Abstract
Cyclospora papionis, Cryptosporidium hominis, and Enterocytozoon bieneusi were detected in 42 (17.9%), 6 (2.6%), and 29 (12.3%) of 235 newly captured baboons in Kenya, respectively. Most C. hominis subtypes and E. bieneusi genotypes found have been detected in humans in the area, suggesting that cross-species transmission of cryptosporidiosis and microsporidiosis is possible.
TEXT
Cryptosporidium spp., Enterocytozoon bieneusi, and Cyclospora cayetanensis, the main causative agents of cryptosporidiosis, microsporidiosis, and cyclosporiasis, respectively, cause self-limiting diarrhea in immunocompetent hosts and persistent and life-threatening diarrhea in immunocompromised persons, especially AIDS patients (7–9, 13). Thus far, very few studies have examined the genetic characteristics of Cryptosporidium, E. bieneusi, and Cyclospora in nonhuman primates. Thus, the role of nonhuman primates in zoonotic transmission of these parasites is not clear. In addition, it remains difficult to conclude with certainty that C. cayetanensis does not infect nonhuman primates, as only a small number of studies have been conducted (8). Nonhuman primates are genetically related to humans and thus may be susceptible to infection with human parasites and serve as zoonotic reservoirs (10).
In this study, we investigated the prevalence of Cryptosporidium, E. bieneusi, and Cyclospora infections in captive baboons in Kenya and characterized the parasites found at the genotype or subtype level.
Baboon specimens.
Fecal specimens were obtained from 235 captive olive baboons (Papio anubis) in 3- to 6-week quarantines during June 2006 to December 2008. The animals were captured in rural or forest areas in two provinces of Kenya, including a Kwetu farm in Thika (Central Province), Mwiga and Lamuria in Aberdares (Rift Valley Province), and Ngurumani (Rift Valley Province). Among them, 92 specimens were collected on 20 June 2006 from baboons captured on 9 May (10 animals), 13 May (7 animals), and 25 May 2006 (75 animals), 77 specimens were collected on 28 August 2007 from baboons captured on 10 August (25 animals), 13 August (25 animals), and 15 August 2007 (27 animals), and 66 specimens were collected on 3 December 2008 from baboons captured on 13 November (1 animal), 19 November (32 animals), 20 November (15 animals), and 22 November 2008 (18 animals). Of the 235 specimens, 230 had corresponding information on animal gender and age and thus were assigned to three age groups as follows: adults (95 females and 58 males with mean body weights of 12.5 ± 2.3 kg and 18.9 ± 5.0 kg, respectively), juveniles (29 females and 35 males with mean body weights of 6.1 ± 1.5 kg and 7.3 ± 2.8 kg, respectively), and infants (8 females and 5 males with mean body weights of 2.2 ± 0.5 kg and 2.5 ± 0.9 kg, respectively). Specimens were stored frozen before analysis.
Pathogen detection, genotyping, and subtyping.
The fecal specimens were analyzed for Cryptosporidium spp., E. bieneusi, and Cyclospora spp. by nested PCR after DNA was extracted from them by use of the FastDNA spin kit for soil (Qbiogene Inc., Carlsbad, CA). Cryptosporidium spp. were detected and differentiated by PCR and sequence analysis of the small-subunit (SSU) rRNA gene (14). Enterocytozoon bieneusi genotypes were detected and differentiated by PCR and sequence analysis of the internal transcribed spacer (ITS) (11). Another intestinal microsporidium, Encephalitozoon intestinalis, was not examined because of its less frequent occurrence in nonhuman primates and lack of genotyping tools. For the detection of Cyclospora spp., a 680-bp polymorphic region of the SSU rRNA gene was amplified by nested PCR using primers APIF1 (5′-AACCTGGTTGATCCTGCCAGT-3′) and CYCR1W (5′-AAAGTTCCGGAACACCAAC-3′) in primary PCR and primers CYCF2W (5′-GCTTGTCTCAAAGATTAAGCCATG-3′) and CYCR2W (5′-AAGGCTACCGGAAGAAAGCC-3′) in secondary PCR. The primary PCR consisted of 35 cycles of 94°C for 45 s, 53°C for 45 s, and 72°C for 60 s, with an initial denaturation (94°C for 5 min) and a final extension (72°C for 10 min). The condition for the secondary PCR was identical to the primary PCR, except that the annealing temperature was increased to 55°C. Cryptosporidium hominis organisms identified were further subtyped by sequence analysis of the 60-kDa glycoprotein (gp60) gene (1). The infection rates were compared by use of a chi-square test, whereas the mean body weights were compared by use of a paired t test. A difference was considered significant when the P value was <0.05. All analyses were done using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Cryptosporidium infection in baboons.
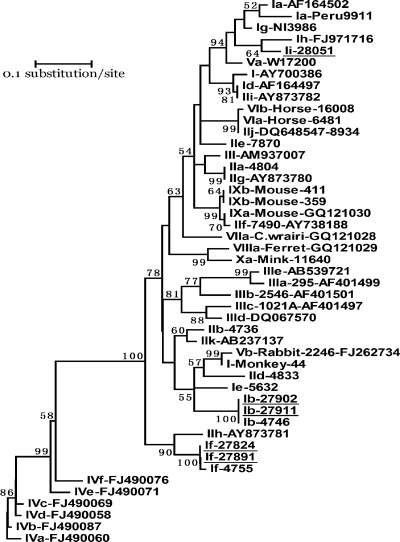
Cryptosporidium was detected in six (2.6%) specimens, all belonging to C. hominis. Infant animals had an infection rate (15.4%) higher than those of juveniles (1.6%) and adults (1.3%) (P > 0.05). Subtyping was successful for five of the specimens; three subtypes in three subtype families, including Ib (IbA9G3), If (IfA12G2), and a novel subtype family, Ii (IiA14), were found (Table 1). Phylogenetic analysis of the gp60 nucleotide sequences showed that the novel subtype family formed a cluster with the C. hominis Ih subtype family and was related to subtype families Ia and Ig of C. hominis and Va of Cryptosporidium cuniculus (Fig. 1).
Table 1.
Distribution of Cryptosporidium hominis subtypes in captive baboons based on sequence analysis of the gp60 gene
| Specimen no. | Animal no. | Gender and agea | Wt (kg) | C. hominis subtype |
|---|---|---|---|---|
| 27824 | 3032 | IfA12G2 | ||
| 27891 | 3134 | FI | 2.2 | IfA12G2 |
| 27902 | 3145 | FI | 2 | IbA9G3 |
| 27911 | 3157 | FA | 14.2 | IbA9G3 |
| 28051 | 3576 | MA | 12.6 | IiA14 |
FA, female adult; FI, female infant; MA, male adult.
Fig. 1.
Phylogenetic relationship of gp60 nucleotide sequences of Cryptosporidium hominis in this study and known Cryptosporidium subtype families, as inferred by a neighbor-joining analysis (Mega 4 software [http://www.megasoftware.net/]) based on genetic distances calculated using the Kimura two-parameter model. Bootstrap values greater than 50% from 1,000 pseudoreplicates are shown. The gp60 tree was rooted with GenBank sequence FJ490060. The name of each subtype family starts with the Cryptosporidium species or genotype designation, with C. hominis, Cryptosporidium parvum, Cryptosporidium meleagridis, Cryptosporidium fayeri, C. cuniculus, horse genotype, Cryptosporidium wrairi, ferret genotype, and mouse genotype I represented by I, II, III, IV, V, VI, VII, VIII, and IX, respectively. Ii near the top of the tree is a new C. hominis subtype family found in this study. Subtype families seen in baboons in this study are underlined.
Enterocytozoon bieneusi infection in baboons.
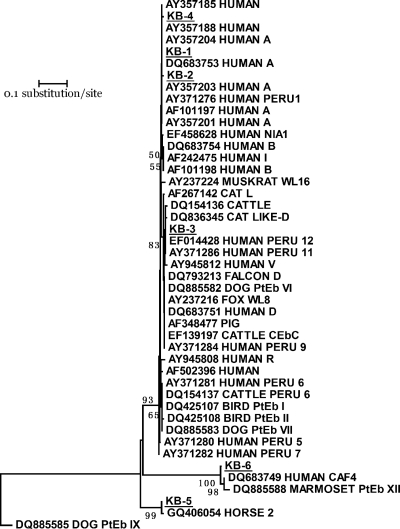
Enterocytozoon bieneusi was identified in 29 (12.3%) of the 235 animals. Juvenile baboons had an infection rate (20.3%) higher than those of adults (9.8%) and infants (7.7%) (P > 0.05). Adult baboons that were positive for E. bieneusi had significantly lower average body weights than those that were negative (12.8 ± 3.0 kg versus 15.1 ± 4.8 kg; P = 0.003), although the differences in body weight between positive and negative animals were not significant in other age groups. All positive animals showed no apparent occurrence of diarrhea. The E. bieneusi belonged to 10 distinct genotypes, 4 of which were known genotypes (A, D, Peru7, and Peru11) and 6 of which represented new genotypes (KB-1 to KB-6) (Table 2). In phylogenetic analysis of the ITS sequences, the genotypes clustered with three groups of existing genotypes (Fig. 2). The first major cluster consisted of genotypes A, D, Peru7, Peru11, and KB-1 to KB-4 from the present study and many other genotypes reported previously in humans, domestic animals, and wild mammals. The second cluster contained KB-6 from this study, genotype CAF4 in humans, and genotype PtEb XII in a marmoset. The third cluster was formed by KB-5 from this study and a recently described genotype 2 in a horse (Fig. 2).
Table 2.
Distribution of Enterocytozoon bieneusi genotypes in captive baboons by sequence analysis of the ITS region of the rRNA gene
| Specimen no. | Animal no. | Gender and agea | Wt (kg) | E. bieneusi genotypeb |
|---|---|---|---|---|
| 27870 | 3110 | FA | 12 | A |
| 27875 | 3115 | MJ | D | |
| 27882 | 3123 | FA | 11.9 | D |
| 27896 | 3139 | FA | 11.4 | D |
| 27897 | 3140 | FI | 3.4 | D |
| 27898 | 3141 | MA | 16.8 | D |
| 28016 | 3538 | MJ | 8.9 | D |
| 28018 | 3541 | MJ | 7 | D |
| 28023 | 3547 | FA | 11 | D |
| 27848 | 3084 | MJ | 7 | Peru7 |
| 27947 | 3384 | MJ | 4.1 | Peru11 |
| 27961 | 3399 | FA | 12 | Peru11 |
| 27976 | 3417 | MA | 21.5 | Peru11 |
| 27985 | 3427 | MJ | 7.3 | KB-1 |
| 27840 | 3076 | FA | 12 | KB-2 |
| 27943 | 3380 | FJ | 7 | KB-3 |
| 27855 | 3093 | FJ | 6.7 | KB-4 |
| 27941 | 3378 | FA | 8.9 | KB-5 |
| 27920 | 3354 | FA | 13 | KB-6 |
| 27922 | 3356 | MJ | 11.6 | KB-6 |
| 27923 | 3357 | FA | 12.2 | KB-6 |
| 27929 | 3365 | FA | 13.8 | KB-6 |
| 27935 | 3372 | FJ | 7.2 | KB-6 |
| 27945 | 3382 | MJ | 4.3 | KB-6 |
| 27946 | 3383 | MJ | 4.5 | KB-6 |
| 27957 | 3395 | MA | 12 | KB-6 |
| 27958 | 3396 | FA | 10 | KB-6 |
| 27963 | 3401 | FA | 13.4 | KB-6 |
| 27984 | 3426 | MJ | 10.5 | KB-6 |
FA, female adult; FJ, female juvenile; FI, female infant; MA, male adult; MJ, male juvenile.
Genotype terminology based on that of Santin and Fayer (9). KB-1 to KB-6 are new genotypes found in this study.
Fig. 2.
Phylogenetic relationship of ITS nucleotide sequences of Enterocytozoon bieneusi in this study and known E. bieneusi genotypes, as inferred by a neighbor-joining analysis (Mega 4 software [http://www.megasoftware.net/]) based on genetic distances calculated using the Kimura two-parameter model. The ITS tree was rooted with GenBank sequence DQ885585. Bootstrap values greater than 50% from 1,000 pseudoreplicates are shown. KB-1 to KB-6 (underlined) are new genotypes found in this study.
Cyclospora infection in baboons.
Cyclospora was detected in 42 (17.9%) of the animals, and the isolates all belonged to one species, Cyclospora papionis. There were no differences in the nucleotide sequences of the SSU rRNA genes obtained in this study and previous studies. Juvenile baboons had an infection rate (26.6%) higher than those of adults (16.3%) and infants (0.0%) (P > 0.05). The average body weight of Cyclospora-positive animals in the adult group was significantly lower than that of negative animals (13.0 ± 3.7 kg versus 15.2 ± 4.8 kg; P < 0.0001). No occurrence of diarrhea was seen in the infected animals.
Public health significance of pathogens in baboons.
Five common C. hominis subtype families, Ia, Ib, Id, Ie, and If, are usually found in children and HIV-infected adults in African countries (13). Within the subtype family Ib, the IbA9G3 subtype found in this study is frequently seen in humans in eastern African countries such as Kenya, Malawi, and Uganda (13). Although the subtype IfA12G2 has thus far not been seen in humans, the If subtype family to which it belongs is known to be prevalent in humans in Africa (5). Thus, captive baboons in Kenya share some of the same C. hominis subtypes circulating in humans locally. The direction of the cross-species transmission of cryptosporidiosis is not clear. It is tempting to conclude that baboons can be reservoirs for human Cryptosporidium infection. However, judged by the common occurrence of IbA9G3 in humans in Kenya and the close contact of baboons with humans, baboons might have acquired the C. hominis infections from humans.
Results of the ITS genotyping showed 10 distinct E. bieneusi genotypes, four of which (A, D, Peru7, and Peru11) have ITS sequences identical to those previously described for humans, including genotype A previously found in AIDS patients in Cameroon, Niger, and Gabon and genotype D previously detected in AIDS patients in Niger, Gabon, Cameroon, and Malawi (9). Of the four reported genotypes, three (genotypes A, Peru7, and Peru11) were previously found only in humans (9). In addition, four of the six new E. bieneusi genotypes in baboons, KB-1 to KB-4, are genetically related to most E. bieneusi genotypes (group 1 by Thellier and Breton [12]) in humans in phylogenetic analysis and thus have zoonotic potential and public health significance. The new genotype KB-6 formed a cluster (group 5 by Thellier and Breton [12]) with the genotype PtEb XII isolated from a marmoset in Portugal (6) and genotype CAF4 from patients in Gabon and Cameroon (2), representing a host-adapted genotype in primates, including humans.
In this study, although a high prevalence of Cyclospora was found in captive baboons, C. papionis was the only species detected, similar to the case in previous reports (3). Despite the detection of many human-pathogenic C. hominis subtypes and E. bieneusi genotypes, the human Cyclospora pathogen, C. cayetanensis, was not found in any of the animals studied. This is in agreement with results of previous characterizations of Cyclospora spp. in nonhuman primates in Ethiopia and Kenya, which also showed the presence of only animal-specific species, including C. papionis, Cyclospora cercopitheci, and Cyclospora colobi, in baboons, vervet monkeys, and colobus monkeys, respectively (3, 4). Thus, baboons are unlikely to be animal reservoirs of the human pathogen C. cayetanensis.
Conclusions.
In summary, results of the present study indicate that newly captive baboons in Kenya harbor human-pathogenic Cryptosporidium subtypes and E. bieneusi genotypes, although this was clearly not the case for Cyclospora. Whether these Cryptosporidium subtypes and E. bieneusi genotypes are circulating within the wild baboon population in isolation from pathogen introduction from humans remains unclear, as, in many places, wild baboons are in close contact with humans, who can be the source of infection in animals, which is the opposite of the more commonly believed direction of transmission. Further studies are also needed to fully elucidate the significance of wild baboons in the epidemiologies of cryptosporidiosis and microsporidiosis in humans. Nevertheless, finding in wild baboons the same major C. hominis subtype and E. bieneusi genotypes that are prevalent in humans living in the same area clearly suggests that efforts to reduce contact between wild nonhuman primates and susceptible human populations and contamination of drinking source water by these reservoir hosts should be made.
Nucleotide sequence accession numbers.
Nucleotide sequences for IfA12G2, IbA9G3, and IiA14 were deposited in GenBank under accession numbers JF681172 to JF681174, respectively, and sequences for KB-1 to KB-6 were deposited under accession numbers JF681175 to JF681180.
Acknowledgments
This study was approved by the Institutional Review Committee (IRC) and the Animal Care and Use Committee (ACUC) of the Institute of Primate Research.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Alves M., et al. 2003. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41: 2744–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breton J., et al. 2007. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. J. Clin. Microbiol. 45: 2580–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eberhard M. L., da Silva A. J., Lilley B. G., Pieniazek N. J. 1999. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n. Emerg. Infect. Dis. 5: 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eberhard M. L., et al. 2001. A survey for Cyclospora spp. in Kenyan primates, with some notes on its biology. J. Parasitol. 87: 1394–1397 [DOI] [PubMed] [Google Scholar]
- 5. Leav B. A., et al. 2002. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect. Immun. 70: 3881–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lobo M. L., et al. 2006. Genotypes of Enterocytozoon bieneusi in mammals in Portugal. J. Eukaryot. Microbiol. 53(Suppl. 1): S61–S64 [DOI] [PubMed] [Google Scholar]
- 7. Mathis A., Weber R., Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ortega Y. R., Sanchez R. 2010. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 23: 218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santín M., Fayer R. 2011. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 90: 363–371 [DOI] [PubMed] [Google Scholar]
- 10. Snowden K. F., Didier E. S., Orenstein J. M., Shadduck J. A. 1998. Animal models of human microsporidial infections. Lab. Anim. Sci. 48: 589–592 [PubMed] [Google Scholar]
- 11. Sulaiman I. M., et al. 2003. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 69: 4495–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thellier M., Breton J. 2008. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite 15: 349–358 [DOI] [PubMed] [Google Scholar]
- 13. Xiao L. 2010. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 124: 80–89 [DOI] [PubMed] [Google Scholar]
- 14. Xiao L., et al. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183: 492–497 [DOI] [PubMed] [Google Scholar]