Abstract
Trichomoniasis is a common sexually transmitted disease associated with preterm birth, low birth weight, and increased susceptibility to infection with other pathogenic sexually transmitted microorganisms. Nucleic acid amplification tests for Trichomonas vaginalis have improved sensitivity for detecting infected individuals compared to existing culture-based methods. This prospective, multicenter U.S. clinical trial evaluated the performance of the automated Aptima T. vaginalis assay for detecting T. vaginalis in 1,025 asymptomatic and symptomatic women. Vaginal swab, endocervical swab, ThinPrep PreservCyt, and urine specimens were collected. Subject infection status was determined by wet-mount microscopy and culture. Aptima T. vaginalis assay performance was determined for each specimen type by comparison to subject infection status. Of 933 subjects analyzed, 59.9% were symptomatic. Aptima T. vaginalis clinical sensitivity and specificity were, respectively, 100% and 99.0% for vaginal swabs, 100% and 99.4% for endocervical swabs, 100% and 99.6% in ThinPrep samples, and 95.2% and 98.9% in urine specimens. Aptima T. vaginalis performance levels were similar in asymptomatic and symptomatic subjects. This study validates the clinical performance of the Aptima T. vaginalis assay for screening asymptomatic and symptomatic women for T. vaginalis infection.
INTRODUCTION
Trichomoniasis, a common sexually transmitted disease (STD) caused by the protozoan Trichomonas vaginalis, affects approximately 180 million persons per year worldwide, making it the most common nonviral STD agent in the world. An estimated 7.4 million new cases occur annually in the United States (1), and the disease has an overall prevalence of 3.1% (24). Both women and men can be infected, although symptoms are more common in women. Symptomatic women have a diffuse, malodorous, yellow-green vaginal discharge with vulvar irritation which may be confused with bacterial vaginosis. Infected men may temporarily have urethral irritation, mild discharge, or slight burning after urination or ejaculation (5). Many infections do not produce symptoms and when left untreated may lead to preterm birth, low birth weight, and pelvic inflammatory disease (5). T. vaginalis infection also increases susceptibility to infection with HIV (14, 22, 23). Effective and inexpensive antibiotic therapy for T. vaginalis infection is readily available, and detection and treatment of T. vaginalis in symptomatic or asymptomatic women with a high risk of STD are important to prevent disease acquisition, transmission, and associated complications.
Currently, the gold standard for the diagnosis of T. vaginalis infection is culture; however, the sensitivity of commercially available culture has been reported to be 75% to 89% compared to amplified methods (13, 20). Tests with improved sensitivity are needed to diagnose this prevalent STD. The Aptima Trichomonas vaginalis assay, an FDA-cleared, fully automated nucleic acid amplification test, has demonstrated high sensitivity and specificity compared to culture (20). Herein, we report the results of a large prospective multicenter trial designed to determine the sensitivity and specificity of this new assay in multiple specimen types from women.
MATERIALS AND METHODS
Subjects and study conduct.
This clinical trial enrolled 1,025 women attending participating U.S. obstetrics and gynecology (OB/GYN), family planning, or STD clinics (collection sites). Subjects 14 years of age or older were enrolled in the study if they demonstrated symptoms consistent with a suspected STD, such as vaginal odor, vaginal discharge, vaginal/vulvar itching, pain/discomfort during sexual intercourse or urination, and/or lower abdominal discomfort; were asymptomatic and had sexual contacts with persons with confirmed or suspected STD(s); were asymptomatic and undergoing screening evaluation for possible STDs; or were undergoing routine Papanicolaou (Pap) screening. Subjects were excluded if they took antibiotic medications within 14 days of enrollment. Subjects signed Institutional Review Board (IRB)-approved informed consent before being enrolled in the study. The protocol of this study was approved by IRBs from all participating sites.
Specimen collection and processing.
At each of the 9 collection sites, 6 specimens were collected from each subject as follows (in order of collection): 1 first-catch urine, 3 vaginal swabs, 1 endocervical swab, and 1 ThinPrep PreservCyt liquid cytology cervical specimen (collected with a broom-like device or an endocervical brush and spatula). Per the study protocol, a 20- to 30-ml urine specimen was self-collected by each subject; all other specimens were clinician collected. One vaginal swab specimen was tested using wet-mount microscopic examination, and another vaginal swab specimen was cultured with the InPouch T. vaginalis test (BioMed Diagnostics, White City, OR) at the collection site's laboratory or a designated laboratory (if the site did not routinely perform culture with the InPouch T. vaginalis test) to determine infection status. The order of the vaginal sample collected for use in the Aptima T. vaginalis assay, wet mount, or culture was randomized for each patient.
One first-catch urine, 1 vaginal swab, 1 endocervical swab, and 1 ThinPrep specimen were processed for Aptima T. vaginalis assay testing by the collection site in accordance with the appropriate package insert instructions as follows. An aliquot of the urine specimen was transferred into the Aptima urine specimen transport tube and stored at 2°C to 30°C. The vaginal swab specimen was placed into an Aptima vaginal swab specimen collection kit tube and stored at 2°C to 30°C. The endocervical swab was placed into an Aptima unisex swab specimen collection kit tube and stored at 2°C to 30°C. The ThinPrep endocervical specimen was processed by immersing and rinsing the collection device (broom-like device or endocervical brush and spatula) in PreservCyt solution in the ThinPrep Pap test vial; an aliquot was transferred into an Aptima specimen transfer kit tube, and the processed ThinPrep sample was stored at 2°C to 8°C. Approximately one-third of all processed samples were then shipped to 1 of 3 designated testing sites.
Sample evaluation. (i) Wet-mount microscopic examination and InPouch T. vaginalis test culture.
Collection site laboratories performed wet-mount microscopic examination using a vaginal swab specimen. In general, the specimen on the swab was treated with a saline solution and examined under a microscope for motile trichomonads. The collection site's laboratory performed culture with the InPouch T. vaginalis test for all except three sites, for which the InPouch T. vaginalis test was performed at an outside laboratory. The pouch was examined daily for the presence of motile trichomonads in accordance with the package insert instructions (2) or for up to 5 days. Technicians performing these procedures were blind to molecular test results.
(ii) Aptima T. vaginalis assay.
The Aptima T. vaginalis assay is a nucleic acid amplification test that utilizes target capture, transcription-mediated amplification (TMA), chemiluminescent probe hybridization, and the automated Tigris DTS system to detect T. vaginalis 18s rRNA. Processed samples were tested in accordance with the investigational Aptima T. vaginalis assay package insert instructions (9). Each Aptima T. vaginalis assay testing site tested approximately one-third of the study samples and used 3 lots of Aptima T. vaginalis assay reagent kits over the course of the study. Operators performing the Aptima T. vaginalis assay were blind to the subjects' clinical data and wet-mount microscopic examination and culture results. Aptima T. vaginalis assay results were not used for determining treatment or patient care.
(iii) Discordant analysis.
Supplemental testing of discordant specimens was conducted using (i) a characterized real-time T. vaginalis PCR assay for the detection of T. vaginalis DNA (10), and (ii) an alternate TMA (Alt-TMA) T. vaginalis assay developed at Gen-Probe which has been previously used to resolve T. vaginalis test discordant results (18–20). This alternate assay uses unique primer, probe, and target capture oligomers to target a different region of the T. vaginalis 18s rRNA (20). A cutoff for T. vaginalis positivity of 100,000 RLU was used with the Alt-TMA T. vaginalis assay for consistency with the Aptima T. vaginalis assay. Both the PCR and Alt-TMA T. vaginalis assays exhibit analytical sensitivity of <1 organism/reaction.
Infection status.
The infection status was established by testing 2 vaginal swab specimens with wet-mount microscopic examination and by culturing with the InPouch T. vaginalis test. The infection status was considered positive if the InPouch T. vaginalis test and/or the wet-mount microscopic examination result was positive. The infection status was considered negative if both InPouch T. vaginalis test and wet-mount microscopic examination results were negative. The infection status was considered indeterminate if either the InPouch T. vaginalis test or the wet-mount microscopic evaluation result was missing and the other test result was negative.
Data analyses: Aptima T. vaginalis assay performance evaluation.
Samples that were not handled in accordance with the Aptima T. vaginalis assay package insert instructions or the clinical protocol and samples with invalid Aptima T. vaginalis assay results or indeterminate infection status were excluded from the analyses (n = 354); this allowed estimation of the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the Aptima T. vaginalis assay. The score method was used for calculating the 2-sided 95% confidence intervals (CIs) for sensitivity and specificity. The exact method was used to compute the 2-sided 95% CIs for PPV and NPV based on the positive and negative likelihood ratios. Analyses were also stratified by collection site, symptom status, and age group (women 14 to 17 years of age versus women 18 years or older).
RESULTS
Subject disposition.
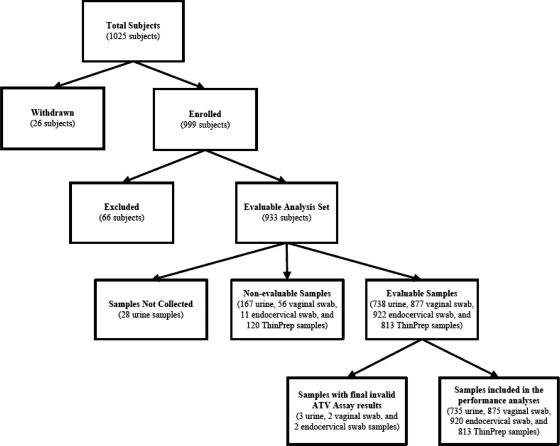
A total of 1,025 women were enrolled at 9 sites; of these women, 26 were withdrawn because they did not meet study eligibility criteria, and 66 were excluded because the samples exceeded the recommended storage period before testing (n = 48) or did not have a conclusive infection status (n = 18). Thus, 933 of 1,025 (91.0%) subjects were included in the evaluable analysis set. These subjects produced 3,343 samples (735 urine, 875 vaginal swab, 920 endocervical swab, and 813 ThinPrep samples) with valid Aptima T. vaginalis assay results, which were included in the final analysis. A detailed subject disposition is presented in Fig. 1.
Fig. 1.
Of 1,025 subjects enrolled at 9 sites, there were 933 evaluable subjects (91.0%), from which we planned to collect 3,732 samples (933 subjects with 4 samples each). Of these, 382 (10.2%) samples were either missing or considered nonevaluable (expired). Of the 3,350 evaluable samples (738 urine, 877 vaginal swab, 922 endocervical swab, and 813 ThinPrep samples), 3,343 (99.8%) had final valid results; seven (0.2%; 3 urine, 2 vaginal swab, and 2 endocervical swab) samples had final invalid results and were not included in the analyses. ATV, Aptima T. vaginalis.
Subject demographic and disease characteristics.
Subjects' demographic and clinical characteristics are presented in Table 1. The median age of the 933 evaluable subjects was 24.0 years (range: 14 to 67 years). Overall, 59.6% of the subjects were black/African American and 33.8% were white. The majority of subjects were symptomatic (59.9%). The most frequently reported symptom was vaginal discharge (75.1%), followed by vaginal odor (43.3%) and vaginal/vulvar itch (32.9%). Overall, 36.0% of the subjects were diagnosed with vaginosis, 15.9% with vaginitis, and 7.1% with cervicitis. The prevalence of T. vaginalis infection in this population ranged from 11.4% in both urine (84/735) and ThinPrep (93/813) samples to 12.7% (111/875) in vaginal swab samples (Table 2).
Table 1.
Subject demographic and clinical characteristics
| Characteristic | Value |
|---|---|
| Total no. | 933 |
| Age, yr | |
| Mean (SD) | 27.0 (9.50) |
| Median | 24.0 |
| Min-max | 14–67 |
| Age groups, no. (%) | |
| 14–17 | 81 (8.7) |
| 18–20 | 177 (19.0) |
| 21–25 | 284 (30.4) |
| 26–30 | 136 (14.6) |
| 31–35 | 88 (9.4) |
| 36–40 | 76 (8.1) |
| >40 | 91 (9.8) |
| Ethnicity, no. (%) | |
| Hispanic or Latino | 93 (10.0) |
| Not Hispanic or Latino | 836 (89.6) |
| Unknown/refused | 4 (0.4) |
| Race, no. (%)a | |
| White | 315 (33.8) |
| Black or African American | 556 (59.6) |
| Asian | 8 (0.9) |
| American Indian/Alaska native | 10 (1.1) |
| Unknown/refused | 58 (6.2) |
| Symptom status, no. (%) | |
| Asymptomatic | 374 (40.1) |
| Symptomatic | 559 (59.9) |
| Reported symptom (symptomatic subjects), no. (%)b | |
| Vaginal odor | 242 (43.3) |
| Vaginal discharge | 420 (75.1) |
| Vaginal/vulvar itch | 184 (32.9) |
| Pain/discomfort during sexual intercourse | 33 (5.9) |
| Lower abdominal discomfort | 99 (17.7) |
| Pain/discomfort during urination | 54 (9.7) |
| Other | 89 (15.9) |
| Clinical diagnosis, no. (%) | |
| Vaginosis | 336 (36.0) |
| Vaginitis | 148 (15.9) |
| Cervicitis | 66 (7.1) |
| Urethritis/cystitis | 12 (1.3) |
| Pelvic inflammatory disease | 8 (0.9) |
| Unknown | 73 (7.8) |
| Otherc | 446 (47.8) |
Subjects may have reported multiple races.
Subjects may have reported multiple symptoms.
Includes diagnosis of “normal.”
Table 2.
Performance of Aptima T. vaginalis assay in the different specimens by symptom status
| Aptima T. vaginalis assay specimen | Symptom status | No. of samplesa |
% Prevalenceb | % Sensitivity (95% CI) | % Specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | TP | FP | TN | FN | |||||||
| Urine | Asymptomatic | 324 | 21 | 3 | 299 | 1 | 6.8 | 95.5 (78.2–99.2) | 99.0 (97.1–99.7) | 87.5 (71.4–96.9) | 99.7 (98.4–100) |
| Symptomatic | 411 | 59 | 4 | 345 | 3 | 15.1 | 95.2 (86.7–98.3) | 98.9 (97.1–99.6) | 93.7 (85.7–98.1) | 99.1 (97.7–99.8) | |
| All women | 735 | 80 | 7 | 644 | 4 | 11.4 | 95.2 (88.4–98.1) | 98.9 (97.8–99.5) | 92.0 (85.1–96.4) | 99.4 (98.5–99.8) | |
| Vaginal swab | Asymptomatic | 345 | 24 | 4 | 317 | 0 | 7.0 | 100 (86.2–100) | 98.8 (96.8–99.5) | 85.7 (70.3–95.6) | 100 (98.9–100) |
| Symptomatic | 530 | 87 | 4 | 439 | 0 | 16.4 | 100 (95.8–100) | 99.1 (97.7–99.6) | 95.6 (89.5–98.8) | 100 (99.2–100) | |
| All women | 875 | 111 | 8 | 756 | 0 | 12.7 | 100 (96.7–100) | 99.0 (97.9–99.5) | 93.3 (87.6–97.0) | 100 (99.5–100) | |
| Endocervical swab | Asymptomatic | 372 | 26 | 1 | 345 | 0 | 7.0 | 100 (87.1–100) | 99.7 (98.4–99.9) | 96.3 (82.4–99.9) | 100 (99.0–100) |
| Symptomatic | 548 | 88 | 4 | 456 | 0 | 16.1 | 100 (95.8–100) | 99.1 (97.8–99.7) | 95.7 (89.6–98.8) | 100 (99.2–100) | |
| All women | 920 | 114 | 5 | 801 | 0 | 12.4 | 100 (96.7–100) | 99.4 (98.6–99.7) | 95.8 (90.7–98.6) | 100 (99.6–100) | |
| ThinPrep | Asymptomatic | 353 | 23 | 0 | 330 | 0 | 6.5 | 100 (85.7–100) | 100 (98.8–100) | 100 (86.2-undc) | 100 (99.0–100) |
| Symptomatic | 460 | 70 | 3 | 387 | 0 | 15.2 | 100 (94.8–100) | 99.2 (97.8–99.7) | 95.9 (88.9–99.1) | 100 (99.1–100) | |
| All women | 813 | 93 | 3 | 717 | 0 | 11.4 | 100 (96.0–100) | 99.6 (98.8–99.9) | 96.9 (91.4–99.3) | 100 (99.5–100) | |
TP, true positive; FP, false positive; TN, true negative; FN, false negative.
(TP + FN)/n.
und, undefined; the upper boundary of the confidence interval could not be calculated.
Aptima T. vaginalis assay performance in different specimen types in all women.
The sensitivity, specificity, NPV, and PPV of the Aptima T. vaginalis assay in the different specimen types are presented in Table 2. Sensitivity values for the detection of T. vaginalis ranged from 95.2% using urine samples to 100% using vaginal swab, endocervical swab, or ThinPrep samples; the negative predictive values for T. vaginalis detection in these sample types ranged from 99.4% (urine) to 100% (vaginal swab, endocervical swab, ThinPrep). The specificity of the Aptima T. vaginalis assay for the detection of T. vaginalis in these samples was also high, ranging from 98.9% (urine) to ≥99% for vaginal swab, endocervical swab, or ThinPrep samples; the positive predictive values for these samples ranged from 92.0% to 96.9%. There was no significant difference between the sensitivity and specificity of detecting T. vaginalis in urine samples and those for other sample types (P > 0.05).
Analysis of false-negative and false-positive Aptima T. vaginalis assay results.
Twenty-seven samples (0.8%) had discordant results in the Aptima T. vaginalis assay compared with the infection status; of these, 4 were false negatives (all urine) and 23 were classified as false positives. The PCR and Alt-TMA T. vaginalis assays were used to provide supplemental information on the 27 samples with discordant Aptima T. vaginalis assay results relative to the established infection status. The testing results were not used to modify the calculated performance characteristics of the Aptima T. vaginalis assay.
(i) Analysis of the 4 false-negative samples.
Four urine specimens were negative by the Aptima T. vaginalis assay despite a positive infection status for these 4 subjects. All 4 subjects had positive Aptima T. vaginalis assay results for the other 3 specimen types. These 4 urine samples were also negative when tested with the Alt-TMA T. vaginalis assay and with PCR (Table 3), suggesting that these samples lacked T. vaginalis nucleic acids.
Table 3.
Discordant test result analysis with PCR and Alt-TMA T. vaginalis assays
| Specimen type | Aptima T. vaginalis assay results | Infection status | Interpretationa | Total no. | No. with indicated T. vaginalis result by: |
|||
|---|---|---|---|---|---|---|---|---|
| PCRb |
Alt-TMA |
|||||||
| Positive | Negative | Positive | Negative | |||||
| Urine | Positive | Negative | FP | 7 | 1 | 6 | 5 | 2 |
| Negative | Positive | FN | 4 | 0 | 4 | 0 | 4 | |
| Vaginal swab | Positive | Negative | FP | 8 | 1 | 7 | 8 | 0 |
| Endocervical swab | Positive | Negative | FP | 5b | 2 | 2 | 5 | 0 |
| ThinPrep | Positive | Negative | FP | 3 | 1 | 2 | 3 | 0 |
FP, false positive; FN, false negative.
One of the 5 false-positive samples was not analyzed by PCR.
(ii) Analysis of false-positive samples.
Twenty-three samples (7 urine, 8 vaginal swab, 5 endocervical swab, and 3 ThinPrep samples) with false-positive results were observed in 10 subjects with negative infection status. Nine of these 10 subjects had positive Aptima T. vaginalis assay results in at least 2 sample types. The T. vaginalis PCR assay also produced 5 positive results for the specimens considered false positive (1/7 for urine, 1/8 for vaginal swab, 2/4 for endocervical swab, and 1/3 for ThinPrep samples) (Table 3). Alt-TMA T. vaginalis assay test results were positive for all vaginal swab, endocervical swab, and ThinPrep samples and for 5 of the 7 urine samples with false-positive Aptima T. vaginalis assay results. Overall, these results indicate the presence of the T. vaginalis rRNA target in those specimens (Table 3).
Aptima T. vaginalis assay performance by symptom status.
Overall, the sensitivities, specificities, and negative predictive values of the Aptima T. vaginalis assay were similar in asymptomatic and symptomatic subjects, despite the prevalence of T. vaginalis infection in symptomatic women (15.1% to 16.4%) being higher than its prevalence in asymptomatic women (6.5% to 7.0%) (Table 2). The PPV for urine and vaginal swabs was slightly lower (by approximately 6 to 10 percentage points) in asymptomatic subjects than in symptomatic subjects (Table 2). The performance of the Aptima T. vaginalis assay levels were very similar for samples collected from adolescent women and adult women and for samples collected from different geographic locations (data not shown).
DISCUSSION
This study was conducted to validate Aptima T. vaginalis assay performance characteristics for the detection of T. vaginalis in self-collected first-catch urine specimens and clinician-collected endocervical swab, vaginal swab, and endocervical ThinPrep samples, with standard wet-mount and culture methods used as the reference. T. vaginalis is widely prevalent in the U.S. population and is associated with important public health sequelae such as preterm birth and acquisition/transmission of HIV. Control of trichomoniasis has been hampered by the lack of sufficiently sensitive diagnostic tests. The most commonly used method is visualization of motile trichomonads in a saline preparation from the vaginal swab (wet-mount microscopic examination). Although this test is rapid and inexpensive, it has two important limitations: it must be performed within 10 to 20 min of collection of the specimen (or the organisms lose viability), and it has a low sensitivity (ranging from 60% to 70%) as compared to culture (5). A commercial culture method (InPouch T. vaginalis test) has a reported sensitivity of approximately 80% and a specificity of 100% compared with wet mount (21) or with Diamond's modified medium (17). Trichomonads may be visualized on the Pap smear, but this also has a limited sensitivity (approximately 60%) (15). Other tests for T. vaginalis include the OSOM Trichomonas rapid test (Genzyme Diagnostics, Cambridge, MA) and the Affirm VP III (Becton Dickenson, San Jose, CA). Both tests are performed on vaginal secretions with a sensitivity of >83% and a specificity of >97% compared with culture or wet mount (3, 4, 8, 12, 13).
In this study, the use of the highly sensitive Aptima T. vaginalis assay found that the prevalence of T. vaginalis infection ranged from 11.4% in urine and ThinPrep specimens to 12.7% in vaginal swabs. These values are higher than the national average of 3.6% (24), possibly because of the high proportion of symptomatic subjects enrolled in this study (55.1%) who presented for screening for a possible STD or who had a partner with an STD. The prevalence values reported here also agree with previously reported T. vaginalis molecular test prevalence rates of 11.3% to 28.7% (11, 13, 19, 20).
The Aptima T. vaginalis assay sensitivity (95.2% in urine samples and 100% in endocervical swab, vaginal swab, and ThinPrep samples) and specificity (∼99%) values reported herein (Table 2) are similar to those reported in previous publications (11, 13, 18, 20). In the present study, the overall Aptima T. vaginalis assay sensitivity (≥95.2% in any specimen type) was higher than published sensitivity estimates for the wet-mount microscopic examination (60% to 70% [6]) and culture using the InPouch T. vaginalis test (81% and 82.4% [17, 21]). This is in agreement with studies that evaluated the analyte-specific-reagent-formatted Aptima T. vaginalis assay and these two reference assays in side-by-side comparisons (13, 20).
The performance levels of the Aptima T. vaginalis assay were nearly identical when testing endocervical ThinPrep specimens, endocervical swab samples, and vaginal swab samples. Performance of the Aptima T. vaginalis assay was somewhat lower in self-collected urine samples; however, the difference in assay sensitivity between vaginal or cervical (100%) and urine samples (95.2%) was not statistically significant. This finding is in agreement with results from Nye et al. (20), who reported a slightly but not significantly lower sensitivity for urine samples (87.5%) than for vaginal and endocervical swab samples (96.6% and 89.8%, respectively) by use of the Aptima T. vaginalis assay in conjunction with a molecular test-resolved algorithm.
In the present study, 4 (0.43%) subjects had urine samples with negative Aptima T. vaginalis assay results and positive results for the other specimen types, indicating that these urine results were false negatives. The rate of urethral colonization in women with a vaginal T. vaginalis infection has been estimated at ∼75% (16). Thus, it is possible that these 4 subjects had T. vaginalis infections in the vagina (they had positive vaginal samples) but were not colonized in the urethra (negative urine samples). Alternatively, it is possible that the presence of only a few trichomonad cells in the urethra or urinary meatus at the time of voiding could have resulted in very low cell concentrations in the urine sample, thus leading to sampling error upon addition of the 2-ml urine aliquot to the urine transport tube. However, in a separate study, we performed quantitative molecular testing for T. vaginalis in 38 urine samples obtained in this trial and found that the median trichomonad cell load was 311 cells/ml of urine, with a mean of 2,040 cells/ml of urine (data not shown). Since the analytical sensitivity of the Aptima T. vaginalis assay is less than 0.1 cell/ml in urine samples, we conclude that these 4 women likely were infected with T. vaginalis in the vagina but not in the urinary tract.
In spite of the slightly lower sensitivity of the Aptima T. vaginalis assay in detecting T. vaginalis in urine specimens, testing of urine is still of clinical benefit due to the high clinical sensitivity shown and the ease of collection of this sample type. Because first-catch urine is a noninvasive, self-collected specimen that does not require a pelvic examination, it represents a convenient specimen collection method for screening large populations, especially in STD clinics and institutional settings.
The specificity of the Aptima T. vaginalis assay was high, ranging from 98.9% in urine samples to 99.6% in ThinPrep samples. False-positive Aptima T. vaginalis assay results were observed in 10 of 933 (1.1%) subjects. However, 9 of these 10 subjects had positive Aptima T. vaginalis assay results in at least 2 other specimen types, suggesting that these 9 subjects were actually infected with trichomonads. Some of these specimens were also found positive by the T. vaginalis PCR assay and most were positive with the Alt-TMA T. vaginalis assay, confirming the presence of T. vaginalis rRNA target and indicating that these samples were likely true positives. These findings imply that the true clinical specificity of the Aptima T. vaginalis assay is actually higher than reported here (98.9% to 99.6%). This issue typically occurs when the performance of the evaluated assay is calculated based on reference assays with inherently lower sensitivity and has been previously reported for TMA-based assays for Chlamydia trachomatis detection (7).
Importantly, the performance levels were of the Aptima T. vaginalis assay were similar in symptomatic and asymptomatic subjects, in adolescent and adult women, and in samples obtained from different collection sites, indicating that the Aptima T. vaginalis assay has broad clinical utility and consistent performance for the detection of T. vaginalis and diagnosis of trichomoniasis in many female populations. In addition, the ability to use multiple specimen types in the Aptima T. vaginalis assay provides clinicians with more options for T. vaginalis testing, which is an important advantage over wet-mount examination or culture methods that use vaginal swabs only. Moreover, the ability to use this highly accurate molecular test on a fully automated instrumentation system represents an effective solution for large-scale screening for T. vaginalis infections in certain populations.
In summary, the results from this study validate the clinical performance characteristics of the Aptima T. vaginalis assay using self-collected first-catch urine specimens and clinician-collected endocervical swab, vaginal swab, and endocervical ThinPrep specimens from symptomatic and asymptomatic women. The superior performance of this method compared to that of the reference tests (wet-mount microscopic examination and culture) should improve the screening, diagnosis, and treatment of T. vaginalis infection.
ACKNOWLEDGMENTS
We thank Rangaraj Selvarangan, Jill Huppert, Neil B. Quigley, Edward Hook, Paul Fine, Kimberle Chapin, Stephen Kasparian, and Mark Martens for assistance with conducting the clinical trial and Rebecca Gunnill and Florence Paillard for assistance with preparation of the manuscript.
Michael G. Catania, Barbara S. Weinbaum, Ann D. Johnson, and Damon K. Getman are employed by Gen-Probe Inc. and hold stock options in that company. Marcia M. Hobbs has received research support from Gen-Probe Inc. Jane R. Schwebke has consulted for Gen-Probe Inc., BioReference Laboratories, Cepheid, and Graceway Pharmaceuticals. Stephanie N. Taylor has supported clinical trials for Gen-Probe, Cepheid, and Roche.
Footnotes
Published ahead of print on 21 September 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. American College of Obstetricians and Gynecologists (ACOG) 2006. ACOG practice bulletin no. 72: vaginitis. Obstet. Gynecol. 107:1195–1206 [DOI] [PubMed] [Google Scholar]
- 2. BioMed Diagnostics 2007. InPouch TV Trichomonas vaginalis test. BioMed Diagnostics, White City, OR [Google Scholar]
- 3. Briselden A. M., Hillier S. L. 1994. Evaluation of affirm VP microbial identification test for Gardnerella vaginalis and Trichomonas vaginalis. J. Clin. Microbiol. 32:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell L., Woods V., Lloyd T., Elsayed S., Church D. L. 2008. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J. Clin. Microbiol. 46:3467–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2006. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm. Rep. 55(RR-11):1–94 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2007. CDC fact sheet: trichomoniasis. http://www.cdc.gov/std/trichomonas/STDFact-Trichomoniasis.htm Accessed 12 September 2010
- 7. Chernesky M. A., Jang D. E. 2006. APTIMA transcription-mediated assays for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Mol. Rev. Diagn. 6:519–525 [DOI] [PubMed] [Google Scholar]
- 8. DeMeo L. R., et al. 1996. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am. J. Obstet. Gynecol. 174:1339–1342 [DOI] [PubMed] [Google Scholar]
- 9. Gen-Probe Inc 2010. APTIMA Trichomonas vaginalis assay for in vitro diagnostic use. Gen-Probe Inc., San Diego, CA: http://www.gen-probe.com/pdfs/pi/502246-EN-RevB.pdf [Google Scholar]
- 10. Hardick J., Yang S., Lin S., Duncan D., Gaydos C. 2003. Use of the Roche LightCycler instrument in a real-time PCR for Trichomonas vaginalis in urine samples from females and males. J. Clin. Microbiol. 41:5619–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hardick A., Hardick J., Wood B. J., Gaydos C. 2006. Comparison between the Gen-Probe transcription-mediated amplification Trichomonas vaginalis research assay and real-time PCR for Trichomonas vaginalis detection using a Roche LightCycler instrument with female self-obtained vaginal swab samples and male urine samples. J. Clin. Microbiol. 44:4197–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huppert J. S., et al. 2005. Use of an immunochromatographic assay for rapid detection of Trichomonas vaginalis in vaginal specimens. J. Clin. Microbiol. 43:684–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huppert J. S., et al. 2007. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin. Infect. Dis. 45:194–198 [DOI] [PubMed] [Google Scholar]
- 14. Laga M., et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95–102 [DOI] [PubMed] [Google Scholar]
- 15. Lara-Torre E., Pinkerton J. S. 2003. Accuracy of detection of trichomonas vaginalis organisms on a liquid-based papanicolaou smear. Am. J. Obstet. Gynecol. 188:354–356 [DOI] [PubMed] [Google Scholar]
- 16. Lawing L. F., Hedges S. R., Schwebke J. R. 2000. Detection of trichomonosis in vaginal and urine specimens from women by culture and PCR. J. Clin. Microbiol. 38:3585–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levi M. H., Torres J., Piña C., Klein R. S. 1997. Comparison of the InPouch TV culture system and Diamond's modified medium for detection of Trichomonas vaginalis. J. Clin. Microbiol. 35:3308–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munson E., et al. 2008. Impact of Trichomonas vaginalis transcription-mediated amplification-based analyte-specific-reagent testing in a metropolitan setting of high sexually transmitted disease prevalence. J. Clin. Microbiol. 46:3368–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munson E., et al. 2010. Trichomonas vaginalis transcription-mediated amplification-based analyte-specific reagent and alternative target testing of primary clinical vaginal saline suspensions. Diagn. Microbiol. Infect. Dis. 68:66–72 [DOI] [PubMed] [Google Scholar]
- 20. Nye M. B., Schwebke J. R., Body B. A. 2009. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am. J. Obstet. Gynecol. 200:188.e1–188.e7 [DOI] [PubMed] [Google Scholar]
- 21. Ohlemeyer C. L., Hornberger L. L., Lynch D. A., Swierkosz E. M. 1998. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J. Adolesc. Health 22:205–208 [DOI] [PubMed] [Google Scholar]
- 22. Schwebke J. R., Burgess D. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17:794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shafir S. C., Sorvilla F. J., Smith L. 2009. Current issues and consideration regarding trichomoniasis and human immunodeficiency virus in African-Americans. Clin. Microbiol. Rev. 22:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sutton M., et al. 2007. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin. Infect. Dis. 45:1319–1326 [DOI] [PubMed] [Google Scholar]