Abstract
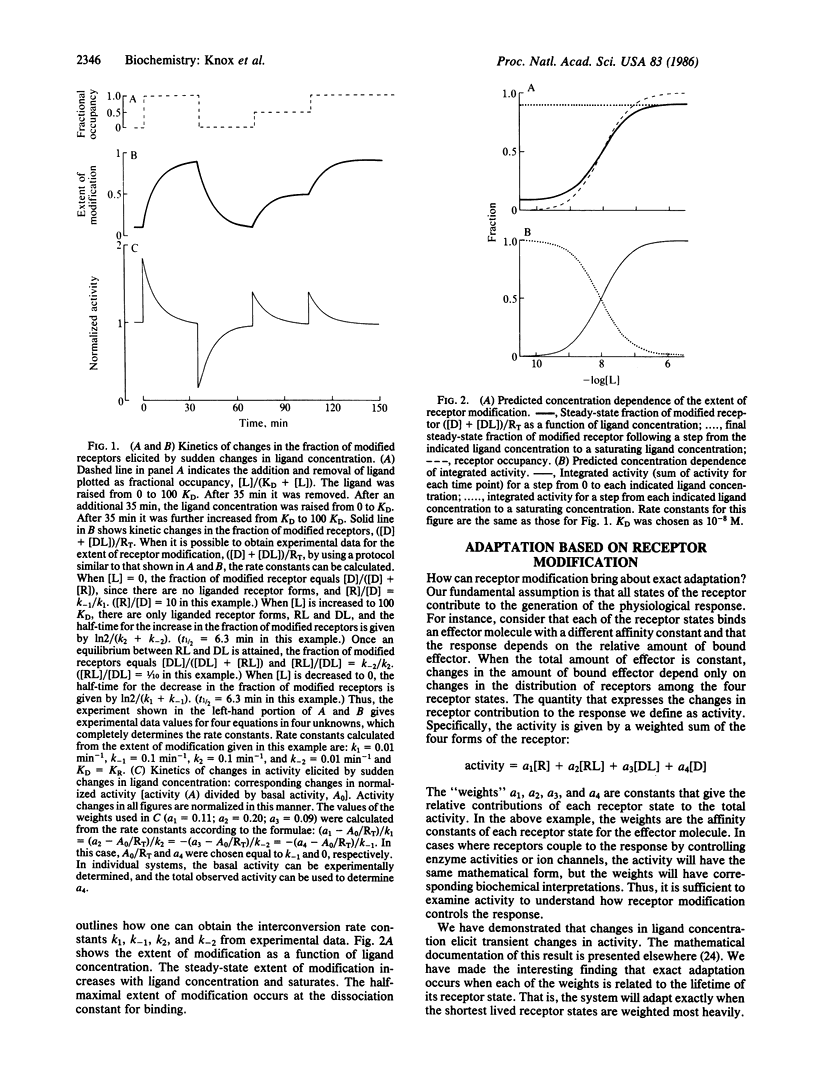
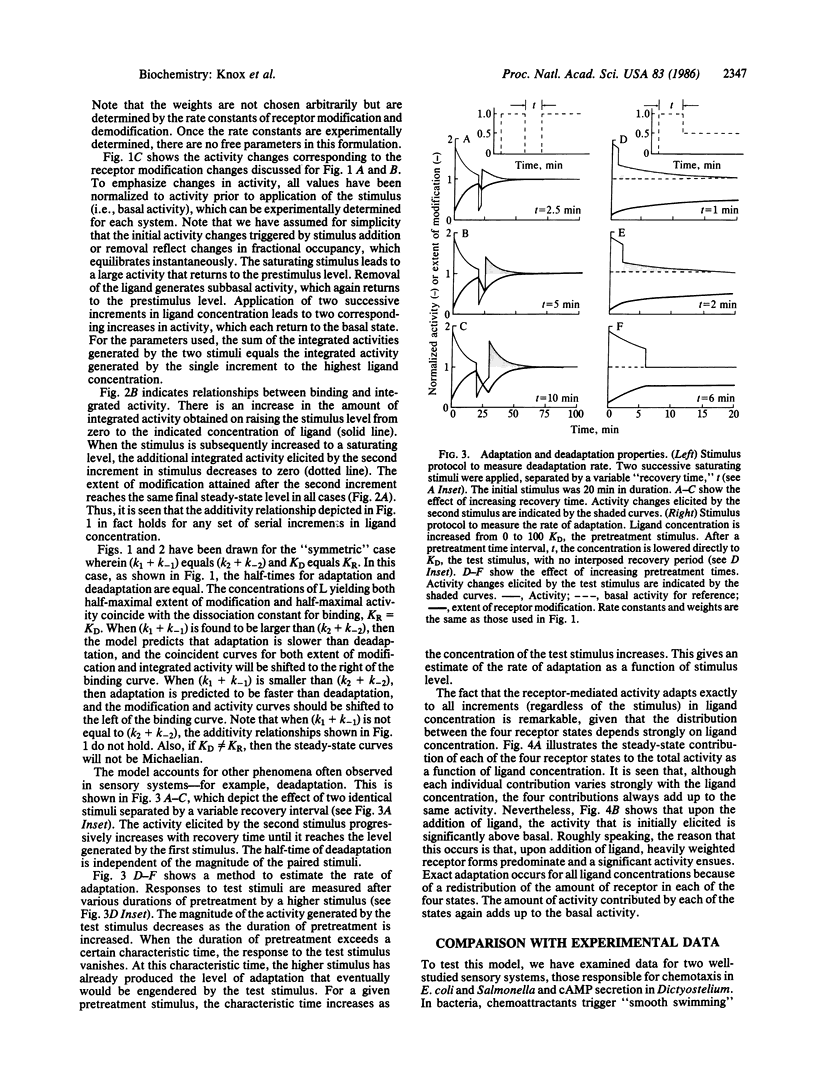
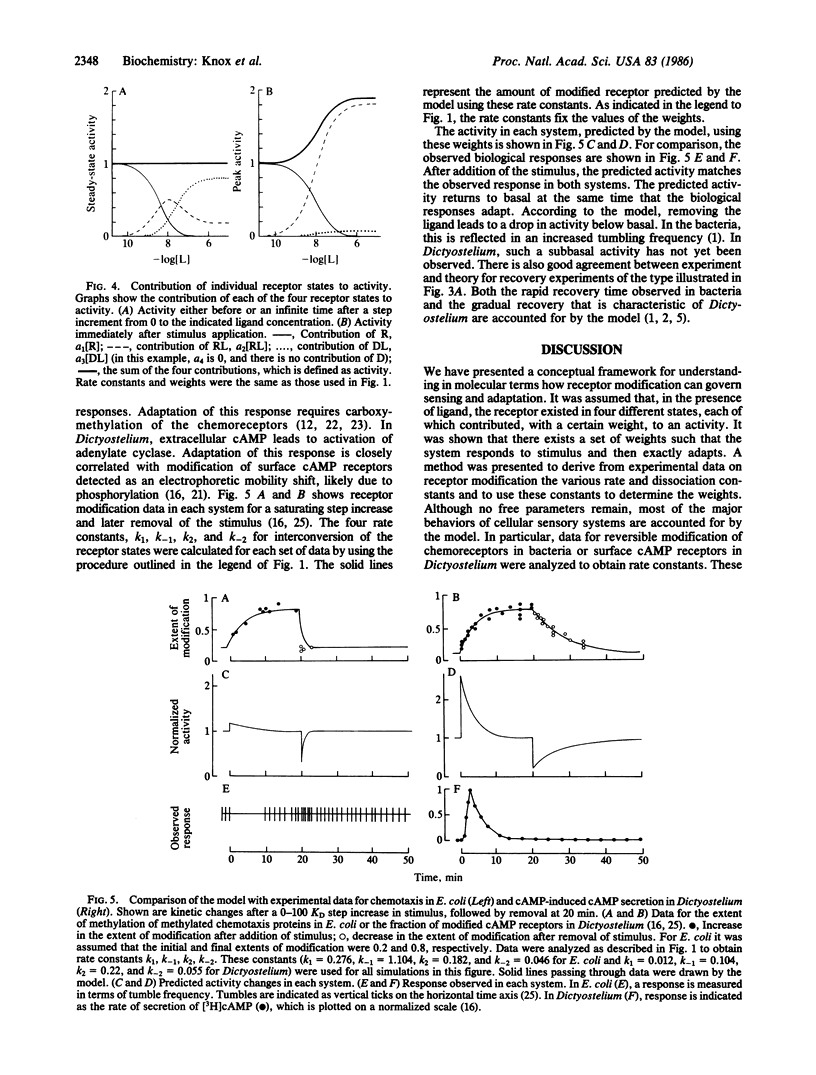
Physiological responses mediated by cell-surface receptors frequently adapt or "desensitize" (i.e., terminate despite persistent occupancy of receptors by ligand). Binding of ligands to the external domains of a wide variety of surface receptors induces covalent modification of their cytoplasmic domains. A mechanism is presented in which the variety of receptor states generated by ligand binding and covalent modification act together to regulate physiological responsiveness. The development of the model is guided by observations of adaptation for chemotaxis in Escherichia coli and adenylate cyclase activation in Dictyostelium. The general features of the marked response and eventual exact adaptation predicted by the model match those observed in the experimental systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Tedesco P. M. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Adaptation kinetics in bacterial chemotaxis. J Bacteriol. 1983 Apr;154(1):312–323. doi: 10.1128/jb.154.1.312-323.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrow S. A., Cohen S., Garbers D. L., Staros J. V. Characterization of the interaction of 5'-p-fluorosulfonylbenzoyl adenosine with the epidermal growth factor receptor/protein kinase in A431 cell membranes. J Biol Chem. 1983 Jun 25;258(12):7824–7827. [PubMed] [Google Scholar]
- Devreotes P. N., Sherring J. A. Kinetics and concentration dependence of reversible cAMP-induced modification of the surface cAMP receptor in Dictyostelium. J Biol Chem. 1985 May 25;260(10):6378–6384. [PubMed] [Google Scholar]
- Devreotes P. N., Steck T. L. Cyclic 3',5' AMP relay in Dictyostelium discoideum. II. Requirements for the initiation and termination of the response. J Cell Biol. 1979 Feb;80(2):300–309. doi: 10.1083/jcb.80.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Steck T. L., Devreotes P. N. Cyclic 3',5'-AMP relay in Dictyostelium discoideum IV. Recovery of the cAMP signaling response after adaptation to cAMP. J Cell Biol. 1980 Aug;86(2):545–553. doi: 10.1083/jcb.86.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Steck T. L., Devreotes P. N. Cyclic 3',5'-AMP relay in Dictyostelium discoideum V. Adaptation of the cAMP signaling response during cAMP stimulation. J Cell Biol. 1980 Aug;86(2):554–561. doi: 10.1083/jcb.86.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Koshland D. E., Jr Simple molecular model for sensing and adaptation based on receptor modification with application to bacterial chemotaxis. J Mol Biol. 1982 Nov 5;161(3):395–416. doi: 10.1016/0022-2836(82)90246-7. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K., Su Y. F., Perkins J. P. Catecholamine-induced desensitization involves an uncoupling of beta-adrenergic receptors and adenylate cyclase. J Cyclic Nucleotide Res. 1979;5(2):99–106. [PubMed] [Google Scholar]
- Hudspeth A. J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Klein C., Lubs-Haukeness J., Simons S. cAMP induces a rapid and reversible modification of the chemotactic receptor in Dictyostelium discoideum. J Cell Biol. 1985 Mar;100(3):715–720. doi: 10.1083/jcb.100.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Revello P. T. Sensory adaptation mutants of E. coli. Cell. 1978 Dec;15(4):1221–1230. doi: 10.1016/0092-8674(78)90048-x. [DOI] [PubMed] [Google Scholar]
- Russo A. F., Koshland D. E., Jr Separation of signal transduction and adaptation functions of the aspartate receptor in bacterial sensing. Science. 1983 Jun 3;220(4601):1016–1020. doi: 10.1126/science.6302843. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979 Jul 26;280(5720):279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Quantitation of the sensory response in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Feb;72(2):710–713. doi: 10.1073/pnas.72.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Lavin T. N., Heald S. L., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase. Structural alterations in the beta-adrenergic receptor revealed by photoaffinity labeling. J Biol Chem. 1982 Aug 25;257(16):9242–9245. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem. 1980 Aug 10;255(15):7410–7419. [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J. Sensory adaptation of leukocytes to chemotactic peptides. J Cell Biol. 1979 Aug;82(2):517–527. doi: 10.1083/jcb.82.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]


