Abstract
Hopanoids are pentacyclic triterpenoids that are thought to be bacterial surrogates for eukaryotic sterols, such as cholesterol, acting to stabilize membranes and to regulate their fluidity and permeability. To date, very few studies have evaluated the role of hopanoids in bacterial physiology. The synthesis of hopanoids depends on the enzyme squalene-hopene cyclase (Shc), which converts the linear squalene into the basic hopene structure. Deletion of the 2 genes encoding Shc enzymes in Burkholderia cenocepacia K56-2, BCAM2831 and BCAS0167, resulted in a strain that was unable to produce hopanoids, as demonstrated by gas chromatography and mass spectrometry. Complementation of the Δshc mutant with only BCAM2831 was sufficient to restore hopanoid production to wild-type levels, while introducing a copy of BCAS0167 alone into the Δshc mutant produced only very small amounts of the hopanoid peak. The Δshc mutant grew as well as the wild type in medium buffered to pH 7 and demonstrated no defect in its ability to survive and replicate within macrophages, despite transmission electron microscopy (TEM) revealing defects in the organization of the cell envelope. The Δshc mutant displayed increased sensitivity to low pH, detergent, and various antibiotics, including polymyxin B and erythromycin. Loss of hopanoid production also resulted in severe defects in both swimming and swarming motility. This suggests that hopanoid production plays an important role in the physiology of B. cenocepacia.
INTRODUCTION
Cholesterol, a characteristic sterol molecule produced by eukaryotic cells, is absent in bacteria. However, bacteria produce pentacyclic triterpenoids called hopanoids that may be surrogates for eukaryotic sterols (19). The absence of sterol production in nearly all prokaryotes, combined with the structural similarities between sterols and hopanoids, has led to the hypothesis that hopanoids have a function similar to that of sterols, acting to stabilize membranes, as well as to regulate their fluidity and permeability (28, 29, 55). The production of hopanoids depends on squalene-hopene cyclase (Shc), which converts the linear precursor squalene to the pentacyclic triterpenoid hopene (Fig. 1A) (31, 53). Hopanoids localize to the cytoplasmic and outer membranes of certain bacteria and, like eukaryotic sterols, exhibit a wide range of structural variation, particularly with respect to functionalization of the side chain (25, 27, 65, 72). Approximately 10% of all sequenced bacterial genomes contain genes necessary to produce hopanoids, with the majority being found in cyanobacteria, acetobacters, streptomycetes, methylotrophs, and purple nonsulfur bacteria (29, 52, 57). Hopanoid production has not been detected in enterobacteria or any other human pathogen, with the exception of Burkholderia gladioli (11, 57). Several studies concerning hopanoid function predict that their production may be linked to environmental and physiological changes, such as the formation of aerial hyphae in Streptomyces spp. (56) or production of hopanoid-coated heterocyst-like vesicles in Frankia spp. (4). It is unknown whether hopanoids play a structural role in these species or function as a barrier protecting bacteria from desiccation or environmental stresses, such as oxidation. One of the few studies utilizing a genetic approach to define the role of hopanoids in membrane structure-function reported that the loss of hopanoid production in Rhodopseudomonas palustris resulted in sensitivity to acidic and alkaline conditions, bile salts, and certain antibiotics (72). This increased sensitivity was not observed in similar experiments performed with Streptomyces scabies (62), demonstrating that hopanoid function varies depending on taxonomy, environment, and physiology.
Fig. 1.
Biosynthesis of hopanoids in B. cenocepacia. (A) Conversion of squalene to the basic saturated and unsaturated hopenes by squalene-hopene cyclase. (B) Schematic representation of the hopanoid synthesis gene cluster as annotated in strain J2315 (which is clonally related to strain MH1K used in this work [20]), containing the shc and hopanoid-associated transporter genes. The shc gene, BCAS0167, located on chromosome 3, is not located within a hopanoid synthesis-associated gene cluster. The arrows representing genes deleted in this study are outlined in black.
Burkholderia cenocepacia is one of at least 17 species of genetically linked bacteria referred to as the B. cepacia complex (Bcc) (38). These bacteria are widespread in the environment, where they degrade pollutants and promote plant growth (38). Unfortunately, these beneficial traits are offset by the ability of Bcc bacteria to cause opportunistic infections in cystic fibrosis (CF) patients and other immune-compromised individuals (67, 69). All Bcc members can cause chronic airway infections in CF patients; however, B. cenocepacia and Burkholderia multivorans are the species most commonly isolated worldwide (39, 68). Infection with B. cenocepacia is a risk factor leading to reduced survival of CF patients, particularly after lung transplantation (7, 44). These adverse outcomes are commonly due to cepacia syndrome, a frequently fatal, necrotizing pneumonia characterized by sepsis (17, 30). Burkholderia species adapt to a wide range of environmental conditions and are intrinsically resistant to most clinically relevant antibiotics, making it very difficult to treat Bcc infections (1, 5, 8, 15, 45). Antibiotic resistance in Burkholderia has been attributed to the presence of multiple efflux pumps, antibiotic-modifying enzymes, and the structure of lipopolysaccharide (LPS) on the outer membrane, but other factors remain to be identified (18, 20, 37, 48).
While all 9 Burkholderia strains tested by Cvejic et al. (11) produced hopanoids, it is unknown whether these compounds are produced by B. cenocepacia. Although Burkholderia species have been analyzed for the presence of hopanoids, there is no study using genetic manipulation to investigate their role in bacterial growth and adaptation to environmental stresses. Here, we report that the deletion of genes encoding the squalene-hopene cyclase in B. cenocepacia K56-2 results in a loss of hopanoid production. The shc mutant is sensitive to acidic pH, detergents, and various antibiotics. The shc mutant also exhibits impaired swimming and swarming motility but is not hindered in its ability to secrete proteases or to replicate and survive within murine macrophages. This study describes for the first time the role of hopanoid production in the opportunistic human pathogen B. cenocepacia K56-2.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria grew in LB broth with shaking or on LB agar plates incubated at 37°C. Escherichia coli cultures were supplemented, as required, with the following final concentrations of antibiotics: 50 μg/ml trimethoprim, 40 μg/ml kanamycin, and 30 μg/ml tetracycline. When needed, B. cenocepacia cultures were supplemented with 100 μg/ml trimethoprim and 150 μg/ml tetracycline.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| B. cenocepacia | ||
| MH1K | ET12 clone related to J2315; CF clinical isolate; made Gms via deletion of an efflux pump | 20 |
| MH1K ΔBCAM2828 | Deletion of BCAM2828 in MH1K | This study |
| MH1K ΔBCAM2831 | Deletion of BCAM2831 in MH1K | This study |
| MH1K ΔBCAS0167 | Deletion of BCAS0167 in MH1K | This study |
| MH1K Δshc | Deletion of BCAM2831 and BCAS0167 in MH1K | This study |
| E. coli | ||
| GT115 | F−mcrA Δ(mrr-hsdRMSmcrBC) φ80′lacZΔM15 ΔlacX74 recA1 rpsL (StrA) endA1 Δdcm uidA(ΔMluI)::pir-116 ΔsbcC-sbcD | Laboratory strain |
| DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Laboratory strain |
| Plasmids | ||
| pBCAM2828 | BCAM2828 in pSCrhaB2 | This study |
| pBCAM2831 | BCAM2831 in pSCrhaB2 | This study |
| pBCAS0167 | BCAS0167 in pSCrhaB2 | This study |
| pDAI-SceI-SacB | oripBBR1 TetrPdhfr mob+; expressing I-SceI; also expresses negative selection marker SacB | 20 |
| pDelBCAM2828 | pGPI-SceI with fragments flanking BCAM2828 | This study |
| pDelBCAM2831 | pGPI-SceI with fragments flanking BCAM2831 | This study |
| pDelBCAS0167 | pGPI-SceI with fragments flanking BCAS0167 | This study |
| pGPI-SceI | oriR6K ΩTprmob+, including an I-SceI restriction site | 13 |
| pRK2013 | oricolE1; RK2 derivative; Kanrmob+tra+ | 67 |
| pSCrhaB2 | oripBBR1rhaR rhaS PrhaB Tprmob+ | 6 |
| pShc | BCAM2831 and BCAS0167 in pSCrhaB2 | This study |
Tpr, trimethoprim resistance; Kanr, kanamycin resistance; Tetr, tetracycline resistance; Gms, gentamicin sensitive.
General molecular techniques.
DNA manipulations and cloning were performed as described previously (60). PCR amplification of DNA was performed using Taq or HotStar HiFidelity DNA polymerases (Qiagen). Table 2 lists the DNA sequences of the primers used in this study. Antarctic phosphatase (New England BioLabs) and T4 DNA ligase (Roche Applied Science) were used as recommended by the manufacturers. E. coli GT115 was transformed via standard electroporation methods (60). DNA sequencing was performed at the sequencing facility at York University (Toronto, Canada). Mobilization of plasmids into B. cenocepacia MH1K was performed by triparental mating using the helper plasmid pRK2013 (10, 12). The sequenced genome of B. cenocepacia strain J2315 was analyzed with the computer programs BLAST and Artemis.
Table 2.
Primers used in this study
| Primer no. | Primer sequence (5′-3′)a | Restriction enzyme |
|---|---|---|
| 4820 | GTCGATGGTACCCTTCGGCAGCCTGTGGCTGTCGCATCAT | KpnI |
| 4821 | GTCGCATCTAGAAGCACGAGATACGGGCCGGACGGCATG | XbaI |
| 4822 | ATCGATAATATTGAACTCGCCGCGCCCGACACGTTCAGCGC | SspI |
| 4823 | GATCGAGGTACCGACGAGCAGCACGTATTCGGCGGGAATCG | KpnI |
| 4824 | GTCGATGGTACCCAGCAGAACGAAGAAGGCCTGTGGGACGA | KpnI |
| 4825 | ATCGATAATATTCAGCACGTCGGTACTCGCGGCCGGATTGG | SspI |
| 4826 | GATCGAGGTACCTCCATGAAATGCATCATCAGGATGTATTC | KpnI |
| 4827 | GTCGATGGTACCGACGGCTTCTGGTGGCACCGCTCGCACAA | KpnI |
| 4828 | GTCGCATCTAGAAAGCCGCGATGTGCTGCATCGTGCCGCGC | XbaI |
| 4829 | GTCGCATCTAGAGGATCGACGATCGCGCGGCACACCGCGAA | XbaI |
| 4858 | ATCGATAATATTGACGATATCGACCGGCGCGCTCTGCGCGAA | SspI |
| 4859 | GATCGAGGTACCCGTAGACGCCGCTGAAGGCGGCGATGACGA | KpnI |
| 4987 | GTACAGCTCCTGCTGCTGTTGCGGCGTG | None |
| 4988 | GCGGCCTGCTTCAGCAGGTCGGTCGCGC | None |
| 4989 | ATTCGCGGCTCGACGCGTTCGAGTTCGG | None |
| 4992 | CGGCTGCTTCAGCAGTTCGCGCAGGATC | None |
| 5097 | CAGCTTCCGAAAGGCCATG | None |
| 5098 | ATCTGCTGCCCGAACTCGTC | None |
| 5268 | ATATATCATATGGCGGACGTGCTCCAGACGCAATAAAGAG | NdeI |
| 5269 | ATATATTCTAGAGCCACGCGTTACATCCCGACCGTCACG | XbaI |
| 5270 | ATATATCATATGACTGCACGTCTCGAGATAAACATTCCG | NdeI |
| 5271 | ATATATTCTAGACCCGGCGCGCGACCGCTCAGGCGAGC | XbaI |
| 5413 | ATATATCATATGGTCGATTATGTAAATATCCCCATACAAATG | NdeI |
| 5414 | ATATATTCTAGAGATTCGCACCTTATTCATTGATTCCTTGCGA | XbaI |
| 5415 | ATATATTCTAGAAGCACTGCACGTCTCGAGATAAACATTCCG | XbaI |
| 5416 | ATATATAAGCTTGCACCCCGGCGCGCGACCGCTCAGGCGAGC | HindIII |
Incorporated restriction enzyme recognition sites are underlined in the primer sequences.
Construction of deletion strains and complementing plasmids.
The method described by Flannagan et al. (13) was used for the construction of unmarked, nonpolar mutant strains. The deletion mutagenesis plasmids were created by amplifying 650-bp DNA fragments flanking the hopanoid-associated transporter gene, BCAM2828, and the Shc-encoding genes BCAM2831 and BCAS0167. The upstream flanking regions were digested with SspI and KpnI, and the downstream flanking regions were digested with KpnI and XbaI (Roche Applied Science). Both digested amplified fragments were ligated into pGPI-SceI to create pDelBCAM2828, pDelBCAM2831, and pDelBCAS0167 deletion plasmids. The mutagenic plasmids were mobilized into B. cenocepacia MH1K by triparental mating, and cointegrants were selected using 100 μg/ml trimethoprim. Selection against E. coli donor and helper strains after the triparental mating was accomplished using 200 μg/ml ampicillin in combination with 25 μg/ml polymyxin B. pDAI-Sce-I-SacB, used in the final stage of mutagenesis to induce the second recombination event leading to unmarked gene deletion, was mobilized into B. cenocepacia MH1K cointegrants, and exconjugants were selected with 150 μg/ml tetracycline. The deletion mutants were cured from the levansucrase-encoding (SacB) plasmid by growing B. cenocepacia MH1K in LB broth overnight and then plating it on salt-free LB agar supplemented with 5% (wt/vol) sucrose. The resulting tetracycline-sensitive colonies were screened by PCR to confirm the presence of the appropriate gene deletions.
Complementing plasmids were constructed by amplifying BCAM2828, BCAM2831, and BCAS0167 with the appropriate primer pairs. PCR products were digested with NdeI-XbaI and cloned into a similarly digested pSCrhaB2, resulting in the creation of pBCAM2828, pBCAM2831, and pBCAS0167. To complement MH1K ΔBCAM2831 ΔBCAS0167 (referred to here as Δshc), pBCAM2831 was linearized with XbaI-HindIII, and a similarly digested BCAS0167 PCR product was inserted into the plasmid, giving rise to pShc, carrying both shc genes, BCAS0167 and BCAM2831. Complementing plasmids were introduced into the desired mutant strains by triparental mating as described above.
GC-MS analysis.
Nonsaponifiable lipids were extracted using the hopanoid detection method of Kuchta et al. (32). Bacteria grown to stationary phase (100-ml culture) were washed twice by centrifugation and resuspended in 0.85% (wt/vol) NaCl, and the resulting pellet was stored at −20°C until analysis. The bacterial pellet was suspended in 20 ml of 10% (wt/vol) KOH in methanol and incubated for 1 h at 65°C. Nonsaponifiable lipids were extracted 3 times using 7 ml of n-hexane, and the solvent was evaporated in a stream of nitrogen gas. The lipid extracts were suspended in 0.2 ml chloroform and analyzed by gas chromatography-mass spectrometry (GC-MS) using a Varian CP-3800 gas chromatograph equipped with a Varian model 220 ion trap mass spectrometer containing a CP-Sil 5 CB low-bleed MS column (WCOT silica; 30 m by 0.25 mm inside diameter (i.d.). To facilitate fragmentation of the hopanoid polar side chains, the injector oven was set to 300°C. After a 1-μl sample injection in splitless mode, hopanoids were eluted using the following program: 120°C held for 4 min and then ramped up to 325°C at 15°C per min and held for 2.33 min for a total run time of 20 min. High-purity He was used as the carrier gas at a flow rate of 1 ml/min. Ions were monitored in the 100 to 400 m/z range.
Environmental stress tests.
The strains grew overnight with shaking in unbuffered LB medium at 37°C. The cultures were adjusted to an optical density at 600 nm (OD600) of 0.005 in the appropriate medium. Buffered LB medium was prepared by adding 100 mM (final concentration) MES (4-morpholineethanosulfonic acid) for pH 4.5, 100 mM MOPS (4-morpholinepropanesulfonic acid) for pH 7.0, or 100 mM bicine [N,N-bis(2-hydroyxethylglycine)] for pH 8.0 and adjusting the medium to the correct pH with concentrated HCl or NaOH before autoclaving. Where appropriate, the pH 7.0 buffered LB medium was supplemented with 0.03% (wt/vol) SDS, 1 mg/ml polymyxin B, 50 μg/ml erythromycin, 5 μg/ml chloramphenicol, 1 μg/ml novobiocin, or 200 μg/ml rifampin. Growth was determined in a 100-well disposable plate using a Bioscreen C automated microbiology growth curve analysis system (MTX Lab Systems). Growth was monitored over 24 h at 37°C.
LPS analysis.
LPS was extracted as described previously (40) and resolved on 16% polyacrylamide gels using a tricine-SDS gel electrophoresis system (34, 61). LPS samples were visualized by silver staining (40).
Electron microscopy.
For membrane analysis, the MH1K and Δshc strains were grown overnight in LB buffered to pH 7.0. Early-exponential-phase cultures (OD600 = 0.7) were diluted to an OD600 of 0.1 and harvested by centrifugation (2,500 × g for 10 min). The cells were then washed twice in 0.1 M sodium cacodylate buffer, pH 7.3, and fixed overnight in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (4°C). The cells were washed in 0.1 M sodium cacodylate and treated with 1% osmium tetroxide for 1 h. The bacteria were embedded in 5% Noble agar and fixed in 2% uranyl acetate for 2 h. The blocks were dehydrated in a graded ethanol series (50%, 70%, 85%, 95%, and 100%), cleared with propylene oxide, and infiltrated overnight in a 50/50 mixture of absolute ethanol/Epon 812 resin for 2.5 h. Samples were embedded for sectioning, and thin sections were stained with 2% uranyl acetate and lead citrate and visualized using a Philips 410 transmission electron microscope operating at 60 kV. For flagellum negative staining, the MH1K and Δshc strains were grown for 18 h in LB buffered to pH 7.0. Formvar-coated copper grids were placed face down on the cell growth to allow cells to adhere. The grids were then washed 4 times in double-distilled H2O (ddH2O) and stained in 1% (wt/vol) uranyl acetate for 45 s. The grids were visualized using a Philips CM 10 transmission electron microscope operating at 60 kV.
Motility and protease secretion assays.
Swimming and swarming motility assays were performed as previously described, with some modifications (3). For swimming assays, 2 μl of overnight culture, adjusted to an OD600 of 1.0, was inoculated within the agar of a swim plate (LB; 0.3% agar). For swarming assays, 2 μl of overnight culture, adjusted to an OD600 of 1.0, was spotted on top of the swarm plate agar (nutrient broth; 0.5% agar, 0.2% glucose). The plates were incubated at 37°C for 24 h, after which the diameters of the swimming and swarming zones were measured. To determine protease activity, overnight cultures grown in LB were diluted to an OD600 of 0.3, and 3 μl of this culture was spotted onto a plate containing dialyzed brain heart infusion agar containing 1.5% skim milk (66). The plates were incubated for 48 h at 37°C, after which the radii of the cleared zones surrounding the colonies were measured.
Intracellular survival in macrophages.
The murine macrophage cell line RAW264.7 (ATCC TIB-71; American Type Culture Collection, Manassas, VA) was maintained in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C in a 95% humidified atmosphere with 5% CO2. Bacterial intracellular survival was assayed as described previously with slight modifications (20). RAW 264.7 macrophages were seeded in 12-well plates at a density of 2 × 106 cells per well and incubated overnight. The bacterial strains were grown overnight in LB broth at 37°C with shaking. The bacterial cultures were washed three times with DMEM-FBS and were used to infect macrophages at a multiplicity of infection (MOI) of 25. After infection, the plates were centrifuged for 1 min at 300 × g and incubated for 1 h at 37°C under 5% CO2. The infected macrophages were washed with PBS three times to remove extracellular bacteria. DMEM-FBS containing 100 μg/ml gentamicin was added to kill any remaining extracellular bacteria. After 1 h, the macrophages were washed twice in PBS, and fresh medium containing 10 μg/ml gentamicin was added for the remainder of the experiment. To enumerate intracellular bacteria, the infected macrophages were lysed with 0.1% (wt/vol) sodium deoxycholate at 0, 24, and 48 h postinfection. The lysates were serially diluted in PBS and plated on LB agar.
RESULTS
A B. cenocepacia squalene-hopene cyclase deletion mutant does not produce hopanoids.
From the sequenced genome of B. cenocepacia J2315, we identified several gene clusters encoding putative hopanoid biosynthesis and transport genes. To date, every bacterium whose genome contains hopanoid biosynthesis genes has been found to produce hopanoids (23, 53, 57, 62, 72). The key step in hopanoid biosynthesis is the conversion of linear squalene to the basic hopene structure, which is catalyzed by squalene-hopene cyclase (Fig. 1A) (31, 53). The B. cenocepacia genome contains 2 copies of the shc gene: BCAM2831, located within a hopanoid biosynthesis gene cluster on chromosome 2, and BCAS0167, located on chromosome 3 (Fig. 1B). According to BLASTP analysis the Shc proteins share 63% similarity and 43% identity. We created unmarked, in-frame deletions in BCAM2831 and BCAS0167 and also a Δshc mutant missing both copies of the shc gene. In addition, we made a deletion of the gene encoding a predicted hopanoid biosynthesis-associated resistance-nodulation-cell division transporter, BCAM2828 (Fig. 1B).
To determine the roles of the deleted gene products in hopanoid biosynthesis, we used a rapid saponification and extraction method to isolate the nonsaponifiable lipids from the wild-type and mutant strains. The lipid samples were analyzed using a sensitive and selective gas chromatography method that makes use of the detection of a characteristic hopanoid fragment ion (m/z = 191) (32). This ion results from thermal decomposition during the high-temperature (300°C) injection, when the polyolic side chain breaks from the hopene molecule (Fig. 2B). The decomposition reduces the number of individual hopanoids and results in increased sensitivity of the method. In the MH1K, ΔBCAM2831, ΔBCAS0167, and ΔBCAM2828 samples, a characteristic m/z 191 peak indicative of hopanoids was detected at approximately 19 min (Fig. 2A to E and data not shown; see Fig. S1 in the supplemental material). This peak also contained an ion with an m/z of 189, which likely corresponds to an unsaturated hopanoid analogue previously detected in other Burkholderia spp. (Fig. 2B) (11). The 19-min (hopanoid) peak was absent in the Δshc strain. Instead, there was an accumulation of a novel m/z 191-containing peak, which eluted at approximately 16.2 min (Fig. 2F; see Fig. S1 in the supplemental material). This novel m/z 191-containing peak is also present in the ΔBCAM2831 strain, but not in the ΔBCAS0167 strain (Fig. 2D and E). While the identification of the 16.2-min peak remains unconfirmed, it may represent an alternative squalene cyclization product that yields an m/z 191 fragment upon thermal decomposition. Complementation of the Δshc mutant with only BCAM2831 was sufficient to restore hopanoid production to wild-type levels, evidenced by the gain of the 19-min peak. In contrast, introducing a copy of BCAS0167 alone into the Δshc mutant produced only very small amounts of the 19-min hopanoid peak (Fig. 2G and H). No peaks corresponding to hopanoids were detected in the hopanoid nonproducer E. coli DH5α (Fig. 2I). The full chromatograms are shown in Fig. S1 in the supplemental material. Together, these results indicate that the shc gene products are essential for the production of hopanoids in B. cenocepacia K56-2 and that the absence of BCAM2831 results in the accumulation of a putative biosynthetic precursor.
Fig. 2.
A Δshc mutant in B. cenocepacia K56-2 does not produce hopanoids. Nonsaponifiable lipids were extracted from B. cenocepacia strains and separated by GC. (A) Total ion chromatogram for MH1K. (B) A full mass spectrum of the 19-min peak seen in panel A, including a structural representation of the hopanoid fragmentation pattern (32). (C to I) Reconstituted ion chromatograms (m/z 191) for MH1K (C), ΔBCAS0167 (D), ΔBCAM2831 (E), Δshc (F), Δshc pBCAS0167 (G), Δshc pBCAM2831 (H), and E. coli DH5α (I). The total ion chromatograms from which the m/z 191 reconstructed ion chromatograms are derived are shown in Fig. S1 in the supplemental material.
The Δshc mutant is sensitive to acidic conditions.
Hopanoids may be involved in bacterial pH tolerance (49, 72). To determine whether hopanoid biosynthesis and transport mutants are affected by acidic and alkaline conditions, their growth was monitored over 24 h. All mutants grew similarly to the wild type in pH 7 buffered medium, indicating that hopanoid production is not required in neutral pH growth environments (Fig. 3B). The Δshc mutant was slightly delayed in reaching the exponential growth phase in pH 8 buffered medium but was able to reach OD600 levels similar to those of the wild type after 24 h (Fig. 3C). Neither the mutant nor the wild-type strain grew in pH 9 buffered medium (data not shown). Growth of the ΔBCAS0167, ΔBCAM2831, and ΔBCAM2828 mutants was not significantly affected in pH 4.5 buffered medium; however, the Δshc mutant exhibited a drastic sensitivity to acidic conditions, growing very poorly at pH 4.5 (Fig. 3A). Complementation of Δshc with pBCAM2831 or pShc restored a growth phenotype similar to that of wild-type MH1K (Fig. 3D).
Fig. 3.
Effect of pH on the growth of hopanoid-defective mutants. (A to C) Representative growth curves of the wild-type and mutant strains in LB medium buffered at pH 4.5 (A), pH 7 (B), and pH 8 (C). (D) Complementation of the Δshc growth defect at pH 4.5 with plasmids expressing BCAS0167, BCAM2831, or both Shc enzymes. Each time point represents the average of two replicate cultures (the error bars represent standard deviations). Each growth curve was repeated three times.
Lack of hopanoid production results in membrane damage.
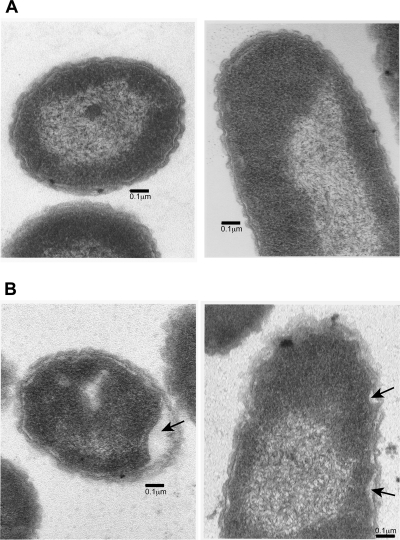
Because hopanoids are hypothesized to act as membrane stabilizers, it is possible that lack of hopanoid production would result in membrane damage. Transmission electron microscopy (TEM) revealed that the Δshc mutant cells have regions where the cytoplasmic membrane appears to be retracted from the outer membrane and disorganized. Although this phenotype was not observed in all cells under low-magnification TEM, all the MH1K cells observed exhibited an intact cell envelope between the outer membrane and the cytoplasmic membrane (Fig. 4). The disorganization of Δshc mutant cell membranes may result in increased membrane permeability and could cause B. cenocepacia to be increasingly sensitive to detergents and antibiotics, well-known indicators of membrane damage in Gram-negative bacteria (2, 58, 72). In contrast to the single shc mutants, ΔBCAS0167 and ΔBCAM2831, the Δshc mutant was unable to grow when exposed to the detergent SDS (Fig. 5A). Introducing pBCAM2831 alone rescued growth of the Δshc mutant strain in SDS (Fig. 5E). The Δshc mutant also displayed increased sensitivity to the antibiotics polymyxin B, erythromycin, and chloramphenicol (Fig. 5B to D). The putative hopanoid transporter mutant, ΔBCAM2828, and the single Shc mutant, ΔBCAM2831, also displayed increased sensitivity to erythromycin and chloramphenicol but were unaffected by the addition of polymyxin B.
Fig. 4.
Loss of hopanoid production results in membrane defects. Shown is transmission electron microscopy of MH1K (A) and Δshc (B) cells grown in pH 7 buffered medium and harvested during early exponential phase. The arrows indicate areas where the inner membrane is retracting from the outer membrane.
Fig. 5.
A B. cenocepacia Δshc mutant exhibits increased sensitivity to detergent and antibiotics. (A to D) Representative growth curves of the wild-type and mutant strains in LB medium buffered to pH 7 and supplemented with 0.03% SDS (A), 1,000 μg/ml polymyxin B (B), 5 μg/ml chloramphenicol (C), or 50 μg/ml erythromycin (D). (E and F) Complementation of the Δshc growth defect in 0.03% SDS and 1,000 μg/ml polymyxin B, respectively. Each time point represents the average of two replicate cultures (the error bars represent standard deviations). Each growth curve was repeated three times.
LPS is a known contributor to antimicrobial peptide resistance in Burkholderia spp. (37, 48). To ensure that the increased antibiotic sensitivity of B. cenocepacia was not due to a major change in LPS, the profiles of the wild-type and mutant strains were compared (data not shown). There was no obvious difference in the appearance or abundance of the LPS molecules between the wild-type, ΔBCAM2828, and Δshc strains.
Hopanoid production is required for motility.
The loss of hopanoid production and the resulting compromise in the structural integrity of the membrane may affect other structures within or spanning the cell envelope. B. cenocepacia relies on membrane-spanning polar flagella for swimming motility, a form of movement regulated by individual cells perceiving chemical signals, which can be measured using characteristic swim agar plates (3, 35). Upon sensing the proper environmental signals, B. cenocepacia modifies its cell morphology to become an elongated, multiflagellated swarm cell (3, 24, 35). Swarming motility is a coordinated social behavior, allowing the bacteria to act as a multicellular population to rapidly colonize nutrient-rich solid substrates, such as swarm agar plates (21, 22, 43, 63). Hence, the wild-type and hopanoid-associated mutant strains were grown on swim and swarm plates to test whether hopanoid production has an effect on either form of motility. The swimming motility of the ΔBCAM2828 strain and the single shc mutants, ΔBCAM2831 and ΔBCAS0167, was not affected within the first 24 h. However, deletion of both shc genes resulted in a mutant that was severely impaired in swimming motility (Fig. 6A and B). At 48 h after inoculation, swimming motility was significantly reduced in several mutant strains, but none so greatly as the Δshc mutant. Swarming motility was significantly reduced in the ΔBCAS0167 mutant and impaired in MH1K Δshc at 24 h postinoculation. Even after 48 h, the Δshc mutant was incapable of swarming motility (Fig. 6C and D). Complementation of the swimming and swarming phenotypes was not possible using the tools currently available, as the introduction of the pSCrhaB2 vector greatly reduced the motility of MH1K (data not shown).
Fig. 6.
Δshc cells are defective for both swimming and swarming motility. (A) The swimming motility of MH1K and hopanoid-defective mutants was measured at 24 and 48 h postinoculation. Only the Δshc mutant showed a significant defect in swimming motility after 24 h (P < 0.0001), whereas the Δshc (P = 0.0055), ΔBCAM2828 (P = 0.0421), and ΔBCAM2831 (P = 0.0396) mutants showed significantly decreased swimming motility 48 h postinoculation. The results represent the averages and standard errors of three independent experiments. (B) Visual representation of swimming motility 24 h postinoculation. (C) The swarming motility of MH1K and hopanoid-defective mutants was measured at 24 and 48 h postinoculation. The Δshc mutant showed a severe defect in swarming motility (P < 0.0001). The results represent the averages and standard errors of three independent experiments. (D) Visual representation of swarming motility 24 h postinoculation. (E and F) Bacterial preparations stained for the detection of flagella are shown in electron micrographs of MH1K (E) and Δshc (F) strains. *, **, and *** indicate P values of <0.05, <0.01, and <0.0001, respectively.
Examination of the Δshc strain by transmission electron microscopy revealed that the strain does not produce any detectable flagella, whereas the wild-type strain, MH1K, produces multiple polar flagella (Fig. 6E and F).
B. cenocepacia possesses various secretion systems, each of which is intimately associated with the bacterial cell envelope. The activity of the B. cenocepacia type II secretion system (T2SS) can be easily measured using a protease secretion assay developed by Sokol et al. (66). All strains produced protease clearance zones that were similar to that of the wild type, MH1K, indicating that the T2SS was functioning properly in all mutants (data not shown).
The Δshc mutant survives and replicates in macrophages.
We reasoned that the increased membrane permeability and pH sensitivity of the Δshc mutant could make it more vulnerable to the intracellular environment in macrophages. Data from our laboratory indicate that BCAM2828 is transcriptionally upregulated during macrophage infection (J. Tolman and M. A. Valvano, unpublished data) and may be important for bacterial adaptation to the intracellular environment. RAW264.7 macrophages were infected with the MHIK parental strain and the corresponding ΔBCAM2828 and Δshc isogenic mutant strains. Bacterial survival and replication were monitored over a period of 48 h. During the first 24 h, there was no significant difference in the survival of mutant strains and that of the wild type, and all strains tested were able to replicate an average of ∼2 log10 units (Fig. 7). All strains exhibited similar levels of intracellular survival after 48 h of infection.
Fig. 7.
Deletion of hopanoid biosynthesis genes does not affect intramacrophage replication or survival of B. cenocepacia K56-2. RAW 264.7 macrophages were infected with the MH1K, ΔBCAM2828, or Δshc strain. The infected cells were lysed at 0, 24, and 48 h postinfection, and intracellular bacteria were enumerated by serial dilution and colony counts on LB agar plates. The results represent the averages and standard errors of three independent experiments. No significant differences in replication or survival were found using an unpaired t test.
DISCUSSION
In this study, we demonstrate that B. cenocepacia K56-2 produces hopanoids in a process that depends on squalene-hopene cyclase enzymes encoded by BCAM2831 and BCAS0167. GC-MS analysis demonstrated that complementation of Δshc with either shc gene restores the hopanoid-containing peak. However, complementation with pBCAS0167 produced a peak of much lower intensity than that produced by pBCAM2831. The addition of pBCAS0167 could not rescue any of the Δshc mutant phenotypes. As ΔBCAM2831 and ΔBCAS0167 produced levels of hopanoid that were similar to those produced by the wild type, this is likely a result of poor expression or improper regulation in the pBCAS0167 complementing plasmid. It is clear that BCAS0167 alone contributes to the membrane stability of B. cenocepacia, as deletion of both shc genes resulted in a mutant that exhibited greater sensitivity to all conditions tested than a mutant lacking only BCAM2831. Studies by Wendt et al. (73, 74) have determined that the functional Shc enzyme is a homodimer and a cytoplasmic integral membrane protein. Although several bacterial species contain two copies of the shc gene, each seemingly of different origin, all Shc enzymes studied to date occur in bacterial species that carry only one copy of the shc gene (64). As B. cenocepacia encodes 2 different Shc proteins, it is possible that the enzyme may function as both a homodimer and a heterodimer, producing different hopanoid derivatives depending on the enzyme composition. The hopanoid derivatives produced by each Shc may differ in their functions, as deletion of BCAS0167 had little effect on sensitivity to pH, SDS, or antibiotics but significantly affected swarming motility.
Unlike other membrane lipids, sterols pass across membranes on their own or using protein carriers (41, 42, 54). Sterol transporters in eukaryotic cells have several functions, including sterol export and maintenance of sterol asymmetry in the lipid bilayer (41, 42). Deletion of the putative hopanoid-associated transporter protein gene BCAM2828 did not always produce effects similar to, or as drastic as, those found in the Δshc strain, implying that hopanoids are likely capable of reaching their proper location in this mutant. Alternatively, other transporters could be involved, making BCAM2828 redundant. Thus, it is unclear whether BCAM2828 is directly involved in hopanoid-associated transport. The ΔBCAM2828 mutant was sensitive to several antibiotics; however, this phenotype may not be attributable to hopanoid function or localization. As a predicted resistance-nodulation-cell division family transporter, BCAM2828 may have other functions in B. cenocepacia, possibly acting as a multidrug efflux pump.
Burkholderia spp. are found in a wide variety of niches ranging from freshwater streams and sediments to the soil rhizosphere (47, 70, 71). They also interact with various plants, fungi, insects, and animal pathogens (38). These diverse interactions require that Burkholderia spp. be highly adaptable and able to compete for resources and to survive exposure to physical and chemical stresses (71). Exposure to pH stresses would certainly be encountered within and while transitioning between these environments, which would result in a change in the pH of the bacterial cytoplasm, compromising the function and integrity of cellular proteins (50, 75). To adjust the pH, the bacterial cell utilizes several transporters and efflux pumps, which move protons in and out of the cytoplasm (9, 16, 36, 51). Some bacteria may have other mechanisms to avoid the loss of these particles. Hopanoid concentrations are greatest in acidophiles and are found almost exclusively in membranes containing large proton gradients (19, 31, 59). Thus, hopanoids may be able to increase the packing of the hydrophobic centers in lipid bilayers, preventing the loss of protons as charged water (19). All but one of the Burkholderia isolates tested by Cvejic et al. contained an unsaturated hopanoid analogue, a feature traditionally found only in acid-tolerant acetic acid bacteria (11, 14). The presence of an m/z 189 ion in the hopanoid-containing peak of B. cenocepacia indicates that this species may also produce unsaturated hopanoid analogues, compounds that may also be important in low-pH tolerance.
Increased sensitivity of Gram-negative bacteria to detergents and antibiotics is indicative of membrane damage. The sensitivity of the Δshc mutant to SDS and several antibiotics implies that the absence of hopanoids affects the stability and permeability of the bacterial membrane. This effect is evident in the transmission electron microscopy images of the Δshc mutant, which clearly show areas of increased separation between the inner and outer membrane. The severe motility defects, due to the observed absence of flagella in the Δshc mutant, may be attributed to this defect in membrane structure, as the flagellar machinery spans the inner and outer membranes. However, flagella may be able to assemble and function under certain conditions in the mutant, as swimming motility occurs in Δshc, albeit at greatly decreased levels. The swarming motility defect in the Δshc strain was much more severe than the swimming motility defect. This is likely because swarming bacteria undergo significant morphological changes with regard to cell shape and hyperflagellation, which may not be possible in hopanoid-defective cells (21, 22, 43, 63). Though the assembly of the flagellum was compromised by the absence of hopanoids, not all membrane-associated structures were similarly affected. For example, every hopanoid-defective mutant, including the Δshc mutant, secreted proteases at levels similar to those of the wild type. In Burkholderia species, the secretion of these proteases depends on the cell envelope-spanning T2SS (46). The absence of hopanoids may not affect the function of this structure, because T2SS proteins are secreted in two steps, first being transported across the inner membrane into the periplasm and then being translocated across the outer membrane (26). It is unknown whether the functions of other B. cenocepacia secretion systems, such as the T4SS and T6SS, are affected by the absence of hopanoids.
Following uptake by macrophages, B. cenocepacia alters the normal phagosome maturation pathway by delaying acidification of the vacuole and preventing fusion with lysosomes (33). However, the pH of the B. cenocepacia-containing vacuole (BcCV) drops considerably during maturation (33), and because the Δshc mutant is sensitive to low pH, we hypothesized that this may affect the ability of the bacteria to survive and replicate within macrophages. However, our macrophage infection revealed that a Δshc mutant replicates and survives within RAW264.7 macrophages at levels similar to those of the parental strain. Though it is possible that intracellular bacteria have an altered hopanoid content and/or localization, other mechanisms may operate in the bacterial adaptation to the intracellular environment. Furthermore, the pH of the BcCV may not decrease to a level that is sufficient to alter the replication and survival of the Δshc or ΔBCAM2828 mutant.
Due to the intrinsic antibiotic resistance of Bcc species, there is a pressing need to develop new ways of treating infections, particularly in CF patients (1, 5, 8, 15, 45). The increased antibiotic sensitivity of the Δshc mutant raises the possibility of utilizing Shc inhibitors, in combination with current antibiotic treatments.
In conclusion, we provide evidence that hopanoids may be important in stabilizing the B. cenocepacia membrane, as well as regulating its fluidity and permeability. It remains uncertain whether these effects are a direct or indirect effect of hopanoid loss, as the absence of hopanoid production may change the protein content or architecture of the bacterial membrane. Further characterization of hopanoid biosynthesis and hopanoid-related cell biology is required to better understand their role in the lifestyle of B. cenocepacia.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Aubert and M. S. Saldías for critical review of the manuscript and J. E. Sholdice of the Department of Microbiology and Immunology Transmission Electron Microscopy Facility for performing the transmission electron microscopy.
This work was supported by grants from Cystic Fibrosis Canada to M.A.V. and from the Natural Sciences and Engineering Research Council of Canada to M.A.B. C.L.S. is supported by a postdoctoral fellowship from Cystic Fibrosis Canada. M.A.V. holds a Canada Research Chair in Infectious Diseases and Microbial Pathogenesis.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Aaron S. D., Ferris W., Henry D. A., Speert D. P., Macdonald N. E. 2000. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 161:1206–1212 [DOI] [PubMed] [Google Scholar]
- 2. Begley M., Gahan C. G., Hill C. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625–651 [DOI] [PubMed] [Google Scholar]
- 3. Bernier S. P., Sokol P. A. 2005. Use of suppression-subtractive hybridization to identify genes in the Burkholderia cepacia complex that are unique to Burkholderia cenocepacia. J. Bacteriol. 187:5278–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berry A. M., et al. 1993. Hopanoid lipids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase. Proc. Natl. Acad. Sci. U. S. A. 90:6091–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burns J. L., Wadsworth C. D., Barry J. J., Goodall C. P. 1996. Nucleotide sequence analysis of a gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob. Agents Chemother. 40:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardona S. T., Valvano M. A. 2005. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 54:219–228 [DOI] [PubMed] [Google Scholar]
- 7. Chaparro C., et al. 2001. Infection with Burkholderia cepacia in cystic fibrosis: outcome following lung transplantation. Am. J. Respir. Crit. Care Med. 163:43–48 [DOI] [PubMed] [Google Scholar]
- 8. Coenye T., LiPuma J. J. 2003. Molecular epidemiology of Burkholderia species. Front. Biosci. 8:e55–e67 [DOI] [PubMed] [Google Scholar]
- 9. Cotter P. A., Chepuri V., Gennis R. B., Gunsalus R. P. 1990. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J. Bacteriol. 172:6333–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Craig F. F., Coote J. G., Parton R., Freer J. H., Gilmour N. J. 1989. A plasmid which can be transferred between Escherichia coli and Pasteurella haemolytica by electroporation and conjugation. J. Gen. Microbiol. 135:2885–2890 [DOI] [PubMed] [Google Scholar]
- 11. Cvejic J. H., et al. 2000. Bacterial triterpenoids of the hopene series as biomarkers for the chemotaxonomy of Burkholderia, Pseudomonas and Ralstonia spp. FEMS Microbiol. Lett. 183:295–299 [DOI] [PubMed] [Google Scholar]
- 12. Figurski D. H., Helinski D. R. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flannagan R. S., Linn T., Valvano M. A. 2008. A system for the construction of targeted unmarked gene deletions in the genus Burkholderia. Environ. Microbiol. 10:1652–1660 [DOI] [PubMed] [Google Scholar]
- 14. Flesch G., Rohmer M. 1988. Prokaryotic hopanoids: the biosynthesis of the bacteriohopane skeleton. Formation of isoprenic units from two distinct acetate pools and a novel type of carbon/carbon linkage between a triterpene and D-ribose. Eur. J. Biochem. 175:405–411 [DOI] [PubMed] [Google Scholar]
- 15. Gold R., Jin E., Levison H., Isles A., Fleming P. C. 1983. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J. Antimicrob. Chemother. 12(Suppl. A):331–336 [DOI] [PubMed] [Google Scholar]
- 16. Goldberg E. B., et al. 1987. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 84:2615–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grimwood K., Kidd T. J., Tweed M. 2009. Successful treatment of cepacia syndrome. J. Cyst. Fibros. 8:291–293 [DOI] [PubMed] [Google Scholar]
- 18. Guglierame P., et al. 2006. Efflux pump genes of the resistance-nodulation-division family in Burkholderia cenocepacia genome. BMC Microbiol. 6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haines T. H. 2001. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 40:299–324 [DOI] [PubMed] [Google Scholar]
- 20. Hamad M. A., Skeldon A. M., Valvano M. A. 2010. Construction of aminoglycoside-sensitive Burkholderia cenocepacia strains for use in studies of intracellular bacteria with the gentamicin protection assay. Appl. Environ. Microbiol. 76:3170–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harshey R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249–273 [DOI] [PubMed] [Google Scholar]
- 22. Harshey R. M. 1994. Bees aren't the only ones: swarming in gram-negative bacteria. Mol. Microbiol. 13:389–394 [DOI] [PubMed] [Google Scholar]
- 23. Härtner T., Straub K. L., Kannenberg E. 2005. Occurrence of hopanoid lipids in anaerobic Geobacter species. FEMS Microbiol. Lett. 243:59–64 [DOI] [PubMed] [Google Scholar]
- 24. Huber B., et al. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147:2517–2528 [DOI] [PubMed] [Google Scholar]
- 25. Jahnke L. L., Stan-Lotter H., Kato K., Hochstein L. I. 1992. Presence of methyl sterol and bacteriohopanepolyol in an outer-membrane preparation from Methylococcus capsulatus (Bath). J. Gen. Microbiol. 138:1759–1766 [DOI] [PubMed] [Google Scholar]
- 26. Johnson T. L., Abendroth J., Hol W. G., Sandkvist M. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255:175–186 [DOI] [PubMed] [Google Scholar]
- 27. Jürgens U. J., Simonin P., Rohmer M. 1992. Localization and distribution of hopanoids in membrane systems of the cyanobacterium Synechocystis PCC 6714. FEMS Microbiol. Lett. 71:285–288 [DOI] [PubMed] [Google Scholar]
- 28. Kannenberg E., Poralla K., Blume A. 1980. A hopanoid from the thermo-acidophilic Bacillus acidocaldarius condenses membranes. Naturwissenschaften 67:458–459 [Google Scholar]
- 29. Kannenberg E. L., Poralla K. 1999. Hopanoid biosynthesis and function in bacteria. Naurwissenschaften 86:168–176 [Google Scholar]
- 30. Kazachkov M., Lager J., LiPuma J., Barker P. M. 2001. Survival following Burkholderia cepacia sepsis in a patient with cystic fibrosis treated with corticosteroids. Pediatr. Pulmonol. 32:338–340 [DOI] [PubMed] [Google Scholar]
- 31. Kleemann G., Kellner R., Poralla K. 1994. Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfur bacterium producing hopanoids and tetrahymanol. Biochim. Biophys. Acta 1210:317–320 [DOI] [PubMed] [Google Scholar]
- 32. Kuchta T., Kubinec R., Farkas P. 1998. Analysis of hopanoids in bacteria involved in food technology and food contamination. FEMS Microbiol. Lett. 159:221–225 [DOI] [PubMed] [Google Scholar]
- 33. Lamothe J., Huynh K. K., Grinstein S., Valvano M. A. 2007. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol. 9:40–53 [DOI] [PubMed] [Google Scholar]
- 34. Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109–117 [DOI] [PubMed] [Google Scholar]
- 35. Lewenza S., Visser M. B., Sokol P. A. 2002. Interspecies communication between Burkholderia cepacia and Pseudomonas aeruginosa. Can. J. Microbiol. 48:707–716 [DOI] [PubMed] [Google Scholar]
- 36. Lewinson O., Padan E., Bibi E. 2004. Alkali tolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. U. S. A. 101:14073–14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loutet S. A., Flannagan R. S., Kooi C., Sokol P. A., Valvano M. A. 2006. A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to antimicrobial peptides and bacterial survival in vivo. J. Bacteriol. 188:2073–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loutet S. A., Valvano M. A. 2010. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 78:4088–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahenthiralingam E., Urban T. A., Goldberg J. B. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144–156 [DOI] [PubMed] [Google Scholar]
- 40. Marolda C. L., Welsh J., Dafoe L., Valvano M. A. 1990. Genetic analysis of the O7-polysaccharide biosynthesis region from the Escherichia coli O7:K1 strain VW187. J. Bacteriol. 172:3590–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maxfield F. R., Wustner D. 2002. Intracellular cholesterol transport. J. Clin. Invest. 110:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mesmin B., Maxfield F. R. 2009. Intracellular sterol dynamics. Biochim. Biophys. Acta 1791:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moens S., Vanderleyden J. 1996. Functions of bacterial flagella. Crit. Rev. Microbiol. 22:67–100 [DOI] [PubMed] [Google Scholar]
- 44. Nash E. F., et al. 2010. Survival of Burkholderia cepacia sepsis following lung transplantation in recipients with cystic fibrosis. Transpl. Infect. Dis. 12:551–554 [DOI] [PubMed] [Google Scholar]
- 45. Nzula S., Vandamme P., Govan J. R. 2002. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J. Antimicrob. Chemother. 50:265–269 [DOI] [PubMed] [Google Scholar]
- 46. O'Grady E. P., Nguyen D. T., Weisskopf L., Eberl L., Sokol P. A. 2011. The Burkholderia cenocepacia LysR-type transcriptional regulator ShvR influences expression of quorum-sensing, protease, type II secretion, and afc genes. J. Bacteriol. 193:163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olapade O. A., Gao X., Leff L. G. 2005. Abundance of three bacterial populations in selected streams. Microb. Ecol. 49:461–467 [DOI] [PubMed] [Google Scholar]
- 48. Ortega X. P., et al. 2007. A putative gene cluster for aminoarabinose biosynthesis is essential for Burkholderia cenocepacia viability. J. Bacteriol. 189:3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ourisson G., Rohmer M., Poralla K. 1987. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu. Rev. Microbiol. 41:301–333 [DOI] [PubMed] [Google Scholar]
- 50. Padan E., Bibi E., Ito M., Krulwich T. A. 2005. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717:67–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Padan E., Maisler N., Taglicht D., Karpel R., Schuldiner S. 1989. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J. Biol. Chem. 264:20297–20302 [PubMed] [Google Scholar]
- 52. Pearson A., et al. 2009. Diversity of hopanoids and squalene-hopene cyclases across a tropical land-sea gradient. Environ. Microbiol. 11:1208–1223 [DOI] [PubMed] [Google Scholar]
- 53. Perzl M., et al. 1998. Cloning of conserved genes from Zymomonas mobilis and Bradyrhizobium japonicum that function in the biosynthesis of hopanoid lipids. Biochim. Biophys. Acta 1393:108–118 [DOI] [PubMed] [Google Scholar]
- 54. Phillips M. C., Johnson W. J., Rothblat G. H. 1987. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim. Biophys. Acta 906:223–276 [DOI] [PubMed] [Google Scholar]
- 55. Poralla K., Kannenberg E., Blume A. 1980. A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett. 113:107–110 [DOI] [PubMed] [Google Scholar]
- 56. Poralla K., Muth G., Hartner T. 2000. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 189:93–95 [DOI] [PubMed] [Google Scholar]
- 57. Rohmer M., Bouvier-Nave P., Ourisson G. 1984. Distribution of hopanoid triterpenes in prokaryotes. J. Gen. Microbiol. 130:1137–1150 [Google Scholar]
- 58. Ruiz N., Falcone B., Kahne D., Silhavy T. J. 2005. Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121:307–317 [DOI] [PubMed] [Google Scholar]
- 59. Sahm H., Rohmer M., Bringer-Meyer S., Sprenger G. A., Welle R. 1993. Biochemistry and physiology of hopanoids in bacteria. Adv. Microb. Physiol. 35:247–273 [DOI] [PubMed] [Google Scholar]
- 60. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 61. Schägger H., von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 62. Seipke R. F., Loria R. 2009. Hopanoids are not essential for growth of Streptomyces scabies 87-22. J. Bacteriol. 191:5216–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shapiro J. A. 1998. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 52:81–104 [DOI] [PubMed] [Google Scholar]
- 64. Siedenburg G., Jendrossek D. 2011. Squalene-hopene cyclases. Appl. Environ. Microbiol. 77:3905–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simonin P., Jurgens U. J., Rohmer M. 1996. Bacterial triterpenoids of the hopane series from the prochlorophyte Prochlorothrix hollandica and their intracellular localization. Eur. J. Biochem. 241:865–871 [DOI] [PubMed] [Google Scholar]
- 66. Sokol P. A., Ohman D. E., Iglewski B. H. 1979. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J. Clin. Microbiol. 9:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Speert D. P. 2002. Advances in Burkholderia cepacia complex. Paediatr. Respir. Rev. 3:230–235 [DOI] [PubMed] [Google Scholar]
- 68. Vandamme P., et al. 2003. Burkholderia cenocepacia sp. nov.—a new twist to an old story. Res. Microbiol. 154:91–96 [DOI] [PubMed] [Google Scholar]
- 69. Vandamme P., et al. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188–1200 [DOI] [PubMed] [Google Scholar]
- 70. Vermis K., Brachkova M., Vandamme P., Nelis H. 2003. Isolation of Burkholderia cepacia complex genomovars from waters. Syst. Appl. Microbiol. 26:595–600 [DOI] [PubMed] [Google Scholar]
- 71. Vial L., Chapalain A., Groleau M. C., Deziel E. 2011. The various lifestyles of the Burkholderia cepacia complex species: a tribute to adaptation. Environ. Microbiol. 13:1–12 [DOI] [PubMed] [Google Scholar]
- 72. Welander P. V., et al. 2009. Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 191:6145–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wendt K. U., Lenhart A., Schulz G. E. 1999. The structure of the membrane protein squalene-hopene cyclase at 2.0 A resolution. J. Mol. Biol. 286:175–187 [DOI] [PubMed] [Google Scholar]
- 74. Wendt K. U., Poralla K., Schulz G. E. 1997. Structure and function of a squalene cyclase. Science 277:1811–1815 [DOI] [PubMed] [Google Scholar]
- 75. Wilks J. C., Slonczewski J. L. 2007. pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 189:5601–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.