Abstract
Remodeling of uterine spiral arteries by trophoblast cells is a requisite process for hemochorial placentation and successful pregnancy. The rat exhibits deep intrauterine trophoblast invasion and accompanying trophoblast-directed vascular modification. The involvement of phosphatidylinositol 3 kinase (PI3K), AKT, and Fos-like antigen 1 (FOSL1) in regulating invasive trophoblast and hemochorial placentation was investigated using Rcho-1 trophoblast stem cells and rat models. Disruption of PI3K/AKT with small-molecule inhibitors interfered with the differentiation-dependent elaboration of a signature invasive-vascular remodeling trophoblast gene expression profile and trophoblast invasion. AKT isoform-specific knockdown also affected the signature invasive-vascular remodeling trophoblast gene expression profile. Nuclear FOSL1 increased during trophoblast cell differentiation in a PI3K/AKT-dependent manner. Knockdown of FOSL1 disrupted the expression of a subset of genes associated with the invasive-vascular remodeling trophoblast phenotype, including the matrix metallopeptidase 9 gene (Mmp9). FOSL1 was shown to occupy regions of the Mmp9 promoter in trophoblast cells critical for the regulation of Mmp9 gene expression. Inhibition of FOSL1 expression also abrogated trophoblast invasion, as assessed in vitro and following in vivo trophoblast-specific lentivirally delivered FOSL1 short hairpin RNA (shRNA). In summary, FOSL1 is a key downstream effector of the PI3K/AKT signaling pathway responsible for development of trophoblast lineages integral to establishing the maternal-fetal interface.
INTRODUCTION
Hemochorial placental development is characterized by close contact between maternal and fetal tissues and occurs in primates and rodents such as the rat and mouse. Trophoblast cells are the functional units of the placenta. One of the key activities of trophoblast cells is remodeling uterine spiral arteries. Specific lineages of trophoblast cells exit the placenta and enter into the uterine parenchyma, where they interact with the vasculature. Vascular remodeling transforms tightly coiled uterine spiral arteries into dilated vessels that are no longer under maternal control (45, 63). Restructuring maternal vasculature is essential for the optimal delivery of nutrients to the fetus. Poor trophoblast invasion is linked to miscarriage, preeclampsia, preterm birth, and fetal growth restriction (36, 45, 63). Hemochorial placentation and especially trophoblast-directed vascular remodeling in the human and the rat are remarkably similar (3, 14, 49, 63, 76). In both species, invasive trophoblast cells penetrate into the uterus through both endovascular and interstitial routes.
In the rat, invasive trophoblast cells arise from the junctional zone and move into the uterus in two waves. The first wave is initiated during midgestation and consists of trophoblast cell invasion into spiral arteries situated within the central area of the uterine mesometrial implantation site (3). These invasive endovascular trophoblast cells effectively replace the maternal endothelium (3, 68). The depth of entry into the uterus is generally restricted to the decidua basalis. The second wave of trophoblast cell invasion begins around gestation day 14.5, exhibiting both endovascular and interstitial courses of entry and characterized by deep penetration into the metrial gland (3, 14, 49, 76). Signaling pathways controlling trophoblast invasion and vascular remodeling are not well understood.
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway has been implicated as a potential regulator of trophoblast invasion. PI3K responds to various extracellular signals, leading to the activation of a serine/threonine kinase termed AKT (13, 15, 29). AKT has a wide range of substrates involved in many cellular processes, including metabolism, cell cycle, survival, protein synthesis, and differentiation (29). There are three AKT isoforms in mammals, AKT1, AKT2, and AKT3 (12). These enzymes possess shared and potentially unique substrate specificities (12, 22). Null mouse models for the three AKT isoforms exhibit a range of phenotypes, characterized by disruptions in placentation, fetal growth, and/or postnatal growth and metabolism (19, 20, 27, 84, 85). PI3K/AKT becomes constitutively activated upon differentiation of trophoblast stem (TS) cells (43) and regulates a set of genes associated with an invasive-vascular remodeling trophoblast phenotype (43, 46, 65, 66, 74). Among these PI3K/AKT-regulated genes is the matrix metallopeptidase 9 gene (Mmp9). MMP9 is a proteolytic enzyme involved in the breakdown of extracellular matrix and tissue remodeling (47, 61). In trophoblast cells, MMP9 is proinvasive (11, 53, 55, 62, 66) and also participates in the regulation of cancer cell invasion and metastasis (23, 47). Downstream molecular mechanisms underlying PI3K/AKT regulation of trophoblast cell invasion have not been elucidated.
Fos-like antigen 1 (FOSL1) is a potential downstream target/effector of the PI3K/AKT signaling pathway leading to the activation of cell invasion (16, 18, 67). FOSL1 is a member of the basic region-leucine zipper transcription factor family and a contributor to the formation of the activator protein-1 (AP-1) transcription factor complex (28). In the human placenta, FOSL1 is expressed in several trophoblast cell types, including the extravillous/invasive trophoblast cell population (9, 57). Fosl1 is also expressed in differentiating rat trophoblast cells (46). Mutant mice possessing a null mutation at the Fosl1 locus die in utero due to placental defects (72). FOSL1 represents a candidate mediator of the PI3K/AKT signaling pathway in trophoblast cells.
In this study, we investigated the involvement of PI3K/AKT and FOSL1 signaling in the regulation of trophoblast cell differentiation, especially the acquisition of the invasive phenotype. In vitro and in vivo research strategies were performed utilizing Rcho-1 TS cells (30, 69) and trophoblast-specific lentivirally mediated gene manipulation (52), respectively. Collectively, the experimental findings demonstrate that PI3K/AKT and FOSL1 participate in a signal transduction pathway controlling a proinvasive/provascular remodeling trophoblast cell phenotype.
MATERIALS AND METHODS
Animals and tissue collection.
Holtzman Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). Animals were housed in an environmentally controlled facility with lights on from 0600 to 2000 h and were allowed free access to food and water. Timed pregnancies were generated by cohabitation of male and female rats. The presence of a seminal plug or sperm in the vaginal smear was designated day 0.5 of pregnancy. Rat placental tissues were collected on gestation days 7.5, 11.5, and 18.5. Tissues for histological analysis were frozen in dry ice-cooled heptane and stored at −80°C until processing. Tissue samples for RNA or protein extraction were frozen in liquid nitrogen and stored at −80°C until processing. Female rats were made pseudopregnant by mating with vasectomized males. The presence of seminal plugs was designated day 0.5 of pseudopregnancy. The University of Kansas Animal Care and Use Committee approved protocols for the care and use of animals.
Maintenance of the Rcho-1 TS cells.
Rcho-1 TS cells were maintained at subconfluent conditions in stem cell medium (RPMI-1640 culture medium [Cellgro, Herndon, VA] supplemented with 20% fetal bovine serum [FBS; Atlanta Biologicals, Norcross, GA], 50 μM 2-mercaptoethanol [Sigma-Aldrich, St. Louis, MO], 1 mM sodium pyruvate [Cellgro], 100 μM penicillin, and 100 U/ml streptomycin [Cellgro]), as previously reported (69). Differentiation was induced by growing cells to near confluence and then replacing the stem cell medium with differentiation medium (NCTC-135 medium supplemented with 1% horse serum [HS; Atlanta Biologicals], 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 10 mM HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [Fisher, Pittsburgh, PA], 38 mM sodium bicarbonate [Fisher], 100 μM penicillin, and 100 U/ml streptomycin [Cellgro]). High cell density and the absence of sufficient growth stimulatory factors (removal of FBS) facilitate trophoblast cell differentiation (69). Trypsin-EDTA (EDTA, in Hanks' balanced salt solution [Cellgro]) was used to passage the cells. Cells in the stem cell condition were grown in stem cell medium and collected 24 h after subculture to restrict the accumulation of spontaneously differentiating cells. Cells in the differentiation condition were grown for 8 days in differentiation medium prior to harvesting unless otherwise noted.
Inhibition of PI3K and AKT.
LY294002 (LY; Calbiochem, La Jolla, CA) was used to inhibit PI3K (77). AKT inhibitor IX (Calbiochem) was used to inhibit AKT activities (42). Rcho-1 TS cells were grown to near confluence and then shifted to differentiation medium containing vehicle (0.1% final concentration of dimethyl sulfoxide [DMSO]), LY (10 μM), or AKT inhibitor IX (10 μM). Cells were harvested after 8 days of treatment. Culture medium and small-molecule inhibitors were replaced daily.
Western blotting.
Whole-cell lysates were prepared in radioimmunoprecipitation buffer (10 mM Tris-HCl, pH 7.2, 1% Triton X-100 or 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml aprotinin) or in cell lysate buffer (Cell Signaling Technology, Danvers, MA). Nuclear lysates were prepared as previously described (24) using an extraction buffer consisting of 25 mM HEPES, pH 7.5, 1 mM dithiothreitol (DTT), 10% glycerol, 0.5 M KCl, 0.5 mM PMSF, 10 mM β-glycerophosphate, 2 mM sodium orthovanadate, 1 mM sodium pyrophosphate, and 10 mM sodium fluoride. Whole-cell lysates or nuclear lysates were fractionated by SDS-PAGE. Separated proteins were electrophoretically transferred to nitrocellulose. Filters were probed with the designated antibodies overnight at 4°C. Antibodies specific for pan-AKT, phospho-Ser 473 AKT, AKT1, and AKT2 were obtained from Cell Signaling Technology. Antibodies to FOSL1 and TATA box binding protein (TBP) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody for ACTB (clone AC-15) was obtained from Sigma-Aldrich. The following antibodies were used at the dilutions indicated (in parentheses): pan-AKT (1:2,000), phospho-Ser 473 AKT (1:2,000), AKT1 (1:1,000), AKT2 (1:1,000), FOSL1 (1:1,000), TBP (1:2,000), and ACTB (1:8,000). Blots were incubated with horseradish peroxidase-conjugated antibodies to rabbit (Cell Signaling Technology) or mouse (Sigma-Aldrich) IgG for 1 h at room temperature. Reaction products were visualized by incubation with enhanced chemiluminescence according to the manufacturer's instructions (ECL, Amersham Pharmacia, Piscataway, NJ).
AKT kinase assay.
The activity of AKT was measured in Rcho-1 TS cell lysates using an AKT kinase assay kit (Cell Signaling Technology). AKT activity was measured in whole-cell lysates from Rcho-1 TS cells treated with LY, AKT inhibitor IX, or vehicle control. The assay was performed according to the manufacturer's instructions. Briefly, phospho-AKT (Ser 473) was immunoprecipitated from 400 μg of Rcho-1 TS cell lysates. Kinase activity was assessed from the immunoprecipitates in the presence of ATP (200 μM) and glycogen synthase kinase 3α/β (GSK3α/β)–glutathione S-transferase fusion protein (1 μg). Phosphorylation (P) of the GSK3α/β fusion protein substrate was identified by Western blotting and represented a measure of AKT kinase activity.
Quantitative reverse transcription-PCR (qRT-PCR).
RNA samples were extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNAs were reverse transcribed from RNA by using reagents from Promega (Madison, WI) according to the manufacturer's instructions. SYBR green PCR master mix (Applied Biosystems, Foster City, CA) was used in the PCR. Reactions were processed using a 7500 real-time PCR system (Applied Biosystems). Conditions included an initial holding stage (50°C for 2 min and 95°C for 10 min) and 40 cycles (95°C for 15 s and 60°C for 1 min) followed by a dissociation stage (95°C for 15 s, 60°C for 1 min, and then 95°C for 15 s). Primers are provided in Table 1. The comparative cycle threshold method (ΔΔCT) was used for relative quantification of the amount of mRNA for each sample normalized to 18S RNA. Values are presented relative to controls for each gene.
Table 1.
qRT-PCR primer sequences
| Gene | GenBank accession no. | Forward primer | Reverse primer |
|---|---|---|---|
| Akt1 | NM_033230 | GGCACCTTTATTGGCTACAAG | AGCACCTGAGTTGTCACTG |
| Akt2 | NM_017093 | TGGCAGGATGTGGTACAGAA | AGGAGAACTGGGGGAAGTGT |
| Akt3 | NM_031575 | TGGTTCGAGAGAAGGCAAGT | ACAGCTCTCCCCCATTAACA |
| Adm | NM_012715 | ACGTCTCGGACTTTCTGCTT | GCTGCTGGACGCTTGTAGTT |
| Cgm4 | NM_012525 | TAGCCCGATACAGAACAGCAA | AGGGTCACAGCATGAGGAAA |
| Ctsd | NM_134334 | TACCTGAACGTCACCCGAAA | CAGGCTGGACACCTTCTCAC |
| Faslg | NM_012908 | TCTGGTTGGAATGGGGTTAG | CTTGGCTTTTTGGTTTCAGAG |
| Fosl1 | NM_012953 | ATCCCCGACCTCTGACCTAT | CAAGGCGTTCCTTCTGCTT |
| Igf2 | NM_031511 | GGAAGTCGATGTTGGTGCTT | CTTGCCCACGGGGTATCT |
| Il17f | NM_001015011 | CAAAACCAGGGCATTTCTGT | GACCAGGATTTCTTGCTGGA |
| Mmp9 | NM_031055 | AACTTCGACGCTGACAAGAA | TTTAGAGCCACGACCATACAGA |
| Prl4a1 | NM_017036 | GACCACCAGATGCCACACTT | CAGGAGCTTTATGTTTGATTCCTT |
| Sema6d | NM_001107768 | GGCCAGTGATGCTGTCATTT | TATGTTCCACGGCGATTTCT |
| Serpine1 | NM_012620 | AGTCTTTCCGACCAAGAGCA | GTGCCGAACCACAAAGAGAA |
| 18S | M11188 | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA |
Matrigel invasion assay.
The invasive ability of Rcho-1 TS cells were measured as previously described (62). Rcho-1 TS cells were differentiated for 6 days, trypsinized, and placed on Matrigel invasion chambers (BD Biosciences, San Jose, CA) at a density of 2.5 × 104 cells per chamber. Cells were initially plated in stem cell medium and then switched to differentiation medium and allowed to invade for 72 h. After 72 h, membranes were collected and stained with Diff-Quick (Dade Behring, Newark, DE). Invading cells were visualized by light microscopy and counted for each sample.
Gelatin zymography.
MMP9 activity was measured in Rcho-1 TS cell-conditioned medium as previously described (62). Conditioned medium was normalized to cell number and run on 8% polyacrylamide gels containing 1 mg/ml porcine gelatin. Gels were washed (wash buffer: 50 mM Tris, pH 8.0, 5 mM calcium chloride, 1 μM zinc chloride, and 0.5% Triton X-100) and incubated with wash buffer without Triton X-100. Proteins were stained using Coomassie staining solution (45% methanol, 10% acetic acid, and 0.2% Coomassie brilliant blue R-250) and incubated in destaining solution (12% methanol and 7% acetic acid).
Immunohistochemistry.
Immunohistochemical analyses were used to localize proteins in placental tissues. Ten-micrometer cryosections of placentation sites were prepared and stored at −80°C until use. Sections were fixed in ice-cold 4% paraformaldehyde and blocked in 10% normal goat serum for 30 min at room temperature. Primary antibody incubation was for 1 h at room temperature with antibodies specific for FOSL1 (dilution of 1:50; Santa Cruz Biotechnology), prolactin family 3, subfamily D, member 1 (PRL3D1, also called placental lactogen-I [PL-I]; 1:50 dilution) (35), prolactin family 3, subfamily B, member 1 (PRL3B1, also called PL-II; 1:50 dilution) (25), or pan-cytokeratin (1:300; Sigma-Aldrich). Sections were incubated with secondary antibodies for 30 min at room temperature. For bright-field immunohistochemical analysis, a secondary goat anti-rabbit antibody conjugated with biotin (Sigma-Aldrich) was used. Avidin-peroxidase was added for 30 min at room temperature followed by color development with an AEC kit (Zymed Laboratories, San Francisco, CA). Tissues were counterstained with Mayer's hematoxylin. For fluorescence detection, a secondary goat anti-rabbit antibody conjugated with cyanine 3 bis-N-hydroxysuccinimide (NHS) ester (Cy3; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) or alternatively a secondary goat anti-mouse antibody conjugated with fluorescein isothiocyanate (FITC; Sigma-Aldrich) was used. Nuclei were visualized with DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes, Carlsbad, CA). Bright-field and fluorescence images were captured using a Leica MZFLIII stereomicroscope or a DMI 4000 microscope equipped with charge-coupled-device (CCD) cameras (Leica Microsystems GmbH, Welzlar, Germany).
shRNA constructs and production of lentivirus.
Akt short hairpin RNA (shRNA) constructs in the pLKO.1 vector were obtained from Open Biosystems (Huntsville, AL). Two Fosl1 shRNAs were designed and subcloned into the pLKO.1 vector by using AgeI and EcoRI restriction sites. Control shRNA that does not target any known mammalian gene, pLKO.1-shSCR (plasmid 1864), was obtained from Addgene (Cambridge, MA) (71). Sequences representing the sense target site for each of the shRNAs used in the analyses are as follows: Akt1, GCACATCAAGATAACGGACTT; Akt2, GATGGATCTTTCATTGGGTAT; Akt3, GCTCTTGATAAAGGATCCAAA; Fosl1 No. 1, GACAAGTTGGAGGATGAGAAAT; Fosl1 No. 2, GTTCCTCAGCCCATCGAAAGAGTA; Control-N, CCTAAGGTTAAGTCGCCCTCG. Third-generation lentiviral packaging vectors were acquired from Addgene and included the following: pMDLg/pRRE (plasmid 12251), pRSV-Rev (plasmid 12253), and pMD2.G (plasmid 12259). Lentiviral particles were produced as previously reported (52). To summarize, 293FT cells (Invitrogen, Carlsbad, CA) were transiently transfected using Lipofectamine 2000 (Invitrogen) with the following plasmids: shRNA containing transducing vector, third-generation packaging system plasmids (pMDLg/pRRE and pRSV-Rev) (26), and a vesicular stomatitis virus glycoprotein (VSVG) envelope plasmid (pMD2.G). After transfection, cells were maintained in 50% Dulbecco's modified Eagle's medium (DMEM) with high glucose (Cellgro), 45% Opti-MEM I (Invitrogen, Carlsbad, CA), and 5% FBS. Culture supernatants containing lentiviral particles were harvested every 24 h for 2 to 3 days. Supernatants were centrifuged to remove cell debris, filter sterilized, concentrated by ultracentrifugation, and stored at −80°C until used. Lentiviral vector titers were determined by measurement of p24 gag antigen by enzyme-linked immunosorbent assay (ELISA) (Advanced Bioscience Laboratories, Kensington, MD).
In vitro lentiviral transduction.
Rcho-1 TS cells were exposed to lentiviral particles, selected with puromycin dihydrochloride (Sigma-Aldrich; 2 μg/μl) for 2 to 4 days, and then maintained in a reduced concentration of the antibiotic (1 μg/μl). The puromycin selective pressure was removed during Rcho-1 TS cell differentiation.
In vivo lentiviral transduction.
Rat embryos were transduced with lentiviral particles as previously described (52). Briefly, rat embryos were collected on gestation day 4.5. Recovered blastocysts were washed with KSOM medium (Millipore). Zonae pellucidae were removed with Pronase (Sigma-Aldrich; 10 mg/ml for 10 min) and incubated with concentrated lentiviral particles (750 ng of p24/ml) for 4.5 h. Transduced blastocysts were transferred to uteri of day 3.5 pseudopregnant rats for subsequent evaluation of gene knockdown and placentation site phenotypes on gestation day 11.5.
Measurement of in vivo FOSL1 expression.
Placentation sites derived from embryos treated with control or Fosl1 shRNAs were processed for the detection of Fosl1 mRNA by qRT-PCR and FOSL1 protein by Western blotting and immunofluorescence analysis. Trophoblast dissections were performed as previously described (5) and qRT-PCR and Western blotting conducted as described above. FOSL1 expression levels detected on tissue sections with immunofluorescence were measured by calculating integrated density using Photoshop CS5 extended edition (Adobe Systems Inc., San Jose, CA). All values were normalized to control samples. Placentation sites exhibiting a decrease in FOSL1 protein expression were included for additional analyses. A decrease in FOSL1 protein expression was defined as FOSL1 protein expression 1 standard deviation below the mean of control samples.
Measurement of in vivo invasion.
Invasive trophoblast cells within placentation sites derived from embryos treated with control or Fosl1 shRNAs were monitored by immunostaining with antibodies to pancytokeratin. An invasion index was calculated by measuring the ratio between the depth of endovascular trophoblast invasion into the decidua and the total depth of the decidua by use of National Institutes of Health ImageJ software (Bethesda, MD). All values were normalized to control samples.
ChIP.
Chromatin immunoprecipitation (ChIP) analysis was performed according to a previously published procedure (50). Briefly, Rcho-1 TS cells stably transduced with control or Fosl1 shRNA-expressing lentivirus were grown to confluence in 150-mm dishes and differentiated for 8 days. Cells were then fixed with 1% formaldehyde, and purified nuclear lysates were sonicated on ice to prepare the DNA fragments at a size of approximately 500 bp. Lysates (300 μg of protein) were immunoprecipitated with 10 μg of FOSL1 antibodies (Santa Cruz Biotechnology). Rabbit IgG (BD Biosciences) was used as a nonspecific control. Immunoprecipitated chromatin fragments were eluted from agarose beads. DNA-protein interactions were reverse cross-linked and purified using a QiaQuick PCR purification kit (Qiagen). Purified DNA fragments were assessed by quantitative PCR (qPCR) using Mmp9 promoter-specific primers and SYBR green PCR master mix (Applied Biosystems). Five putative AP-1 response elements (TGAGTCA) were identified within 5 kbp upstream of the Mmp9 translation start site using rVista 2.0 (54) (http://rvista.dcode.org/). Two of the AP-1 response elements, located at −107 bp and −533 bp (upstream of the translation start site), were conserved in rat, mouse, and human and were examined for FOSL1 occupancy. Specific primers were used for qPCR measurements of AP-1 site A (−107 to −101) occupancy (forward, GAGTCAGCGTAAGCCTGGAG; reverse, AGCAGAATTTGCGGAGGTTT; 81-bp product spanning −105 to −26) and AP-1 site B (−533 to −527) occupancy (forward, AAAGAGCCTGCTCCCAGAG; reverse, CATTCCCACCCCCTGTTAGT; 66-bp product spanning −567 to −502). Relative occupancy/enrichment was normalized to input samples by use of the ΔΔCT method.
Mmp9 promoter analysis.
Rat Mmp9 5′-flanking DNA spanning −1107 to +120 (relative to the translation start site NC_005102.2) was PCR amplified using genomic DNA templates isolated from Rcho-1 TS cells (forward primer, GGAGGCTCAATCAGAACAGCTT; reverse primer, GAGGTTGGAGGTTTTCAGGTCT). PCR amplified 5′-flanking regions were initially cloned into the TA cloning vector (Invitrogen) for sequence confirmation and then subcloned (−1024 to +47) into KpnI and BglII sites (−1024 Mmp9 KpnI, GGAAGAGGGAAGGTACCGAGGCT; +47 Mmp9 BglII, TGAGGGGCAGCAAAGATCTAGCCTA) of the pGL3 basic luciferase vector (Promega, Madison, WI) for in vitro assays using Rcho-1 TS cells. The two conserved AP-1 response elements, located at −107 bp and −533 bp (upstream of the translation start site) were modified (CAGGACA) by site-directed mutagenesis. Activities of wild-type and mutant promoter-reporter constructs were assessed as previously described (61). Briefly, cells grown in 6-well plates were cotransfected with 500 ng of reporter vector and 50 ng of control Renilla vector by use of Lipofectamine 2000 (Invitrogen). Twelve hours after transfection, the medium was changed, and incubations were continued for an additional 48 h before passive cell lysis and processing with a standard dual luciferase assay (Promega). Firefly luciferase activities were normalized to the cotransfected Renilla luciferase reporter activities. Results were expressed as the relative luciferase activity of the Mmp9 promoter-reporter constructs compared to the luciferase activity of the empty (promoterless) luciferase reporter construct.
Statistical analyses.
Statistical comparisons of two means were performed with Student's t test or Welch's t test. Comparisons of multiple groups were evaluated with analysis of variance. The source of variation from significant F-ratios was determined with Tukey's honestly significant difference (HSD) multiple comparison test. Statistical analyses were performed using the R statistical package (http://www.r-project.org/).
RESULTS
Trophoblast AKT expression and disruption with small-molecule inhibitors.
The PI3K/AKT pathway is activated during trophoblast differentiation (43). In the following experiments, we examined roles for the PI3K/AKT pathway in the regulation of trophoblast cell differentiation and the invasive trophoblast phenotype.
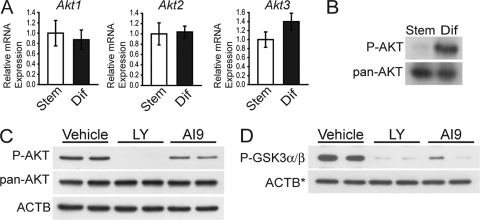
Each of the three isoforms of Akt (Akt1, Akt2, and Akt3) is expressed in Rcho-1 TS cells (Fig. 1A). AKT is activated upon phosphorylation of serine 473. In Rcho-1 TS cells, phosphorylation of AKT on serine 473 AKT was increased following differentiation (Fig. 1B) (43), indicating that AKT is activated in differentiated Rcho-1 TS cells.
Fig. 1.
AKT expression and activity in trophoblast cells. (A) qRT-PCR analysis of Akt1, Akt2 and Akt3 in stem (Stem) and differentiated (Dif) Rcho-1 TS cells. Sample size: n = 4. Bars represent means ± standard error of the mean (SEM). (B) Western blot analysis of total AKT (pan-AKT) and phosphorylated AKT (P-AKT) in stem and differentiated Rcho-1 TS cells. (C) Analysis of AKT phosphorylation state in differentiated Rcho-1 TS cells treated with vehicle, LY, or AI9. P-AKT, Western blot of phosphorylated AKT. pan-AKT, Western blot analysis of total AKT. ACTB was used as a loading control. (D) Analysis of AKT kinase activity in differentiated Rcho-1 TS cells treated with vehicle (0.01% DMSO), LY294002 (LY; 10 μM), or AKT inhibitor IX (AI9; 10 μM). P-GSK3α/β, GSK3α/β–glutathione S-transferase fusion protein was used as a substrate to monitor AKT kinase activity (P-GSK3α/β, phosphorylated GSK3α/β). ACTB was used as a loading control for the input Rcho-1 TS cell lysates used for immunoprecipitation of phospho-AKT (Ser 473) and the subsequent kinase assay (ACTB*).
AKT activity was blocked using a small-molecule inhibitor of PI3K (LY) (78) or using an inhibitor that blocks the activity of all AKT isoforms (AKT inhibitor IX) (42). Treatment of Rcho-1 TS cells with LY decreased both AKT phosphorylation and kinase activity (Fig. 1C and D), while cells treated with AKT inhibitor IX retained AKT phosphorylation but lost AKT kinase activity (Fig. 1C and D). This strategy for using small-molecule inhibitors to disrupt PI3K and/or AKT was used in experiments described below to investigate the role of the PI3K/AKT signaling pathway in the regulation of the invasive trophoblast-vascular remodeling cell phenotype.
PI3K/AKT signaling: regulation of invasion-vascular remodeling genes.
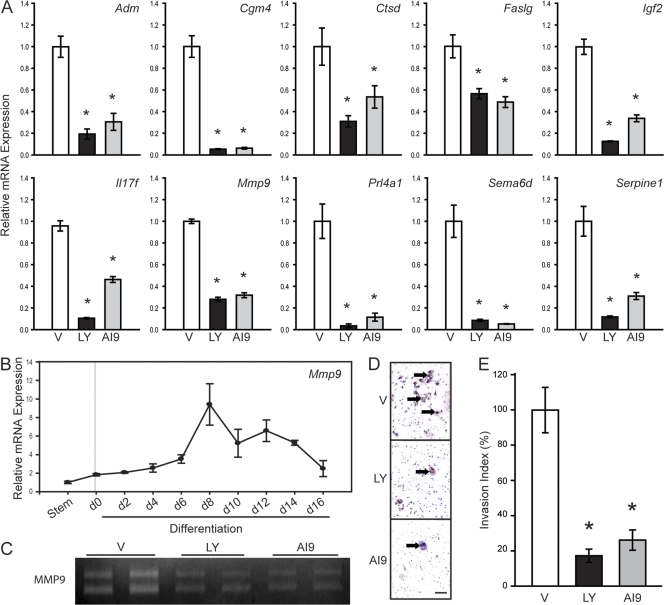
Previous gene profiling in trophoblast cells led to the identification of a subset of genes that are upregulated during differentiation and sensitive to PI3K inhibition (46). In this experiment, we focused on a group of 10 PI3K-sensitive genes that are potential regulators of trophoblast invasion and/or vascular remodeling (Adm [44, 81], Cgm4 [82], Ctsd [59], Faslg [8, 37], Igf2 [7, 40], Il17f [32], Mmp9 [53, 62], Prl4a1 [4, 6, 60], Sema6d [75], and Serpine1 [33]) (Table 2). In Fig. 2A, we show that these 10 genes are sensitive to disruption of AKT activation. These observations are consistent with a role for the PI3K/AKT signaling pathway in the regulation of invasive and vascular remodeling trophoblast cell phenotypes.
Table 2.
Potential regulators of trophoblast invasion and/or vascular remodeling
| Gene name | Abbreviation | Synonym(s) | Functional group | GenBank accession no. |
|---|---|---|---|---|
| Adrenomedullin | Adm | Hypotensive peptide | NM_012715 | |
| Carcinoembryonic antigen gene family 4 | Cgm4 | Psg16 | Secretory protein, unknown function | NM_012525 |
| Cathepsin D | Ctsd | CD, CatD | Lysosomal aspartic endopeptidase | NM_134334 |
| Fas ligand (TNF superfamily, member 6) | Faslg | Faslg, Tnfsf6 | Ligand/membrane anchored | NM_012908 |
| Insulin-like growth factor 2 | Igf2 | Igf-II | Ligand, growth factor | NM_031511 |
| Interleukin 17F | Il17f | ML1 | Ligand/cytokine | NM_001015011 |
| Matrix metallopeptidase 9 | Mmp9 | Gelatinase B | Extracellular matrix remodeling | NM_031055 |
| Prolactin family 4, subfamily a, member 1 | Prl4a1 | PLP-A | Ligand/cytokine | NM_017036 |
| Sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6D | Sema6d | Receptor | NM_001107768 | |
| Serine (or cysteine) peptidase inhibitor, clade E, member 1 | Serpine1 | Pai1, Planh | Blood coagulation, angiogenesis | NM_012620 |
Fig. 2.
PI3K and AKT regulate the expression of invasion-vascular remodeling genes and invasive abilities of trophoblast cells. (A) qRT-PCR analysis of invasion-vascular remodeling genes in differentiated Rcho-1 TS cells treated with vehicle (V; 0.01% DMSO), LY294002 (LY; 10 μM), or AKT inhibitor IX (AI9; 10 μM). Experiments were performed with a sample size ranging from 4 to 6. (B) qRT-PCR analysis of Mmp9 expression during Rcho-1 TS cell differentiation. Experiments were performed in triplicate. (C) Measurement of MMP9 activity by gelatin zymography in conditioned media from differentiated Rcho-1 TS cells treated with V, LY, or AI9. (D and E) Invasiveness of differentiated Rcho-1 TS cells treated with V, LY, or AI9. Invasion was measured using the Matrigel chamber assay. (D) Representative filters showing trophoblast invasion through Matrigel in V, LY, or AI9 treated cultures are presented (cells are marked with arrows). Bar = 100 μm. (E) Graphic presentation of results from the Matrigel invasion chamber assays. Cells were counted from nine replicates and normalized to control samples. Invasion index represents the number of invading cells in each group relative to the number of cells invading in the control group. Bars represent means ± SEM. Values significantly different from controls are indicated (∗, P < 0.05).
PI3K/AKT signaling: MMP9 activity and trophoblast invasion.
Of the 10 invasion-vascular remodeling genes, 1 gene in particular, Mmp9, which encodes matrix metallopeptidase 9 (MMP9), has been associated with invasive behavior of trophoblast (11, 53, 55, 62, 66) and cancer (23, 47) cells. Mmp9 mRNA expression increases during trophoblast differentiation (Fig. 2B). MMP9 gelatinolytic activity is decreased in conditioned medium from cells treated with PI3K or AKT inhibitors (Fig. 2C). Disruption of PI3K and/or AKT activities impaired trophoblast cell invasion as assessed using Matrigel invasion chamber assays (Fig. 2D and E). These experimental results indicate that PI3K/AKT signaling promotes the trophoblast cell-invasive phenotype, potentially through modulation of the expression of Mmp9 and other proinvasive genes.
AKT isoforms: modulation of the trophoblast phenotype.
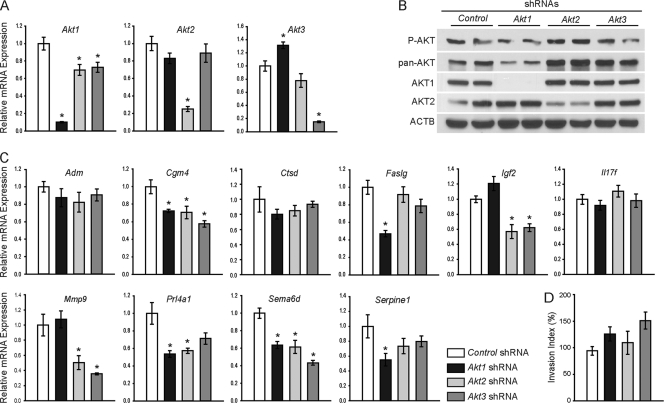
Cellular AKT activity is the net result of the activities of three AKT isoenzymes (AKT1, AKT2, AKT3) (12, 13). To investigate relationships between AKT isoforms and expression of the invasion-vascular remodeling genes, the expression of each AKT isoform was specifically disrupted using lentiviral delivery of isoform-specific shRNAs. Confirmation that isoform-specific shRNAs were effective in inhibiting expression at transcript and protein levels is shown in Fig. 3. Total AKT was affected by AKT1 knockdown but changed minimally following the knockdown of AKT2 or AKT3 (Fig. 3B), supporting earlier reports suggesting that AKT1 is the predominant isoform in the placenta (43, 85). Disruption of each individual AKT isoform yielded an isoform-specific effect on expression of the invasion-vascular remodeling genes. Knockdown of AKT1 resulted in a significant decrease in Cgm4, Faslg, Prl4a1, Sema6d, and Serpine1, while AKT2 disruption inhibited Cgm4, Igf2, Mmp9, Prl4a1, and Sema6d, and AKT3 contributed to the regulation of Cgm4, Igf2, Mmp9, and Sema6d (Fig. 3C). Expression of some invasion-vascular remodeling genes (Adm, Ctsd, and Il17f) was not affected by single AKT isoform disruption (Fig. 3C). Knockdown of individual AKT isoforms was not sufficient to disrupt the invasive abilities of trophoblast cells as assessed in Matrigel invasion chamber assays (Fig. 3D). Stable double-AKT-isoform knockdowns had effects on growth and survival and were problematic to establish. Trophoblast cell phenotypes resulting from the knockdown of individual AKT isoforms provide further support for the involvement of the PI3K/AKT signaling pathway in the regulation of trophoblast invasion and vascular remodeling. However, the results also demonstrate that there is a potentially complex interplay of each isoform in modulating the trophoblast cell phenotype.
Fig. 3.
Role of AKT isoforms in the regulation of the invasive trophoblast phenotype. Differentiated Rcho-1 TS cells expressing control, Akt1, Akt2, or Akt3 shRNAs were evaluated. (A) qRT-PCR analysis for Akt1, Akt2, and Akt3. Experiments were performed in quadruplicate. (B) Western blot analysis of phosphorylated AKT (P-AKT), total AKT (pan-AKT), AKT1, and AKT2. ACTB was included as a loading control. (C) qRT-PCR analyses of invasion-vascular remodeling genes. All samples were normalized to control samples. Experiments were performed in quadruplicate. (D) Invasion was assessed using Matrigel chamber assays. Cells were counted from eight replicates and normalized to control samples. Bars represent means ± SEM. Values significantly different from controls are indicated (*, P < 0.05).
FOSL1: expression during trophoblast differentiation and regulation by PI3K/AKT.
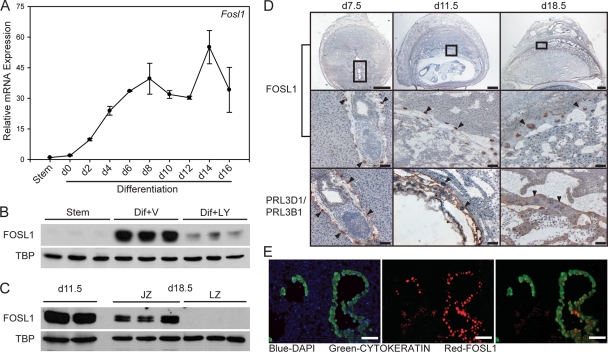
We next sought to identify potential downstream mediators of the PI3K/AKT signaling pathway responsible for regulating the invasive trophoblast and vascular remodeling phenotype. In cancer cells, FOSL1 is regulated by PI3K/AKT signaling (16, 18, 67). FOSL1 has also been linked to placentation (72) and is expressed in a differentiation-dependent pattern in rat trophoblast cells (46). Expression of FOSL1 was examined in trophoblast cell populations maintained in vitro. FOSL1 transcripts and protein increase during Rcho-1 TS cell differentiation (Fig. 4A and B). To determine if the PI3K/AKT signaling pathway can regulate FOSL1, we examined the presence of FOSL1 protein in nuclear lysates from control and PI3K/AKT-disrupted Rcho-1 TS cells. FOSL1 expression was greatly diminished in nuclear lysates of cells treated with the PI3K inhibitor (Fig. 4B). These findings indicate that the PI3K/AKT signaling pathway regulates nuclear accumulation of FOSL1, the site of FOSL1 action.
Fig. 4.
Expression of FOSL1 in Rcho-1 TS cells and rat placentation sites. (A) qRT-PCR analysis of Fosl1 expression during Rcho-1 TS cell differentiation. Experiments were performed in triplicate. (B) Western blot analysis of FOSL1 protein in nuclear lysates of stem (Stem) or differentiating Rcho-1 TS cells treated with vehicle (Dif+V; 0.01% DMSO) or LY294002 (Dif+LY; 10 μM). TBP was included as a loading control. (C) Western blot analysis of protein levels for FOSL1 in nuclear lysates from gestation days 11.5 and 18.5 placental tissues. Placental tissues isolated from gestation day 18.5 were dissected into junctional zone (JZ) and labyrinth zone (LZ) compartments. TBP was included as a loading control. (D) Immunohistochemical localization of FOSL1 in gestation days 7.5, 11.5, and 18.5 rat placentation sites. FOSL1 is localized to the nucleus of trophoblast giant cells (arrowheads). PRL3D1 (PL-I, day 7.5) and PRL3B1 (PL-II, days 11.5 and 18.5) are expressed in trophoblast giant cells (arrowheads) and were used as positive controls. Top bars = 1 mm; middle and bottom bars = 100 μm. (E) Immunofluorescence colocalization of FOSL1 and cytokeratin in gestation day 11.5 placentation sites. Nuclei were visualized with DAPI (blue). (Left) Cytokeratin and DAPI; (middle) FOSL1 and DAPI; (right) cytokeratin and FOSL1. Please note that FOSL1 colocalizes with cytokeratin in invasive endovascular trophoblast cells. Bar = 100 μm.
FOSL1 expression at the placentation site.
FOSL1 is expressed in the rat placenta on gestation days 11.5 and 18.5 as assessed by Western blotting. At day 18.5, FOSL1 protein was restricted to the junctional zone of the chorioallantoic placenta (Fig. 4C). FOSL1 was localized to the nuclei of trophoblast giant cells by immunohistochemistry at gestation days 7.5, 11.5, and 18.5. Trophoblast giant cells were identified morphologically and by their expression of PRL3D1 (PL-I) and PRL3B1 (PL-II) (Fig. 4D). Costaining with anti-FOSL1 and anti-cytokeratin (an epithelial cell marker routinely used to identify trophoblast cells) demonstrated that FOSL1 is also expressed in the nuclei of invasive endovascular trophoblast cells on gestation day 11.5 (Fig. 4E). Thus, FOSL1 is a candidate regulator of trophoblast cell invasion and/or uterine spiral remodeling.
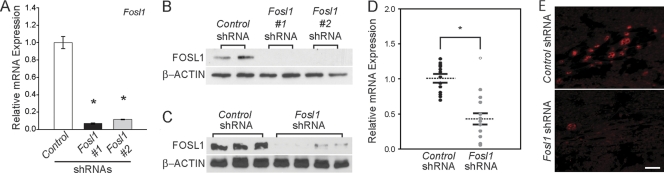
In vitro and in vivo lentiviral knockdown of FOSL1.
In the next series of experiments, we disrupted FOSL1 by using both in vitro and in vivo model systems for the purpose of investigating the biology of FOSL1 in trophoblast cells. Fosl1 mRNA and FOSL1 protein were effectively decreased in Rcho-1 TS cells using lentiviral delivery of two independent shRNAs (Fig. 5A and B). In vivo FOSL1 knockdown was achieved using a trophoblast cell-specific lentiviral delivery of Fosl1-specific shRNAs (52). Gestation day 4.5 rat embryos were exposed to lentiviral particles expressing control shRNA or shRNA specific for Fosl1 and then transferred to day 3.5 pseudopregnant female rats and allowed to develop until day 11.5. The in vivo lentiviral Fosl1 shRNA delivery system significantly knocked down Fosl1 mRNA (Fig. 5C) and FOSL1 protein (Fig. 5D and E) expression in gestation day 11.5 trophoblast tissue.
Fig. 5.
Analysis of shRNA-mediated FOSL1 knockdown. (A and B) In vitro analyses. qRT-PCR measurements of Fosl1 mRNA (A) and Western blot analysis of FOSL1 protein (B) in differentiated Rcho-1 TS cells expressing control or Fosl1 shRNAs. The qRT-PCR measurements were performed in quadruplicate. (C to E) In vivo analyses. (C) Western blot analysis of FOSL1 protein in dissected placental samples exposed to control or Fosl1 shRNAs. ACTB was included as a loading control. (D) qRT-PCR analysis of Fosl1 mRNA in dissected placental samples exposed to control or Fosl1 shRNAs. Dashed lines mark the means and the solid lines indicate the SEMs. Sample sizes: control, n = 14; Fosl1 shRNA, n = 12. Fosl1 shRNA treatment significantly decreased Fosl1 mRNA expression (*, P < 0.05). The open circle represents a Fosl1 shRNA sample that escaped knockdown and was not included in the statistical analysis. (E) FOSL1 immunohistochemistry of rat placentation sites exposed to control or Fosl1-specific shRNAs.
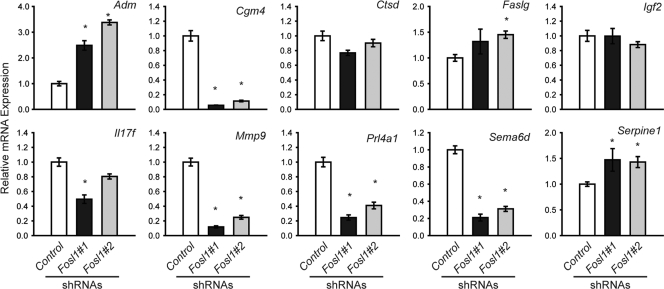
FOSL1: regulation of invasion-vascular remodeling genes.
Knockdown of FOSL1 in vitro resulted in a decrease in expression of several invasion-vascular remodeling genes, including Cgm4, Mmp9, Prl4a1, and Sema6d (Fig. 6). Other invasion-vascular remodeling genes were not consistently affected by FOSL1 knockdown (Ctsd, Faslg, Igf2, and Il17f), while expression of Adm and Serpine1 was significantly upregulated (Fig. 6).
Fig. 6.
Role of FOSL1 in the regulation of invasion-vascular remodeling gene expression. qRT-PCR analysis of invasion-vascular remodeling genes in differentiated Rcho-1 TS cells expressing control or Fosl1 shRNAs. All samples were normalized to control samples. Experiments were performed in quadruplicate. Bars represent means ± SEM. Values significantly different from controls are indicated (*, P < 0.05).
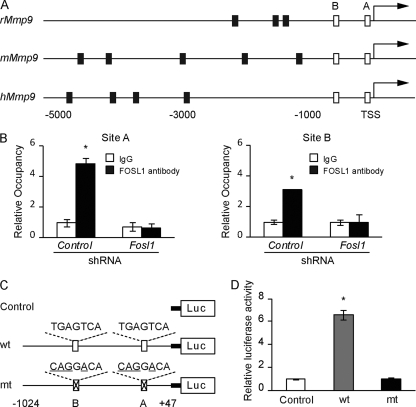
FOSL1 regulation of Mmp9.
FOSL1 has been implicated as a direct regulator of Mmp9 gene expression in cancer cells (16, 67), which prompted an assessment of FOSL1 interactions with the Mmp9 promoter in trophoblast cells. FOSL1 binds to AP-1 elements (28). The rat Mmp9 promoter possesses two conserved AP-1 elements (site A: −107 to −101; site B: −533 to −527) (Fig. 7A). In differentiated Rcho-1 TS cells, FOSL1 was specifically bound to both conserved AP-1 elements, as determined by ChIP analysis (Fig. 7B). These two AP-1 elements are essential for the activation of the Mmp9 promoter in differentiating Rcho-1 TS cells (Fig. 7C and D). Thus, the data are consistent with FOSL1 directly activating the Mmp9 gene promoter during trophoblast cell differentiation.
Fig. 7.
FOSL1 occupancy on AP-1 elements within the Mmp9 promoter. (A) Schematic representation of putative AP-1 elements (black and white boxes) within Mmp9 promoters of the rat (rMmp9), mouse (mMmp9), and human (hMmp9). Sites A and B (white boxes) were conserved in all three species. Putative AP-1 elements demarcated by black boxes were not conserved. TSS, translation start site. (B) ChIP determination of the occupancy of FOSL1 on sites A and B of the rat Mmp9 promoter in Rcho-1 TS cells expressing control or Fosl1 shRNAs. IgG was included as a negative control. Values significantly different from control shRNA are indicated (*, P < 0.05). (C and D) Activities of an empty luciferase reporter construct (control), a wild-type rat Mmp9 promoter (−1024 to +47)-luciferase reporter construct (wt), or a mutant rat Mmp9 promoter (−1024 to +47)-luciferase reporter construct with AP-1 sites A and B mutated (mt). All constructs were evaluated in Rcho-1 TS cells. The wt Mmp9 promoter was active and dependent upon AP-1 sites A and B (*, P < 0.05). All experiments were performed in triplicate. Bars represent the mean ± SEM.
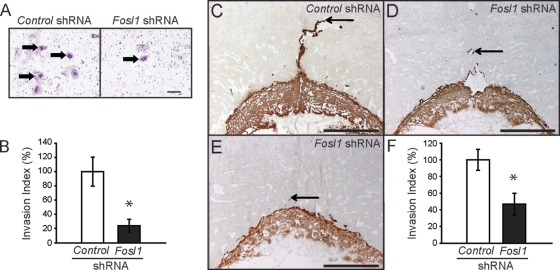
FOSL1 modulates trophoblast cell invasion.
The effects of FOSL1 on the regulation of a subset of invasion-vascular remodeling genes prompted an analysis of the role of FOSL1 in the regulation of in vitro trophoblast cell invasion. Similar to the modulation of the PI3K/AKT signaling pathway, the loss of FOSL1 decreased the in vitro invasive trophoblast ability as assessed using Matrigel chamber invasion assays (Fig. 8A and B). The results indicate that FOSL1 mediates some actions of the PI3K/AKT signaling pathway in vitro, including invasive cell behavior.
Fig. 8.
FOSL1 regulates trophoblast invasion as assessed in vitro and in vivo. (A and B) The invasive abilities of Rcho-1 TS cells expressing control or Fosl1 shRNAs were measured using Matrigel chamber assays. (A) Representative filters showing trophoblast invasion through Matrigel in control and FOSL1 knockdown cultures (cells are marked with arrows). Bar = 100 μm. (B) Graphic presentation of results from the Matrigel invasion chamber assays. Cells were counted from nine replicates and normalized to control samples. (C to F) Evaluation of the impact of FOSL1 knockdown on trophoblast invasion in vivo. Control or Fosl1 shRNAs were delivered to the trophectoderm of gestation day 4.5 blastocysts by use of a lentiviral delivery system and transferred to pseudopregnant rats, and placentation sites were evaluated on gestation day 11.5. (C to E) Immunohistochemistry of rat placentation sites was performed using anti-cytokeratin to identify trophoblast. Representative sections of gestation day 11.5 placentation sites for control shRNA (C) and Fosl1 shRNA (D and E) samples are shown. Bars = 1 mm. (F) Quantification of the invasion index (see Materials and Methods). Sample sizes: control, n = 12; Fosl1 shRNA, n = 9. Bars represent means ± SEM. Values significantly different from controls are indicated (*, P < 0.05).
We next evaluated a role for FOSL1 in regulating in vivo trophoblast cell invasion. Histological analysis demonstrated that the trophoblast invasion index (depth of invasion/height of decidua) was significantly less at placentation sites with FOSL1 knockdown (Fig. 8C to F), implicating FOSL1 as a component of the in vivo regulatory pathway controlling trophoblast cell invasion.
Collectively, our findings indicate that a regulatory pathway involving PI3K/AKT and FOSL1 controls the invasive trophoblast phenotype.
DISCUSSION
Intrauterine trophoblast invasion and remodeling of the uterine vasculature are important features of hemochorial placentation. Blood vessels are restructured, permitting increased blood flow and nutrient delivery to the placenta and ultimately the fetus. In this study, we have identified a cellular signaling pathway involving PI3K/AKT and FOSL1 that regulates invasive trophoblast and vascular remodeling phenotypes. Small-molecule inhibitors were effective in interfering with the PI3K/AKT signaling pathway. This strategy of nullifying the actions of PI3K or AKT in trophoblast cells led to the inhibition of proinvasion and vascular remodeling genes. Blockade of the PI3K/AKT pathway also decreased nuclear accumulation of the FOSL1 protein. Disruptions of PI3K, AKT, or FOSL1 interfered with trophoblast cell invasion.
PI3K/AKT signaling activates the expression of a set of 10 genes encoding proteins implicated in cell invasion and/or vascular remodeling. The invasion-vascular remodeling set of genes can be placed into two groups, genes encoding proteins that potentially affect trophoblast movement and genes encoding proteins that are postulated to alter the vasculature. In the first group, the genes encode proteins that can induce cell movement (IGF2 [7, 40]), guide cells (SEMA6D [75]), and remodel the extracellular matrix (CTSD [59], MMP9 [53, 62], and SERPINE1 [33]). The second group consists of genes encoding proteins that directly affect endothelial cells and blood vessels (ADM [44, 81], CGM4 [82], FASLG [8, 37], and IL17F [32]) or indirectly modulate blood vessel remodeling by altering immune cell cytokine production (CGM4 [34, 79, 80], IL17F [32], and PRL4A1 [4, 6, 60]). Although each of the 10 proteins encoded by these genes has the potential to influence trophoblast invasion and/or vascular remodeling, the relative roles of each regulator and their specific orchestration during gestation are unknown.
Three AKT isoforms are expressed in trophoblast cells and regulate trophoblast cell biology in a unique and complicated overlapping manner. Knockdown of individual AKT isoforms affected subsets of PI3K/AKT-sensitive invasion-vascular remodeling genes and led to a unique expression profile. Expression of some invasion-vascular remodeling genes (Adm, Ctsd, and Il17f), nuclear accumulation of FOSL1 protein, and trophoblast cell invasion were not affected by interfering with the expression of a single AKT isoform. The retention of these functions in single-AKT-knockdown cells was associated with the presence of active cellular AKT, suggesting a potentially complex interplay of each AKT isoform.
Even though knockdown of individual AKT isoforms was not sufficient to decrease trophoblast cell invasion and FOSL1 nuclear protein accumulation, we gained some insight into the role of individual AKT isoforms in regulating expression of the invasion-vascular remodeling genes. Two of the 10 invasion-vascular remodeling-related genes (Cgm4 and Sema6d) are sensitive to disruptions in any one of the three AKT isoforms, indicating that a high level of AKT activity is needed for regulating their expression. Other invasion-vascular remodeling genes were sensitive to disruption of a single isoform. Faslg and Serpine1 were sensitive only to interference of AKT1, implying that there must be some AKT1 substrate specificity associated with their regulation. Prl4a1 was sensitive to either AKT1 or AKT2 knockdown, and Mmp9 and Igf2 were sensitive to either AKT2 or AKT3 knockdown. These findings indicate a mixture of substrate specificity and promiscuity. Adm, Ctsd, and Il17f were exquisitely sensitive to small-molecule inhibition of PI3K or AKT but were not affected by knockdown of any single AKT isoform. Following the above logic, we would propose that the activation of this subset of genes requires lower levels of AKT activity and/or exhibits complete AKT substrate promiscuity. The same conclusion could be made for the regulation of FOSL1 protein nuclear accumulation and trophoblast invasion. The balance of each individual AKT isoform may also be critical for the regulation of the trophoblast cell phenotype, as has been shown in breast cancer cells (39). Disruption of multiple AKT isoforms in the trophoblast cells may provide additional insights; however, based on initial experiments, this approach is challenging due to effects on cell survival. Finally, the subcellular location of AKT isoforms could be informative in discerning roles for each isoform in trophoblast cells (70). Intracellular AKT isoform location impacts access to substrates and the activation of specific regulatory pathways.
PI3K/AKT signaling regulates FOSL1 in trophoblast cells. Similar findings have been reported for other cell types (16, 18, 67). FOSL1 has also been shown to be a downstream mediator of the PI3K/AKT signaling pathway in regulating the expression of proinvasion genes, including Mmp9, and cellular invasion (16, 18, 67). The precise mechanism of PI3K/AKT regulation of FOSL1 has not been clarified, but here we show that loss of PI3K/AKT signaling results in decreased nuclear accumulation of FOSL1 protein. FOSL1 may be a substrate for AKT, and its phosphorylation may influence its nuclear accumulation and/or activity. Alternatively, the role of the PI3K/AKT signaling pathway in regulating FOSL1 may be indirect. Cross talk between PI3K/AKT and other cellular signaling pathways in trophoblast has been described previously (65, 66, 74) and may contribute to the stabilization of FOSL1 protein in the nucleus (10). FOSL1 undergoes ubiquitin-independent degradation by the proteasome, which can be prevented by ERK1/2 phosphorylation within the C-terminal stabilizing region of the FOSL1 protein (10). Whether AKT can act similarly or whether it activates another kinase (such as extracellular signal-regulated kinase 1/2 [ERK1/2]) to stabilize FOSL1 remains to be determined.
FOSL1 has been implicated as a key regulator of placentation. A null mutation in the Fosl1 gene disrupts morphogenesis of the mouse placenta and results in prenatal lethality (72). Defective vascularization of the labyrinth zone of the mouse chorioallantoic placenta was viewed as the insult responsible for the prenatal demise of the fetus. In our report, invasive trophoblast cell populations were shown to express FOSL1, including those possessing an endovascular location, and FOSL1 was demonstrated to regulate the expression of a subset of trophoblast invasion-vascular remodeling genes (Cgm4, Mmp9, Prl4a1, and Sema6d). FOSL1-responsive genes include genes sensitive to knockdown of any AKT isoform (Cgm4 and Sema6d), a gene regulated by AKT1 and AKT2 (Prl4a1), and a gene regulated by AKT2 and AKT3 (Mmp9). Both in vitro and in vivo experiments implicated FOSL1 as a regulator of the invasive trophoblast phenotype. These FOSL1 actions are consistent with its prominent role in regulating cancer cell invasion (51, 58, 77, 86). FOSL1 occupies critical AP-1 elements within the Mmp9 promoter essential for gene regulation in trophoblast cells. In vivo trophoblast-specific knockdown of FOSL1 led to significantly decreased intrauterine endovascular trophoblast invasion. Disruptions in labyrinthine vascularization may have also been evident in the FOSL1 hypomorphs but were not examined in our report. Trophoblast invasion is less prominent in the mouse than in the rat (2, 3, 21). In the Fosl1 null mouse, trophoblast invasion was probably of secondary significance to interruptions in nutrient and waste exchange (72).
Regulation of the invasive trophoblast lineage is complex, involving a network of signaling pathways and transcriptional regulators (48). Mitogen-activated protein kinases, PI3K/AKT, focal adhesion kinase, Rho proteins, and Wnt signaling pathways have all been implicated in regulating various aspects of trophoblast cell motility and invasion (1, 17, 38, 56, 65, 66, 73, 74). These signaling molecules potentially impinge on a set of transcription factors to direct the development and function of the invasive trophoblast lineage. Thus far, inhibitor of differentiation 2, signal transducer and activator of transcription 3, Ikaros, and peroxisome proliferator-activated receptor-gamma have been shown to possess putative modulatory roles on the invasive trophoblast phenotype (31, 41, 64, 83). In this report, we have generated experimental evidence indicating that the PI3K/AKT/FOSL1 signaling pathway is involved in regulating invasive trophoblast and vascular remodeling phenotypes. FOSL1 is shown to be integral to trophoblast-directed uterine spiral remodeling and establishment of the maternal-fetal interface.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (HD20676).
We thank Warren Nothnick and Adam Krieg (University of Kansas Medical Center, Kansas City, KS) for advice with the zymography and ChIP analyses, respectively.
Footnotes
Published ahead of print on 26 September 2011.
REFERENCES
- 1. Abell A. N., et al. 2009. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol. Cell. Biol. 29: 2748–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamson S. L., et al. 2002. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 250: 358–373 [DOI] [PubMed] [Google Scholar]
- 3. Ain R., Canham L. N., Soares M. J. 2003. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev. Biol. 260: 176–190 [DOI] [PubMed] [Google Scholar]
- 4. Ain R., Dai G., Dunmore J. H., Godwin A. R., Soares M. J. 2004. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc. Natl. Acad. Sci. U. S. A. 101: 16543–16548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ain R., Konno T., Canham L. N., Soares M. J. 2006. Phenotypic analysis of the rat placenta. Methods Mol. Med. 121: 295–313 [DOI] [PubMed] [Google Scholar]
- 6. Ain R., Tash J. S., Soares M. J. 2003. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol. Cell. Endocrinol. 204: 65–74 [DOI] [PubMed] [Google Scholar]
- 7. Aplin J. D., et al. 2000. Growth factor-extracellular matrix synergy in the control of trophoblast invasion. Biochem. Soc. Trans. 28: 199–202 [DOI] [PubMed] [Google Scholar]
- 8. Ashton S. V., et al. 2005. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler. Thromb. Vasc. Biol. 25: 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bamberger A. M., et al. 2004. Expression pattern of the activating protein-1 family of transcription factors in human placenta. Mol. Hum. Reprod. 10: 223–228 [DOI] [PubMed] [Google Scholar]
- 10. Basbous J., Jariel-Encontre I., Gomard T., Bossis G., Piechaczyk M. 2008. Ubiquitin-independent- versus ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1 transcription factors: is there a unique answer? Biochimie 90: 296–305 [DOI] [PubMed] [Google Scholar]
- 11. Bischof P., Meisser A., Campana A. 2002. Control of MMP-9 expression at the maternal-fetal interface. J. Reprod. Immunol. 55: 3–10 [DOI] [PubMed] [Google Scholar]
- 12. Brazil D. P., Hemmings B. A. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26: 657–664 [DOI] [PubMed] [Google Scholar]
- 13. Brazil D. P., Yang Z.-Z., Hemmings B. A. 2004. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29: 233–242 [DOI] [PubMed] [Google Scholar]
- 14. Caluwaerts S., Vercruysse L., Luyten C., Pijnenborg R. 2005. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta 26: 574–584 [DOI] [PubMed] [Google Scholar]
- 15. Cantley L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296: 1655–1657 [DOI] [PubMed] [Google Scholar]
- 16. Cao H., Dronadula N., Rao G. N. 2006. Thrombin induces expression of FGF-2 via activation of PI3K-Akt-Fra-1 signaling axis leading to DNA synthesis and motility in vascular smooth muscle cells. Am. J. Physiol. 290: C172–C182 [DOI] [PubMed] [Google Scholar]
- 17. Chakraborty C., Gleeson L. M., McKinnon T., Lala P. K. 2002. Regulation of human trophoblast migration and invasiveness. Can. J. Physiol. Pharmacol. 80: 116–124 [DOI] [PubMed] [Google Scholar]
- 18. Chandrasekar B., et al. 2006. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-κB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J. Biol. Chem. 281: 15099–15109 [DOI] [PubMed] [Google Scholar]
- 19. Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. 2001. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276: 38349–38352 [DOI] [PubMed] [Google Scholar]
- 20. Cho H., et al. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292: 1728–1731 [DOI] [PubMed] [Google Scholar]
- 21. Coan P. M., Conroy N., Burton G. J., Ferguson-Smith A. C. 2006. Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn. 235: 3280–3294 [DOI] [PubMed] [Google Scholar]
- 22. Coffer P. J., Jin J., Woodgett J. R. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coussens L. M., Fingleton B., Matrisian L. M. 2002. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295: 2387–2392 [DOI] [PubMed] [Google Scholar]
- 24. Daggett M. A., Rice D. A., Heckert L. L. 2000. Expression of steroidogenic factor 1 in the testis requires an E box and CCAAT box in its promoter proximal region. Biol. Reprod. 62: 670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deb S., et al. 1989. Antipeptide antibodies reveal structural and functional characteristics of rat placental lactogen-II. Mol. Cell. Endocrinol. 63: 45–56 [DOI] [PubMed] [Google Scholar]
- 26. Dull T., et al. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72: 8463–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dummler B., Hemmings B. A. 2007. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 35: 231–235 [DOI] [PubMed] [Google Scholar]
- 28. Eferl R., Wagner E. F. 2003. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer 3: 859–868 [DOI] [PubMed] [Google Scholar]
- 29. Engelman J. A., Luo J., Cantley L. C. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7: 606–619 [DOI] [PubMed] [Google Scholar]
- 30. Faria T. N., Soares M. J. 1991. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology 129: 2895–2906 [DOI] [PubMed] [Google Scholar]
- 31. Fournier T., et al. 2002. The role of PPAR-gamma/RXR-alpha heterodimers in the regulation of human trophoblast invasion. Ann. N. Y. Acad. Sci. 973: 26–30 [DOI] [PubMed] [Google Scholar]
- 32. Gaffen S. L. 2008. An overview of IL-17 function and signaling. Cytokine 43: 402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graham C. H. 1997. Effect of transforming growth factor-beta on the plasminogen activator system in cultured first trimester human cytotrophoblasts. Placenta 18: 137–143 [DOI] [PubMed] [Google Scholar]
- 34. Ha C. T., Waterhouse R., Wessells J., Wu J. A., Dveksler G. S. 2005. Binding of pregnancy-specific glycoprotein 17 to CD9 on macrophages induces secretion of IL-10, IL-6, PGE2, and TGF-beta1. J. Leukoc. Biol. 77: 948–957 [DOI] [PubMed] [Google Scholar]
- 35. Hamlin G. P., Lu X. J., Roby K. F., Soares M. J. 1994. Recapitulation of the pathway for trophoblast giant cell differentiation in vitro: stage-specific expression of members of the prolactin gene family. Endocrinology 134: 2390–2396 [DOI] [PubMed] [Google Scholar]
- 36. Harris L. K. 2010. Trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta 31 (Suppl.): S93–S98 [DOI] [PubMed] [Google Scholar]
- 37. Harris L. K., et al. 2006. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a Fas ligand-dependent mechanism. Am. J. Pathol. 169: 1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ilić D., et al. 2001. Plasma membrane-associated pY397FAK is a marker of cytotrophoblast invasion in vivo and in vitro. Am. J. Pathol. 159: 93–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iliopoulos D., et al. 2009. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci. Signal. 2: ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Irwin J. C., Suen L. F., Martina N. A., Mark S. P., Giudice L. C. 1999. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum. Reprod. 14 (Suppl. 2): 90–96 [DOI] [PubMed] [Google Scholar]
- 41. Janatpour M. J., et al. 2000. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 127: 549–558 [DOI] [PubMed] [Google Scholar]
- 42. Jin X., et al. 2004. Inhibition of AKT survival pathway by a small molecule inhibitor in human endometrial cancer cells. Br. J. Cancer 91: 1808–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kamei T., et al. 2002. The phosphatidylinositol 3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol. Endocrinol. 16: 1469–1481 [DOI] [PubMed] [Google Scholar]
- 44. Kato J., Tsuruda T., Kita T., Kitamura K., Eto T. 2005. Adrenomedullin: a protective factor for blood vessels. Arterioscler. Thromb. Vasc. Biol. 25: 2480–2487 [DOI] [PubMed] [Google Scholar]
- 45. Kaufmann P., Black S., Huppertz B. 2003. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 69: 1–7 [DOI] [PubMed] [Google Scholar]
- 46. Kent L. N., Konno T., Soares M. J. 2010. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev. Biol. 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kessenbrock K., Plaks V., Werb Z. 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141: 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knöfler M. 2010. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int. J. Dev. Biol. 54: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Konno T., Rempel L. A., Arroyo J. A., Soares M. J. 2007. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol. Reprod. 76: 709–718 [DOI] [PubMed] [Google Scholar]
- 50. Krieg A. J., et al. 2010. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol. Cell. Biol. 30: 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kustikova O., et al. 1998. Fra-1 induces morphological transformation and increases in vitro invasiveness and motility of epithelioid adenocarcinoma cells. Mol. Cell. Biol. 18: 7095–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee D.-S., Rumi M. A. K., Konno T., Soares M. J. 2009. In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis 47: 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Librach C. L., et al. 1991. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 113: 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Loots G. G., Ovcharenko I. 2004. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 32: W217–W221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luo J., Qiao F., Yin X. 2011. Impact of silencing MMP9 gene on the biological behaviors of trophoblasts. J. Huazhong Univ. Sci. Technol. Med. Sci. 31: 241–245 [DOI] [PubMed] [Google Scholar]
- 56. MacPhee D. J., et al. 2001. Focal adhesion kinase is a key mediator of human trophoblast development. Lab. Invest. 81: 1469–1483 [DOI] [PubMed] [Google Scholar]
- 57. Marzioni D., et al. 2010. Activating protein-1 family of transcription factors in the human placenta complicated by preeclampsia with and without fetal growth restriction. Placenta 31: 919–927 [DOI] [PubMed] [Google Scholar]
- 58. Milde-Langosch K. 2005. The Fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 41: 2449–2461 [DOI] [PubMed] [Google Scholar]
- 59. Mohamed M. M., Sloane B. F. 2006. Cysteine cathepsins: multifunctional enzymes in cancer. Nat. Rev. Cancer 6: 764–775 [DOI] [PubMed] [Google Scholar]
- 60. Müller H., et al. 1999. Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology 140: 2711–2720 [DOI] [PubMed] [Google Scholar]
- 61. Page-McCaw A., Ewald A. J., Werb Z. 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peters T. J., et al. 1999. Differentiation-dependent expression of gelatinase B/matrix metalloproteinase-9 in trophoblast cells. Cell Tissue Res. 295: 287–296 [DOI] [PubMed] [Google Scholar]
- 63. Pijnenborg R., Vercruysse L., Hanssens M. 2006. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27: 939–958 [DOI] [PubMed] [Google Scholar]
- 64. Poehlmann T. G., et al. 2005. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta 26 (Suppl. A): S37–S41 [DOI] [PubMed] [Google Scholar]
- 65. Qiu Q., Yang M., Tsang B. K., Gruslin A. 2004. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol. Hum. Reprod. 10: 677–684 [DOI] [PubMed] [Google Scholar]
- 66. Qiu Q., Yang M., Tsang B. K., Gruslin A. 2004. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 128: 355–363 [DOI] [PubMed] [Google Scholar]
- 67. Ramachandran A., et al. 2010. An Akt- and Fra-1-dependent pathway mediates platelet-derived growth factor-induced expression of thrombomodulin, a novel regulator of smooth muscle cell migration. Am. J. Pathol. 177: 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rosario G. X., Konno T., Soares M. J. 2008. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev. Biol. 314: 362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sahgal N., Canham L. N., Canham B., Soares M. J. 2006. Rcho-1 trophoblast stem cells: a model system for studying trophoblast cell differentiation. Methods Mol. Med. 121: 159–178 [PubMed] [Google Scholar]
- 70. Santi S. A., Lee H. 2010. The Akt isoforms are present at distinct subcellular locations. Am. J. Physiol. Cell Physiol. 298: C580–C591 [DOI] [PubMed] [Google Scholar]
- 71. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101 [DOI] [PubMed] [Google Scholar]
- 72. Schreiber M., et al. 2000. Placental vascularisation requires the AP-1 component fra1. Development 127: 4937–4948 [DOI] [PubMed] [Google Scholar]
- 73. Shiokawa S., et al. 2002. Small guanosine triphospatase RhoA and Rho-associated kinase as regulators of trophoblast migration. J. Clin. Endocrinol. Metab. 87: 5808–5816 [DOI] [PubMed] [Google Scholar]
- 74. Sonderegger S., et al. 2010. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology 151: 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Toyofuku T., et al. 2004. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 6: 1204–1211 [DOI] [PubMed] [Google Scholar]
- 76. Vercruysse L., Caluwaerts S., Luyten C., Pijnenborg R. 2006. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta 27: 22–33 [DOI] [PubMed] [Google Scholar]
- 77. Verde P., Casalino L., Talotta F., Yaniv M., Weitzman J. B. 2007. Deciphering AP-1 function in tumorigenesis. Fra-ternizing on target promoters. Cell Cycle 6: 2632–2639 [DOI] [PubMed] [Google Scholar]
- 78. Vlahos C. J., Matter W. F., Hui K. Y., Brown R. F. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-mopholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269: 5241–5248 [PubMed] [Google Scholar]
- 79. Waterhouse R., Ha C., Dveksler G. S. 2002. Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J. Exp. Med. 195: 277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wessells J., et al. 2000. Pregnancy specific glycoprotein 18 induces IL-10 expression in murine macrophages. Eur. J. Immunol. 30: 1830–1840 [DOI] [PubMed] [Google Scholar]
- 81. Wilson C., Nikitenko L. L., Sargent I. L., Rees M. C. P. 2004. Adrenomedullin: multiple functions in human pregnancy. Angiogenesis 7: 203–212 [DOI] [PubMed] [Google Scholar]
- 82. Wynne F., et al. 2006. Mouse pregnancy-specific glycoproteins: tissue-specific expression and evidence of association with maternal vasculature. Reproduction 131: 721–732 [DOI] [PubMed] [Google Scholar]
- 83. Yamamoto E., et al. 2005. Ikaros is expressed in human extravillous trophoblasts and involved in their migration and invasion. Mol. Hum. Reprod. 11: 825–831 [DOI] [PubMed] [Google Scholar]
- 84. Yang Z.-Z., et al. 2005. Dosage-dependent effects of Akt1/protein kinase Bα (PKBα) and Akt3/PKBγ on thymus, skin, and cardiovascular and nervous system development in mice. Mol. Cell. Biol. 25: 10407–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang Z.-Z., et al. 2003. Protein kinase Bα/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278: 32124–32131 [DOI] [PubMed] [Google Scholar]
- 86. Young M. R., Colburn N. H. 2006. Fra-1 a target for cancer prevention or intervention. Gene 379: 1–11 [DOI] [PubMed] [Google Scholar]