. No change in CsgD and CsgA levels is detected in the mltE and mltC single mutants. The strains UMR1 ΔcsgD and UMR1 ΔcsgA were used as negative controls (n.c.) for CsgD and CsgA detection, respectively. Strains were grown on LB without NaCl agar at 28°C for 17 h.
To investigate whether the lack of the phenotype of the ΔmltE mutant was due to functional redundancy among lytic transglycosylases, we deleted genes encoding family I lytic transglycosylases in the wild-type, UMR1, and ΔmltE genetic backgrounds. A homology search identified seven lytic transglycosylases belonging to family I in S. Typhimurium (Fig. 2A and B). Six single deletion mutants (mltE, mltC, PSLT072, STM4217, mltD, and slt mutants), excluding the most distantly related yfhD (mltF) mutant, were investigated and showed no change in rdar morphotype expression (data not shown). The analysis of all possible double mutants, however, revealed a significant downregulation of the rdar morphotype in the ΔmltE ΔmltC double mutant, which manifested as a white and smooth colony appearance after 24 h of growth (Fig. 1A). We also overexpressed the remaining five lytic transglycosylases individually (mltC, PSLT072, STM4217, mltD, and slt), but rdar morphotype expression was not altered compared to that of the wild type (data not shown). These results demonstrate a specific involvement of MltE and MltC in rdar morphotype expression.
Fig. 2.
Phylogenetic analysis of the transglycosylase SLT domains of the lytic transglycosylases of S. Typhimurium. (A) Amino acid alignment of transglycosylase SLT domains of S. Typhimurium. The catalytic E-S motif is a characteristic of SLT family I domains. (B) Phylogenetic tree indicating the relatedness of SLT domains of lytic transglycosylases. MltE and MltC lytic transglycosylase SLT domains are highly related. The tree was constructed using Dendroscope. (C) Domain architecture of MltE and MltC. MltE contains only a transglycosylase SLT domain. MltC is composed of an N-terminal DUF3393 domain and a C-terminal transglycosylase SLT domain. The diamond indicates the catalytic residues of MltE and MltC (E64 and E219, respectively).
MltE and MltC have different domain structures. While MltE contains only the transglycosylase SLT domain, MltC is composed of two domains, the domain of unknown function, DUF3393, at the N terminus and the SLT domain at the C terminus (Fig. 2C). The SLT domains of MltE and MltC share 38% identity, which is the highest identity between transglycosylase SLT domains in S. Typhimurium (Fig. 2A and B). Therefore, it is possible that the close phylogenetic relationship between the SLT domains of MltE and MltC is the basis for their apparent functional redundancy in rdar morphotype expression.
MltE and MltC regulate expression of the extracellular matrix components through CsgD.
To gain insight into the mechanism responsible for the effect of MltE and MltC on biofilm formation, we measured expression levels of the master transcriptional activator CsgD and the levels of one of the main matrix components, curli fimbriae. The expression of curli fimbriae was monitored by the assessment of the amount of monomeric CsgA, the structural subunit released following formic acid-mediated fimbriae depolymerization. The ΔmltE or ΔmltC single mutant showed no differences from the wild-type UMR1, while the ΔmltE ΔmltC double mutant showed a clear reduction in CsgD and CsgA protein levels (Fig. 1C). CsgD expression levels were reduced to approximately 30% in the ΔmltE ΔmltC double mutant compared to that of wild-type UMR1.
To investigate at which level MltE and MltC regulate CsgD expression, the csgD mRNA levels were determined by real-time RT-PCR (Fig. 3A). The csgD mRNA levels in the ΔmltE ΔmltC double mutant were about 47% of the levels of the wild type. Therefore, MltE and MltC appear to regulate CsgD expression at the transcriptional and posttranscriptional levels.
Fig. 3.
Complementation of the S. Typhimurium UMR1 mltE mltC double mutant by MltE. (A) Expression of csgD in the wild-type S. Typhimurium UMR1 and the mltE mltC double mutant was determined by quantitative real-time RT-PCR. csgD mRNA levels decreased in the mltE mltC double mutant compared to those of wild-type UMR1. The expression of MltE from a plasmid restored csgD mRNA to wild-type UMR1 levels. VC, vector control pBad30. A decrease in csgD mRNA levels in the MAE52 mltE mltC double mutant also was observed. The strain UMR1 ΔompR was used as a negative control (45). Bacteria were grown on LB agar plates without salt at 28°C for 17 h. The experiment was performed with three biological replicates, using the mean expression values from four technical replicates. (B) Rdar morphotype expression in the double mutant UMR1 ΔmltE ΔmltC is complemented with a plasmid expressing MltE but not MltC. Strains were grown on Congo red agar at 28°C for 24 h. (C) CsgD and CsgA expression levels in the double mutant UMR1 ΔmltE ΔmltC complemented with pMltE and pMltC. The expression of MltE strongly increases the amount of CsgD and CsgA in the double mutant, while MltC expression leads to the minor upregulation of CsgD and CsgA. The relative protein levels compared to that of S. Typhimurium UMR1, which was set at 100%, are indicated. VC, vector control pBad30. The strains UMR1 ΔcsgD and UMR1 ΔcsgA were used as negative controls (n.c.) for CsgD and CsgA detection.
When MltE was expressed from a plasmid in the ΔmltE ΔmltC double mutant, the csgD mRNA levels were restored to wild-type levels (Fig. 3A). Also, rdar morphotype expression and CsgD and CsgA protein levels reached or exceeded wild-type levels in the ΔmltE ΔmltC double mutant upon the overexpression of MltE but not MltC (Fig. 3B and C and data not shown). An investigation of the level of MltC expression revealed that MltC is expressed at significantly lower levels than MltE despite the fact that they had identical promoters and ribosome-binding sites (data not shown). Low MltC from plasmid expression may be responsible for the lack of restoration of rdar morphotype expression.
The catalytic activities of MltE and MltC are responsible for the effect on the rdar morphotype.
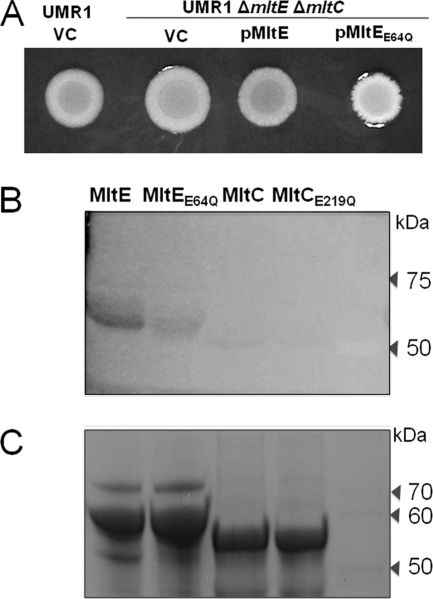
To investigate whether the transglycosylase activities of MltE and MltC were required for rdar morphotype expression, we constructed point mutations in both proteins that were expected to decrease or abolish their activities. Lytic transglycosylases of family I share a conserved E-S motif (4), where the glutamyl residue is directly involved in catalysis (58). We replaced the glutamyl residues in the E-S motif with glutamines (E65→Q in MltE and E219→Q in MltC). When the mutant MltE and MltC proteins were expressed from plasmids (pCPM14 and pCPM15, respectively), they could not restore the rdar morphotype in the ΔmltE ΔmltC double mutant (Fig. 4A and data not shown). Interestingly, the overexpression of MltEE65Q resulted in a mucoid phenotype (Fig. 4A), suggesting that the expression of the protein lacking the catalytic activity imposed envelope stress (35, 36).
Fig. 4.
Catalytic activity of the transglycosylase SLT domain of MltE is required for rdar morphotype expression. (A) Morphotypes of UMR1 ΔmltE ΔmltC overexpressing pMltE or pMltEE64Q. MltE expressed from a plasmid leads to an upregulation of the morphotype compared to that of strain UMR1 ΔmltE ΔmltC, while the expression of MltEE64Q showed a white and mucoid colony. VC, vector control pBad30. Strains were grown on Congo red agar plates for 24 h at 28°C. (B) Cell wall hydrolytic activity of lytic transglycosylases MltE and MltC. SLT domains from MltE and MltC were partially purified and resolved on SDS-PAGE gel containing M. luteus cells as substrates for lytic transglycosylase activity. The lytic transglycosylase activity of MltE was higher than the activity of MltC. The MltE and MltC proteins containing mutations in the conserved glutamyl residues show only residual hydrolytic activity. (C) An SDS-PAGE gel stained with Coomassie blue as a loading control. Lanes: 1, MltE; 2, MltEE64Q; 3, MltC; 4, MltCE219Q; and 5, molecular mass standard (in kDa).
To verify the effect of the E→Q mutations on catalytic activities of MltE and MltC, we overexpressed the SLT domains from these proteins as fusions to the maltose-binding protein, MBP. The purified MBP-MltESlt and MBP-MltCSlt proteins were used in a zymogram assay for the degradation of the PG-rich Micrococcus cell wall. Wild-type MltE and MltC have PG hydrolytic activity, although the SLT domain of MltC proved to be much less active than the SLT domain of MltE in the zymogram assays. The catalytic-site E→Q mutants of MltE and MltC had significantly reduced activities compared to the activities of the wild-type proteins, but their activities were not fully abolished (Fig. 4B and C). It is unclear whether the residual activities observed in the zymogram assays also are present in the full-length proteins in vivo. Taken together, however, these results demonstrate that the catalytic activities of MltE and MltC were responsible for the positive effect of these lytic transglycosylases on the rdar morphotype expression.
The stress sigma factors RpoS and OmpR are not involved in regulation of CsgD expression by lytic transglycosylases.
The stress sigma factor RpoS is required for CsgD expression (45). RpoS expression is regulated by a complex network involving a multitude of components and regulatory pathways. We investigated whether RpoS expression is involved in CsgD downregulation in the ΔmltE ΔmltC double mutant. The overexpression of RpoS from a plasmid did not restore rdar morphotype expression in the ΔmltE ΔmltC double mutant. In addition, in strain MAE52, CsgD expression is independent of RpoS due to a single point mutation in the csgD promoter resulting in semiconstitutive, temperature-independent CsgD and rdar morphotype expression (49). In the MAE52 ΔmltE ΔmltC double mutant, the rdar morphotype was downregulated when grown at 28 and 37°C (data not shown). The csgD expression of the MAE52 ΔmltE ΔmltC double mutant was reduced to 59% of that of MAE52 (Fig. 3A), and CsgD protein levels were even more reduced (data not shown). Because a similar reduction was observed in the UMR1 background, where csgD expression is RpoS dependent, we conclude that CsgD regulation by MltE and MltC occurs independently of RpoS. OmpR is another global transcriptional regulator required for csgD expression (45). The overexpression of OmpR did not restore the CsgD expression of the ΔmltE ΔmltC double mutant, suggesting that OmpR does not limit CsgD expression in the ΔmltE ΔmltC double mutant (data not shown).
MltE and MltC do not work through the Rcs and Cpx two-component systems.
Two membrane-bound sensory systems monitor cell envelope stress. The complex two-component system Rcs senses perturbations in the PG layer (33), with the response regulator proteins RcsB and RcsA serving as the output (35). CpxR, the response regulator of the Cpx two-component surface-sensing system, suppresses CsgD expression and activates the expression of cell wall-degrading proteins (28, 59). To investigate whether PG degradation by MltE and MltC is sensed through either of the two signaling systems, we expressed MltE and MltEE65Q in the respective response regulator mutants. We also constructed response regulator ΔmltE ΔmltC triple mutants. The upregulation of rdar morphotype expression was observed upon the overexpression of MltE in all response regulator mutants, i.e., rcsA, rcsB, and cpxR mutants (see Fig. S1A in the supplemental material; data not shown). Further, the deletion of the response regulator genes in the ΔmltE ΔmltC background did not prevent rdar morphotype downregulation (see Fig. S1B; data not shown). Consequently, the regulation of the rdar morphotype by MltE and MltC occurs independently of the Rcs and Cpx two-component systems. Interestingly, however, the expression of MltEE65Q in the rcsB mutant did not stimulate a mucoid phenotype (i.e., overproduction of colanic acid) (see Fig. S1A). This finding demonstrates that a specific envelope stress signal was induced by the catalytically inactive MltE, but it was not induced by the active form of MltE.
c-di-GMP can partially compensate for the downregulation of rdar morphotype and CsgD expression in the mltE mltC deletion mutants.
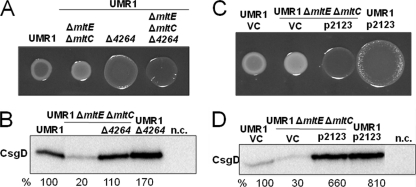
The second messenger c-di-GMP is known to regulate rdar morphotype expression (29, 47). We investigated whether c-di-GMP signaling is involved in rdar morphotype and CsgD expression through MltE and MltC. Six proteins that affect the c-di-GMP metabolism in S. Typhimurium were described to be specifically involved in CsgD regulation. Among them are the GGDEF-EAL domain diguanylate cyclase STM2123 and the EAL domain phosphodiesterase STM4264 (29, 54). To investigate the effect of elevated c-di-GMP concentrations, we deleted the phosphodiesterase STM4264 or overexpressed the di-guanylate cyclase STM2123, which leads to elevated CsgD expression in wild-type S. Typhimurium UMR1. CsgD expression in the ΔmltE ΔmltC ΔSTM4264 triple mutant was increased compared to that of the ΔmltE ΔmltC double mutant, but it did not reach the level of the STM4264 single mutant (Fig. 5A and B). Consistently with this result, CsgD expression was upregulated upon the overexpression of the diguanylate cyclase STM2123 in the ΔmltE ΔmltC mutant (Fig. 5C and D). We conclude that while higher intracellular c-di-GMP levels can partially compensate for the effect of MltE and MltC on rdar morphotype and CsgD expression, the effect of MltE and MltC is largely c-di-GMP independent.
Fig. 5.
Effect of c-di-GMP signaling on the restoration of rdar morphotype expression in the UMR1 ΔmltE ΔmltC double mutant. (A) Deletion of STM4264, encoding a c-di-GMP-dependent phosphodiesterase, in the mltE mltC double mutant upregulated rdar morphotype expression compared to that of the mltE mltC double mutant. As previously shown (45), the STM4264 mutant displayed an upregulated rdar morphotype compared to that of wild-type UMR1. (B) CsgD expression in wild-type UMR1 and mutants. The mltE mltC STM4264 triple mutant shows an increase in CsgD levels compared to that of UMR1 ΔmltE ΔmltC, as CsgD expression increased in S. Typhimurium UMR1 compared to that of its STM4264 mutant. The relative protein levels compared to that of S. Typhimurium UMR1, which was set at 100%, are indicated. n.c., negative-control MAE50 ΔcogD. (C) UMR1 and the mltE mltC double mutant harboring p2123 showed upregulated rdar morphotype expression compared to that of the vector control (VC) pBAD30. (D) CsgD expression in UMR1 and UMR1 ΔmltE ΔmltC containing p2123. CsgD expression increased upon the expression of STM2123 in the wild-type UMR1 and the UMR1 ΔmltE ΔmltC mutant. n.c., negative-control MAE50 ΔcogD. For phenotype observation (A and C), strains were grown on Congo red agar at 28°C for 24 h. To detect CsgD expression (B and D), wild-type UMR1 and mutants were grown on LB without NaCl agar at 28°C for 17 h.
Morphological characterization of ΔmltE ΔmltC mutants.
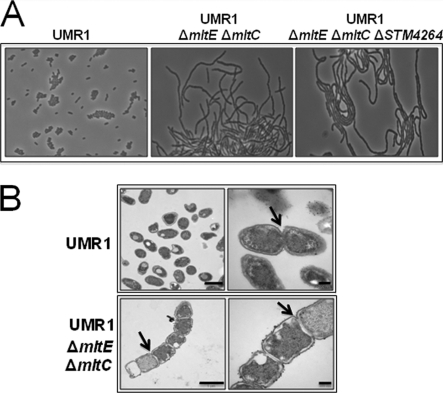
Lytic transglycosylases are responsible for the cleavage of the bacterial PG, which is essential for the separation of the daughter cells following cell division (19). Therefore, the deletion of such a general function might have pleiotropic effects. However, we observed no difference in growth rates between the wild type and the ΔmltE ΔmltC double mutant when these strains were grown in LB medium at 37°C (data not shown). Interestingly, the double mutant showed severe morphological alteration, i.e., the formation of long chains, when grown at 28°C in LB without salt (Fig. 6A; also see Fig. S2 in the supplemental material). These chains of cells subsequently were analyzed by TEM, which revealed that chain formation is due to the impairment of the cleavage of the PG septum (Fig. 6B). The severity of the morphological alterations varied with growth conditions. When grown at 37°C in LB medium, the ΔmltE ΔmltC double mutant showed only a few longer cells but no extended chains (data not shown).
Fig. 6.
Morphological characterization of mltE and mltC mutants. (A) Light microscopy of the mltE mltC and mltE mltC STM4264 mutants. The mltE mltC double mutant and the mltE mltC STM4264 triple mutant display the formation of long chains when grown at 28°C in LB without salt medium. The wild-type UMR1 shows rod-shaped cells of standard length. Magnification, ×630. (B) Transmission electron microscopy (TEM) of the ΔmltE ΔmltC double mutant. Long chains of cells are observed in the ΔmltE ΔmltC double mutant due to an impairment of the cleavage of the PG septum (arrow). The wild-type UMR1 shows rod-shaped cells of 0.8 to 1 μm standard length without impairment in the cleavage of the PG septum. Bars indicate 1 (left panel) or 0.2 μm (right panel).
The ΔmltE ΔmltC ΔSTM4264 triple mutant, with an upregulated rdar morphotype (due to higher intracellular c-di-GMP levels) compared to that of the ΔmltE ΔmltC double mutant, also formed long chains at 28°C in LB without salt medium (Fig. 6A), while the ΔSTM4264 mutant looked like wild-type S. Typhimurium UMR1. As CsgD expression is upregulated in the ΔmltE ΔmltC ΔSTM4264 triple mutant (Fig. 6B), these results indicate that long-chain formation and rdar morphotype expression represent two uncoupled phenomena. Long-chain formation also was observed in E. coli when six murein hydrolases were deleted (19). Note that no morphological alterations were detected for the ΔmltE and ΔmltC single mutants (data not shown).
As another phenotype that can result from defective lytic transglycosylases, the integrity of the cell envelope was tested. Increased cell membrane permeability leads to susceptibility to bile acids. The ΔmltE ΔmltC double mutant, respective single mutants, and the ΔmltE ΔmltC ΔSTM4264 triple mutant were grown on MacConkey agar, which contains bile salts. The ΔmltE ΔmltC double mutant and the ΔmltE ΔmltC ΔSTM4264 triple mutant were unable to grow on MacConkey agar plates (data not shown), showing that the deletion of these two lytic transglycosylases increases the permeability of the cell envelope. S. Typhimurium, like other Gram-negative bacteria, is resistant to vancomycin, as the outer membrane is a barrier for this high-molecular-weight antibiotic. However, resistance to vancomycin was not affected by the ΔmltE ΔmltC mutations (data not shown), which demonstrates that membrane integrity is not entirely lost.
DISCUSSION
In this study, we identified two lytic transglycosylases of family I, the catalytic activities of which specifically affect rdar morphotype expression (biofilm formation) in S. Typhimurium. We established that these transglycosylases act upon the expression of CsgD, the key rdar morphotype transcription factor. In total, we identified nine lytic transglycosylases in S. Typhimurium. In contrast to E. coli, which harbors five transglycosylases of family I (30, 52), in S. Typhimurium seven belong to family I, while MltA belongs to family II and MltB to family III. This functional redundancy makes the assignment of specific functions complex. In this work, we demonstrate that two lytic transglycosylases of family I, MltE and MltC, are specifically involved in the expression of the rdar morphotype.
MltE and MltC have different domain structures. While MltE contains an SLT domain only, MltC contains an additional domain of unknown function, DUF3393. The SLT domains of MltE and MltC show closer similarity to each other than to other lytic transglycosylases in S. Typhimurium. MltE and MltC may be redundant with respect to the regulation of CsgD expression, i.e., only the absence of both proteins has an effect on CsgD and rdar morphotype expression. However, while MltE overexpression fully complemented the ΔmltE ΔmltC double mutant, MltC overexpression complemented it only partially. This may be explained by significantly higher levels of MltE compared to those of MltC despite identical expression sequences. An alternative is that the functions of MltC and MltE are complementary because MltE is believed to be an endo-acting transglycosylase (2), whereas MltC is an exoenzyme (32).
Ours is not the first report linking lytic transglycosylases (also known as autolysins) to biofilm formation. For example, the mltE gene was found to promote biofilm formation in a recent screen of E. coli mutants (38). Furthermore, in firmicutes (low-GC Gram-positive bacteria), interference with PG turnover alters surface adherence and biofilm formation (1, 9, 20).
Several possibilities may be considered for how MltE and MltC affect CsgD expression. First, activities of these lytic transglycosylases can be sensed in the cytoplasm via PG degradation products, anhydromuropeptide monomers, which are transported to the cytoplasm primarily by the permease AmpG (27). The deletion of AmpG, however, had no effect on rdar morphotype expression (data not shown). Similarly, the deletion of NagE and ManYZ transporters for PG degradation products such as GlcNac and glucosamine, as well as an ampG nagE manYZ triple mutant, had no effect on CsgD expression or rdar morphotype formation (data not shown). Therefore, cytosolic anhydromuropeptides or other PG degradation products do not seem to be involved in signaling to regulate CsgD expression.
Second, extracytoplasmic stress has been shown to downregulate CsgD expression and rdar morphotype (43, 61). Anhydromuropeptide monomers or subsequent PG degradation products may initiate signaling in the periplasm. However, neither the well-studied Rcs phosphorelay system, the major signaling pathway that senses perturbations in PG turnover (6, 24, 33), nor the Cpx phosphorelay system was found to contribute to the downregulation of CsgD by MltE/MltC. Consistently with this finding, susceptibility to selected β-lactam antibiotics was not changed in the ΔmltE ΔmltC double mutant (data not shown). Overexpressed catalytically inactive MltE, however, is sensed through the Rcs phosphorelay system, leading to a mucoid phenotype. It is feasible that MltE binding to its substrates without degradation causes periplasmic stress. Alternatively, overexpressed, catalytically impaired MltE may interfere with other periplasmic proteins.
Third, altered membrane permeability may affect CsgD levels via an as-yet uncharacterized signaling pathway. In E. coli, the deletion of six lytic transglycosylases has been shown to alter membrane permeability (19). In S. Typhimurium, the deletion of MltE and MltC was, under the growth conditions used here, already sufficient to alter membrane permeability to some extent. Consistent with the idea that altered membrane permeability affects rdar morphotype is the observation that in E. coli, the loss of outer membrane integrity due to the deletion of the Tol system resulted in the downregulation of the curli-mediated biofilm (61). Also in line with this possibility is our observation that the periplasmic lipoprotein NlpI represses CsgD expression and rdar morphotype expression via an unknown mechanism (50).
Fourth, the connection between MltE and MltC with CsgD and rdar morphotype expression can be a structural one, as lytic transglycosylases have a role in the assembly of macromolecular complexes (30).
As we could not identify an already known regulatory pathway involved in the regulation of rdar morphotype expression by MltE and MltC, we screened for suppressor mutants of the ΔmltE ΔmltC double mutant phenotype by random transposon mutagenesis. We identified suppressor mutants in STM2680, STM2773, and STM4363 which restored rdar morphotype expression to wild-type levels. None of these genes is described to be involved in rdar morphotype and CsgD expression, suggesting that the lytic transglycosylases MltE and MltC regulate rdar morphotype and CsgD expression through a novel pathway.
In summary, the complex regulation of the rdar morphotype underscores the importance of the biofilm mode of growth. For the first time, we uncovered a specific signaling role of two family I lytic transglycosylases in S. Typhimurium that communicate the status of cell wall turnover to the master regulator of biofilm formation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the European Commission under contract number MEST-CT-2004-008475 (to U.R.), the Swedish Research Council Natural Science (621-2007-6509) (to U.R.), and the U.S. National Science Foundation (MCB 0645876 and 1052575) (to M.G.).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Ahn S. J., Burne R. A. 2006. The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis. J. Bacteriol. 188:6877–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Artola-Recolons C., et al. 2011. High-resolution crystal structure of MltE, an outer membrane-anchored endolytic peptidoglycan lytic transglycosylase from Escherichia coli. Biochemistry 50:2384–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernadsky G., Beveridge T. J., Clarke A. J. 1994. Analysis of the sodium dodecyl sulfate-stable peptidoglycan autolysins of select gram-negative pathogens by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 176:5225–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blackburn N. T., Clarke A. J. 2001. Identification of four families of peptidoglycan lytic transglycosylases. J. Mol. Evol. 52:78–84 [DOI] [PubMed] [Google Scholar]
- 5. Boehm A., et al. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116 [DOI] [PubMed] [Google Scholar]
- 6. Callewaert L., Vanoirbeek K. G., Lurquin I., Michiels C. W., Aertsen A. 2009. The Rcs two-component system regulates expression of lysozyme inhibitors and is induced by exposure to lysozyme. J. Bacteriol. 191:1979–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collinson S. K., Clouthier S. C., Doran J. L., Banser P. A., Kay W. W. 1996. Salmonella enteritidis agfBAC operon encoding thin, aggregative fimbriae. J. Bacteriol. 178:662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubrac S., Boneca I. G., Poupel O., Msadek T. 2007. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J. Bacteriol. 189:8257–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finn R. D., et al. 2008. The Pfam protein families database. Nucleic Acids Res. 36:D281–D288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerstel U., Römling U. 2003. The csgD promoter, a control unit for biofilm formation in Salmonella typhimurium. Res. Microbiol. 154:659–667 [DOI] [PubMed] [Google Scholar]
- 12. Gerstel U., Römling U. 2001. Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella typhimurium. Environ. Microbiol. 3:638–648 [DOI] [PubMed] [Google Scholar]
- 13. Girardin S. E., et al. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702–41708 [DOI] [PubMed] [Google Scholar]
- 14. Goldman W. E., Klapper D. G., Baseman J. B. 1982. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect. Immun. 36:782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodell E. W. 1985. Recycling of murein by Escherichia coli. J. Bacteriol. 163:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grantcharova N., Peters V., Monteiro C., Zakikhany K., Römling U. 2010. Bistable expression of CsgD in biofilm development of Salmonella enterica serovar Typhimurium. J. Bacteriol. 192:456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guzman L. M., Belin D., Carson M. J., Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammar M., Arnqvist A., Bian Z., Olsen A., Normark S. 1995. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 18:661–670 [DOI] [PubMed] [Google Scholar]
- 19. Heidrich C., Ursinus A., Berger J., Schwarz H., Höltje J. V. 2002. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184:6093–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heilmann C., Hussain M., Peters G., Götz F. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013–1024 [DOI] [PubMed] [Google Scholar]
- 21. Holmqvist E., et al. 2010. Two antisense RNAs target the transcriptional regulator CsgD to inhibit curli synthesis. EMBO J. 29:1840–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Höltje J. V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:181–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Höltje J. V., Mirelman D., Sharon N., Schwarz U. 1975. Novel type of murein transglycosylase in Escherichia coli. J. Bacteriol. 124:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Y. H., Ferrieres L., Clarke D. J. 2006. The role of the Rcs phosphorelay in Enterobacteriaceae. Res. Microbiol. 157:206–212 [DOI] [PubMed] [Google Scholar]
- 25. Huson D. H., et al. 2007. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobs C., Frere J. M., Normark S. 1997. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram-negative bacteria. Cell 88:823–832 [DOI] [PubMed] [Google Scholar]
- 27. Jacobs C., Huang L. J., Bartowsky E., Normark S., Park J. T. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 13:4684–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jubelin G., et al. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 187:2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kader A., Simm R., Gerstel U., Morr M., Römling U. 2006. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 60:602–616 [DOI] [PubMed] [Google Scholar]
- 30. Koraimann G. 2003. Lytic transglycosylases in macromolecular transport systems of Gram-negative bacteria. Cell Mol. Life Sci. 60:2371–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koropatnick T. A., et al. 2004. Microbial factor-mediated development in a host-bacterial mutualism. Science 306:1186–1188 [DOI] [PubMed] [Google Scholar]
- 32. Kraft A. R., Templin M. F., Holtje J. V. 1998. Membrane-bound lytic endotransglycosylase in Escherichia coli. J. Bacteriol. 180:3441–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laubacher M. E., Ades S. E. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 190:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindquist S., et al. 1993. AmpG, a signal transducer in chromosomal beta-lactamase induction. Mol. Microbiol. 9:703–715 [DOI] [PubMed] [Google Scholar]
- 35. Majdalani N., Gottesman S. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59:379–405 [DOI] [PubMed] [Google Scholar]
- 36. Mariscotti J. F., Garcia-Del Portillo F. 2008. Instability of the Salmonella RcsCDB signalling system in the absence of the attenuator IgaA. Microbiology 154:1372–1383 [DOI] [PubMed] [Google Scholar]
- 37. Melly M. A., McGee Z. A., Rosenthal R. S. 1984. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149:378–386 [DOI] [PubMed] [Google Scholar]
- 38. Niba E. T., Naka Y., Nagase M., Mori H., Kitakawa M. 2007. A genome-wide approach to identify the genes involved in biofilm formation in E. coli. DNA Res. 14:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ogasawara H., Yamada K., Kori A., Yamamoto K., Ishihama A. 2010. Regulation of the Escherichia coli csgD promoter: interplay between five transcription factors. Microbiology 156:2470–2483 [DOI] [PubMed] [Google Scholar]
- 40. Oh H. S., Kvitko B. H., Morello J. E., Collmer A. 2007. Pseudomonas syringae lytic transglycosylases coregulated with the type III secretion system contribute to the translocation of effector proteins into plant cells. J. Bacteriol. 189:8277–8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park J. T., Uehara T. 2008. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 72:211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paul K., Nieto V., Carlquist W. C., Blair D. F., Harshey R. M. 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol. Cell 38:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prigent-Combaret C., et al. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Römling U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell Mol. Life Sci. 62:1234–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Römling U., Bian Z., Hammar M., Sierralta W. D., Normark S. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Römling U., et al. 2003. Dissection of the genomic pathway leading to multicellular behaviour in Salmonella enterica serotype Typhimurium and other Enterobacteriaceae, p. 231–261 In Wilson M., Devin D. (ed.), Medical implications of biofilm. Cambridge University Press, Cambridge, MA [Google Scholar]
- 47. Römling U., Gomelsky M., Galperin M. Y. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57:629–639 [DOI] [PubMed] [Google Scholar]
- 48. Römling U., Rohde M., Olsen A., Normark S., Reinköster J. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10–23 [DOI] [PubMed] [Google Scholar]
- 49. Römling U., Sierralta W. D., Eriksson K., Normark S. 1998. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249–264 [DOI] [PubMed] [Google Scholar]
- 50. Rouf S. F., et al. 2011. Opposing contributions of polynucleotide phosphorylase and the membrane protein NlpI to biofilm formation by Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryjenkov D. A., Simm R., Römling U., Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310–30314 [DOI] [PubMed] [Google Scholar]
- 52. Scheurwater E. M., Clarke A. J. 2008. The C-terminal domain of Escherichia coli YfhD functions as a lytic transglycosylase. J. Biol. Chem. 283:8363–8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmieger H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75–88 [DOI] [PubMed] [Google Scholar]
- 54. Simm R., Lusch A., Kader A., Andersson M., Römling U. 2007. Role of EAL-containing proteins in multicellular behavior of Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:3613–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simm R., Morr M., Kader A., Nimtz M., Römling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134 [DOI] [PubMed] [Google Scholar]
- 56. Solano C., et al. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43:793–808 [DOI] [PubMed] [Google Scholar]
- 57. Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thunnissen A. M., et al. 1994. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature 367:750–753 [DOI] [PubMed] [Google Scholar]
- 59. Weatherspoon-Griffin N., et al. 2010. The CpxR/CpxA two-component system upregulates two Tat-dependent peptidoglycan amidases to confer bacterial resistance to antimicrobial peptide. J. Biol. Chem. 286:5529–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Viala J., et al. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166–1174 [DOI] [PubMed] [Google Scholar]
- 61. Vianney A., et al. 2005. Escherichia coli tol and rcs genes participate in the complex network affecting curli synthesis. Microbiology 151:2487–2497 [DOI] [PubMed] [Google Scholar]
- 62. Viollier P. H., Shapiro L. 2003. A lytic transglycosylase homologue, PleA, is required for the assembly of pili and the flagellum at the Caulobacter crescentus cell pole. Mol. Microbiol. 49:331–345 [DOI] [PubMed] [Google Scholar]
- 63. Zakikhany K., Harrington C. R., Nimtz M., Hinton J. C. D., Römling U. 2010. Unphosphorylated CsgD controls biofilm formation in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 77:771–786 [DOI] [PubMed] [Google Scholar]
- 64. Zogaj X., Nimtz M., Rohde M., Bokranz W., Römling U. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452–1463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.