Abstract
Invasive group A streptococcal (GAS) infections cause significant morbidity and mortality. A national survey was initiated to assess the burden of invasive GAS infections in France, describe their clinical characteristics, and assess the molecular characteristics of GAS strains responsible for these infections. The survey was conducted in 194 hospitals, accounting for 51% of acute care hospital admissions in France. Clinical data, predisposing factors, and demographic data were obtained, and all GAS isolates were emm sequence typed. We identified 664 cases of invasive GAS infections, with an annual incidence of 3.1 per 100,000 population. The case-fatality ratio was 14% and rose to 43% in the case of streptococcal toxic shock syndrome. Bacteremia without identified focus (22%) and skin/soft tissue infections (30%) were the most frequent clinical presentations. Necrotizing fasciitis was frequent in adults (18%) and uncommon in children (3%). The 3 predominant emm types were emm1, emm89, and emm28, accounting for 33%, 16%, and 10% of GAS isolates, respectively. The emm1 type was associated with fatal outcomes and was more frequent in children than in adults. Six clusters of cases were identified, with each cluster involving 2 invasive cases due to GAS strains which shared identical GAS emm sequence types. Four clusters of cases involved eight postpartum infections, one family cluster involved a mother and child, and one cluster involved two patients in a nursing home. Invasive GAS infection is one of the most severe bacterial diseases in France, particularly in persons aged ≥50 years or when associated with toxic shock syndrome.
INTRODUCTION
Streptococcus pyogenes (group A streptococcus [GAS]) causes a wide variety of diseases ranging from mild pharyngitis and impetigo to severe invasive infections, including streptococcal toxic shock syndrome (TSS) and necrotizing fasciitis. The lethality of severe GAS infections remains high, ranging from 14% to 19% in high-income countries (8, 11, 12, 16, 22, 29). In addition, outbreaks of invasive GAS infections have been described in the community, in nursing homes, and in hospitals (7, 13, 17, 24, 26). Although rarely reported, secondary transmission occurs among household contacts (9, 18, 25).
In France, invasive GAS infection surveillance relies on the Epibac national hospital-based laboratory network and on the characterization of GAS strains sent to the French National Reference Center for Streptococci. Since 1987, the Epibac network has been collecting data from participating hospital laboratories on bacteremic infections and meningitis due to 6 bacterial species, including GAS. These infections are defined as the isolation of the bacterium from blood (bacteremia) or cerebrospinal fluid (CSF; meningitis). The participating hospitals account for more than 75% of French acute care admissions, as described at http://www.invs.sante.fr/surveillance/epibac/default.htm.
The French National Reference Center for Streptococci has been collecting GAS strains isolated from invasive and noninvasive GAS infections since 1995 (28).
According to Epibac data, between 2000 and 2006, the incidence of GAS bacteremia and meningitis increased by 32% in France, from 1.5 to 2.0 cases per 100,000 population (French Institute for Public Health Surveillance, unpublished data). National guidelines for the prevention of secondary cases of invasive GAS infection in the community and hospitals were issued in 2005 and 2006, respectively (5, 6). Antibiotic prophylaxis is recommended for all household contacts of a patient with invasive GAS infection when one of them presents with a predisposing factor to invasive GAS infection.
In order to better characterize the epidemiology of invasive GAS infections, we conducted a national prospective survey of invasive GAS infections in metropolitan France. The main goals were to (i) estimate the burden of invasive GAS infections with or without a positive blood culture, (ii) characterize the clinical presentations, (iii) assess predisposing factors and outcomes, (iv) describe the molecular characteristics and antibiotic susceptibility of GAS strains isolated from invasive infections, and (v) assess the level of implementation of the recommendations on antibiotic prophylaxis among household contacts (6).
MATERIALS AND METHODS
Design.
We conducted a cross-sectional survey over a 1-year period from November 2006 to November 2007. Among the 332 hospital laboratories eligible for Epibac surveillance, 194 laboratories located throughout the 22 French administrative regions participated on a voluntary basis. The participating hospitals accounted for 51% of French acute care inpatient admissions in 2007.
Case definition.
GAS invasive infection was defined as the isolation of the bacterium from a usually sterile site (e.g., blood, cerebrospinal fluid, joint, bone, or synovial fluid) or from samples obtained from deep-body-site aspirates, intraoperative specimens, or a nonsterile site in association with one of the following clinical conditions: necrotizing fasciitis, clinically ascertained pneumonia, endometritis, salpingitis, or TSS not attributable to any other cause and defined according to the U.S. Working Group on Severe Streptococcal Infections definitions (31).
Invasive GAS infections identified during the hospital stay or within the 7 days following the hospital discharge were presumed to be nosocomial if they occurred at least 48 h after the time of admission or if the patient underwent a surgical operation during the 7 days preceding the onset of GAS infection. Postpartum infections were presumed to be nosocomial if they occurred during the hospital stay or within 7 days after discharge.
A confirmed GAS infection in a case contact was defined as isolation of GAS from the site of infection or, in case of acute pharyngitis, as a positive rapid antigen detection test for GAS.
Data collection.
Cases were identified by the local microbiologist, who completed a standardized questionnaire for each case meeting the case definition with the support of the local infectious diseases specialist and of the attending physician. Data collected included age, sex, clinical presentations, predisposing factors, and outcomes at the time of discharge from hospital. Information regarding the occurrence of a GAS infection among close contacts 30 days before or after the onset of disease of the identified case was also collected. Close contacts were defined as individuals living in the same household or institution as the case or having close and/or repeated contact during the 7 days preceding the onset of the index case (5). In hospitals, close contacts were patients hospitalized in the same ward (6). Additional information regarding possible clusters of nosocomial invasive GAS infection was retrieved from mandatory notifications of nosocomial invasive GAS infection and from available investigation reports. The survey's questionnaires were sent to the French Institute for Public Health Surveillance staff, who reviewed the inclusion criteria and the completeness of the data. The microbiologists were reminded to report cases by regular phone calls and mailings.
Microbiological methods.
GAS isolates were confirmed to be S. pyogenes by beta-hemolysis on sheep blood agar, presence of Lancefield group A antigen, and production of pyrrolydonyl arylamidase (20). Susceptibility to penicillin, amoxicillin, vancomycin, erythromycin, tetracycline, and clindamycin was determined according to the French Society for Microbiology guidelines described at http://www.sfm.asso.fr. All available isolates were also screened for streptococcal pyrogenic exotoxin speA, speB, and speC genes and ssa genes by multiplex PCR assay. When identical isolates on these markers were obtained from any given patient, only the first invasive isolate was further characterized by molecular typing. The emm gene sequencing was performed as described by Beall et al. (1) with the modifications described at www.cdc.gov/ncidod/biotech/strep.htm.
Data analysis.
The incidence of invasive GAS infections in France was estimated by applying a correcting factor yielded by the survey to the incidence of GAS bacteremic infections and meningitis in 2007 ascertained by Epibac surveillance (IncEPI). IncEPI is calculated by dividing the number of reported cases by the population covered by Epibac surveillance, corrected for the rates of underreporting of cases, which is evaluated by 3 sources of capture-recapture analysis (2, 3, 10, 14, 23). The population coverage was assessed to be 78% in 2007, and a 20% underreporting rate was assumed.
Invasive GAS incidence was therefore computed as IncEPI/(1 − k), where k is the correcting factor corresponding to the proportion of invasive GAS infections in the survey where GAS has not been isolated from blood or cerebrospinal fluid, and IncEPI is the incidence estimated through Epibac. Age-specific incidence rates for invasive GAS infections were estimated by applying to the specific age groups considered the same methodology described above for the all-age invasive GAS infection incidence rate.
A cluster of invasive GAS infections was defined as the occurrence of ≥2 invasive GAS infection cases in close contacts within 30 days for community-acquired invasive GAS infections and within 6 months for institutionalized invasive GAS infection cases. Clusters were confirmed if the related invasive GAS infection cases were due to GAS strains of identical emm sequence types.
Associations between outcomes, clinical presentations, predisposing factors, and strain characteristics were tested using Fisher's exact test for binomial data. Associations between each specific clinical presentation or predisposing factor with death were tested irrespective of the presence of other clinical presentations or predisposing factors. Death predictors were evaluated using logistic regression modeling. Statistical analysis was performed using Stata (version 9.2) software (Stata Corporation, College Station, TX).
RESULTS
Number of cases, demographic characteristics, and incidence.
We identified 664 invasive GAS infections meeting the case definition. GAS strains were isolated from blood cultures alone (n = 345, 52%), blood cultures and other sterile sites (n = 47, 7%), blood cultures and nonsterile sites (n = 75, 11%), sterile sites other than blood (n = 127, 19%), and nonsterile sites (n = 70, 11%); cases where strains were isolated from nonsterile sites were included on the basis of the presence of at least one of the following clinical manifestations: toxic shock syndrome (n = 21), necrotizing fasciitis (n = 22), endometritis (n = 23), salpingitis (n = 2), or pneumonia (n = 2).
The median age of the patients was 55 years (range, 28 days to 103 years). The male-to-female ratio was 0.9.
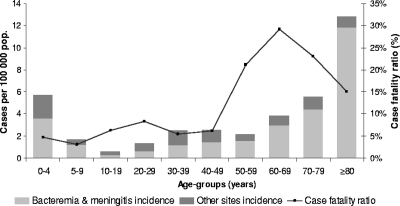
The estimates of invasive GAS infection incidence in 2007 by age group are presented in Fig. 1. On the basis of Epibac surveillance, the incidence of GAS bacteremic infections and meningitis was 2.2 cases per 100,000 population in 2007. Moreover, GAS invasive infections where GAS was not isolated from blood or cerebrospinal fluid accounted for 29% (n = 193) of the total invasive GAS infections reported in the survey. Therefore, the overall incidence of invasive GAS infections in France was estimated to be 3.1 (95% confidence interval [CI], 2.9 to 3.2) cases per 100,000 population in 2007. The highest incidence occurred in children <5 years of age (5.7 per 100,000) and in adults ≥70 years of age (8.4 per 100,000). Incidence rates were similar for men and women except in those aged 50 to 69 years, where the incidence was higher in men than in women (3.5 versus 2.2 per 100,000; P < 0.001).
Fig. 1.
Age-specific rates and case-fatality ratios of invasive group A streptococcal infections in France in 2007.
Clinical presentations.
Among the 664 invasive GAS infections included, various clinical presentations were reported (Table 1). Nonnecrotic skin or soft tissue infections were the most frequent, accounting for 30% of the cases; blood cultures were positive in 82% of these cases. Necrotizing fasciitis was reported in 16% of cases, pleuropulmonary infection in 11%, septic arthritis in 9%, and postpartum sepsis in 5%. Other clinical presentations, such as intra-abdominal infections, osteomyelitis, and gynecological infections, were reported in less than 5% of cases. No identified focus of invasive GAS infection was reported in 22% of cases, although the portal of entry was clinically suspected in 71% (n = 105) to be the skin in 60%, the respiratory tract in 30%, or other sites in 10%.
Table 1.
Clinical presentations, incidence of positive blood cultures, and outcomes among 664 invasive group A streptococcal infection cases
| Clinical presentation | No. (%) of cases |
No. of cases with fatal outcome/total no. (%)c | ||
|---|---|---|---|---|
| Totala | With positive blood cultureb | Presenting with a TSSb | ||
| Bacteremia without identified focus | 147 (22) | 147 (100) | 24 (16) | 32/144 (22)f |
| Skin or soft tissue infection | 196 (30) | 160 (82) | 29 (15) | 17/193 (9)g |
| Necrotizing fasciitis | 104 (16) | 49 (47) | 45 (43) | 22/102 (22)f |
| Pleuropulmonary infectiond | 71 (11) | 44 (62) | 24 (34) | 15/68 (22) |
| Postpartum infection | 32 (5) | 12 (38) | 0 (0) | 0/32 (0)g |
| Intra-abdominal infection | 24 (4) | 11 (46) | 5 (21) | 3/23 (13) |
| Septic arthritis | 59 (9) | 22 (37) | 7 (12) | 2/54 (4)g |
| Osteomyelitis | 22 (3) | 13 (59) | 0 (0) | 0/22 (0) |
| Other clinical presentatione | 48 (7) | 30 (63) | 10 (21) | 5/45 (11) |
The total number does not add up to 664, as certain patients presented with more than one clinical presentation; percentages are given with respect to the 664 cases.
Percentages are of cases presenting with the clinical presentation.
Outcomes were available for a total of 646 cases. The total number of cases does not add up to 646, as certain patients presented with more than one clinical presentation.
Cases presenting a pleuropulmonary infection (n = 71) included 48 cases presenting with pneumonia, 12 cases presenting with a primary pleural infection, and 11 cases presenting with pneumonia and pleural infection.
Other clinical presentations included 48 patients presenting 51 clinical presentations: meningitis (n = 7), salpingitis/endometritis (n = 14), endocarditis (n = 7), other upper respiratory tract infection (n = 10), brain abscess (n = 3), renal abscess/pyelonephritis (n = 4), vascular catheter infection (n = 2), urinary catheter infection (n = 2), hip prosthesis infection (n = 1), and bacteremia from digestive tract infection (n = 1).
P < 0.05 for a positive association between the presence of the specific clinical presentation and death (Fisher exact test for binary data).
P < 0.05 for a negative association between the presence of the specific clinical presentation and death (Fisher exact test for binary data).
Septic arthritis, osteomyelitis, and pleural infection were observed more frequently in children (age, <15 years; n = 109) than in adults (age, ≥15 years; n = 554), being reported in 20%, 15%, and 12% of children, respectively, and in 7%, 1%, and 2% of adults, respectively (P < 0.001 each). Necrotizing fasciitis was mostly observed in adults and uncommon in children (18% versus 3%; P < 0.001). Postpartum cases accounted for 32% (32/100) of cases in women of childbearing age (15 to 49 years).
A TSS was associated in 20% of cases (Table 2). It was 3- and 1.9-fold more frequent in the presence of necrotizing fasciitis and pulmonary infection, respectively (P < 0.001 and P < 0.01), than in other clinical presentations. TSS was reported in children (15%) and in adults (21%), but it was more frequent in persons aged 50 to 69 years than in other age groups (31% versus 17%; P < 0.001) (Table 2).
Table 2.
Ages and outcomes among 664 invasive group A streptococcal infection cases
| Age group (yr) | No. (%) of cases |
No. of cases with fatal outcome/total no. (%)a | |
|---|---|---|---|
| Total | Presenting with a TSS | ||
| 0-14 | 109 (17) | 16 (15) | 4/105 (4) |
| 15-49 | 188 (28) | 27 (14) | 12/184 (7) |
| 50-69 | 142 (21) | 44 (31) | 35/138 (25)b |
| ≥70 | 224 (34) | 44 (20) | 40/218 (18)b |
| All agesc | 664 (100) | 131 (20) | 91/646 (14) |
Outcomes were available for 646 cases.
P < 0.05 for the test of a difference in the frequency of death compared with that for the 0- to 14-year-old age group, used as the reference group (Fisher exact test for binary data).
Includes one patient without age information.
Predisposing factors.
Predisposing factors were ascertained for 657 (99%) cases; 507 (77%) presented at least one predisposing factor for invasive GAS infection (Table 3). A cutaneous pathology (e.g., burns, varicella, skin disease, and wounds) was reported in 247 (38%) cases and in 114 (52%) of those aged ≥70 years. A chronic medical condition such as diabetes, malignancy, or liver or cardiac chronic disease was reported in 234 (36%) cases. Among children (age, <15 years), 55 (51%) presented a predisposing factor; varicella was reported in 20 (19%), primarily in those under 5 years of age (19/20), and some other lesion or disease of the skin was reported in 19 (18%). Only 6 (6%) children presented with a chronic medical condition.
Table 3.
Predisposing factors and outcomes among invasive GAS infection cases
| Predisposing factora | No. (%) of cases |
No. of cases with fatal outcome/total no. (%)d | |
|---|---|---|---|
| Totalb | Presenting with a TSSc | ||
| Immunosuppression | 49 (7) | 9 (18) | 9/47 (19) |
| Steroid use | 30 (5) | 8 (27) | 6/28 (21) |
| Chemotherapy | 18 (3) | 6 (33) | 7/16 (44)g |
| Injection drug use | 9 (1) | 1 (11) | 1/9 (11) |
| Skin disease or wounde | 247 (38) | 48 (19) | 33/242 (14) |
| Varicella | 20 (3) | 3 (15) | 1/20 (5) |
| Skin wound | 193 (29) | 41 (21) | 27/188 (14) |
| Skin disease | 46 (7) | 8 (17) | 6/46 (13) |
| Burn | 3 (0) | 0 (0) | 0/3 (0) |
| Chronic medical conditionf | 234 (36) | 56 (24) | 56/226 (25)g |
| Diabetes | 88 (13) | 22 (25) | 21/86 (24)g |
| Malignancy | 42 (6) | 12 (29) | 11/39 (28)g |
| Hepatic disease | 28 (4) | 6 (21) | 10/28 (36)g |
| Renal impairment | 25 (4) | 6 (24) | 7/25 (28) |
| Heart disease | 44 (7) | 10 (23) | 10/43 (23) |
| Other chronic medical condition | 31 (5) | 8 (26) | 6/29 (21) |
| Alcoholism | 34 (5) | 14 (41) | 7/33 (21) |
| Surgery <8 days earlier | 18 (3) | 4 (22) | 2/16 (13) |
| Childbirth <4 wk earlier | 32 (5) | 0 (0) | 0/32 (0)h |
| No predisposing factor reported | 150 (23) | 28 (19) | 13/149 (9)h |
Information on the presence of a predisposing factor was available for 657 patients.
Patients may have more than one predisposing factor; percentages are calculated using the 657 cases as the denominator.
Percentages are calculated using the number of cases presenting the specific predisposing factor as the denominator.
Outcomes were available for 646 cases. The total number of cases does not add up to 646, as certain patients presented more than one predisposing factor.
The total of the subcategories does not add up to 247, as certain patients presented more than one type of skin disease or wound.
The total of the subcategories does not add up to 234, as certain patients presented more than one chronic medical condition.
P < 0.05 for a positive association between the presence of the specific predisposing factor and death (Fisher exact test for binary data).
P < 0.05 for a negative association between the presence of the specific predisposing factor and death (Fisher exact test for binary data).
Ninety-six cases (15%) were considered nosocomial; among them, 32 (5%) were postpartum infection cases.
emm sequence typing and antimicrobial susceptibility.
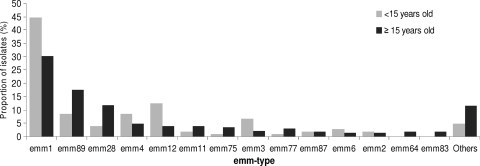
Among the 664 cases, at least one GAS strain for 623 cases (94%) was sent to the National Reference Center for Streptococci, which analyzed only one representative strain per case. A total of 48 different emm types were identified, and 3 types, emm1 (n = 203, 33%), emm89 (n = 100, 16%), and emm28 (n = 65, 10%), accounted for 59% of isolates, followed by emm4 (n = 34, 5%) and emm12 (n = 33, 5%) (Fig. 2). No association between the distribution of emm types and geographical location was observed. emm1, emm3, and emm12 types were more frequently identified in children than in adults (45% versus 30% [P < 0.05], 7% versus 2% [P < 0.05], and 12% versus 4% [P < 0.01], respectively), while the emm89 type was less frequently identified in children than in adults (9% versus 18% [P < 0.05]).
Fig. 2.
Distribution of emm types among 623 GAS strains responsible for invasive infections in children and adults.
The speA, speB, speC, and ssa genes were detected in 37%, 100%, 49%, and 12% of strains, respectively. The speA gene was carried by 97% of emm1 strains (196/203) and 100% of emm3 strains (18/18), whereas it was carried by only 4% (16/399) of strains of other emm types.
The emm1 type was associated with a higher frequency of TSS (P < 0.001) and fatal outcomes (P < 0.001) than other emm types in univariate analysis. The presence of the speA gene was also associated with a higher frequency of TSS (P < 0.001) and fatal outcomes (P < 0.001).
All GAS strains were susceptible to penicillin, amoxicillin, and vancomycin; 8% were resistant to erythromycin, 13% to tetracycline, and 6% to clindamycin. Among GAS isolates from children (n = 105), 4%, 3%, and 1% were resistant to erythromycin, tetracycline, and clindamycin, respectively. A higher prevalence of resistance was observed among GAS isolates from adults (n = 518), with 9%, 15%, and 7% being resistant to erythromycin, tetracycline, and clindamycin, respectively.
Outcomes.
Overall, 31% (n = 209) of cases required intensive care unit admission and 28% (n = 183) underwent surgery. The outcome at the time of discharge from hospital was available for 646 cases (Table 2). The overall in-hospital lethality was 14% (95% CI, 11% to 17%), reaching 43% (56/129) in patients who presented with TSS. More than two-thirds of the deaths (62/91, 68%) occurred within the 4 days following the onset of infection.
Among 104 necrotizing fasciitis cases, 64% required intensive care unit admission and 69% underwent surgery, 22% (22/102) died, and 31% (32/102) had a permanent complication at the time of discharge from hospital, such as an amputation (11%) or skin or soft tissue or muscular defects (14%) due to necrosis. Overall, only 46% (47/102) of the patients who presented with a case of necrotizing fasciitis recovered without sequelae.
Bacteremia without an identified focus, necrotizing fasciitis, pneumonia, and TSS were all associated with a higher risk of death in univariate analysis (P < 0.01, P < 0.05, P < 0.05, and P < 0.001, respectively).
Death was associated with the presence of a predisposing factor (16% versus 9%, P < 0.05). Among predisposing factors, chemotherapy, diabetes, malignancy, and hepatic disease were all associated with death in univariate analysis (P < 0.01, P < 0.01, P < 0.05, and P < 0.01, respectively).
According to the results of multivariate logistic regression analysis, age ≥50 years, hepatic disease, TSS, bacteremia without an identified focus, and emm1 type were all independently associated with an increased risk of a fatal outcome (Table 4).
Table 4.
Results of multivariate logistic regression analysis of factors associated with death in invasive GAS infectiona
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Age <50 yr | Reference | |
| Age ≥50 yr | 2.9 (1.5-5.6) | <0.01 |
| Toxic shock syndrome | 9.9 (5.6-17.5) | <0.001 |
| Bacteremia without identified focus | 2.5 (1.4-4.7) | <0.01 |
| Chemotherapy | 3.9 (0.9-16.2) | 0.06 |
| Diabetes | 1.8 (0.9-3.6) | 0.10 |
| Liver disease | 3.7 (1.3-10.5) | 0.02 |
| emm1 type | 2.2 (1.2-3.8) | <0.01 |
Data are for 600 cases. Clinical presentations, predisposing factors, emm types, and patient characteristics were entered in the model if associated with an increased or a decreased frequency of death on univariate analysis (P < 0.20) and retained in the model if associated with death (P < 0.10). Finally, factors were considered to be independently associated with death if associated with a P value of 0.05 or less (Wald test). Patients with missing data (n = 64) were excluded from the analysis. Two-way interactions were evaluated.
Antibiotic prophylaxis and cluster of GAS infections in close contacts.
Information on the prescription of an antibiotic prophylaxis to household contacts was available for 451 (68%) cases. Among them, 50 (11%) reported having one or more of their household contacts who presented with a predisposing factor for invasive GAS infection; 266 (59%) cases did not report such a factor among their contacts, and 135 (30%) lived alone. In following French recommendations, an antibiotic prophylaxis was to be prescribed to each member of these 50 households (i.e., 103 household contacts); the recommendations were actually fully respected for all the household contacts in only 7 households (including 11 household contacts) and partially realized in 1 household (1 antibiotic was prescribed to 1 child with varicella, while the 5 other household contacts did not receive any). No prophylaxis at all was conducted in 42 households (including 86 household contacts). Despite a poor compliance with the recommendations, no subsequent case of invasive GAS infection was reported in the 103 household contacts of these 50 households.
Information concerning the occurrence of a GAS infection in close contacts of the case was available for 518 (78%) cases. A noninvasive GAS infection was confirmed in a close contact of 11 (2%) cases; the contact case was a member of the household (8 cases), shared the same hospital room (1 case), or was a resident of the same nursing home (2 cases).
Subsequent invasive GAS infection cases were identified in the 30 days following the onset of a case among the close contacts of 7 (1%) cases; for 5 of these cases, GAS strains were of identical emm type, and for 2 they were of different emm types. In addition to these 5 clusters, a cluster of 3 cases of GAS infection (2 invasive cases and 1 noninvasive case) which shared identical emm89 GAS strains was identified with a delay of 77 days in 3 residents of the same nursing home.
Overall, 6 clusters involving 12 invasive GAS infections cases were confirmed on the basis of epidemiological links and isolation of GAS strains of identical emm types (Table 5). One family cluster due to an emm1 strain (cluster 1) occurred in a 34-year-old mother and her 3-year-old daughter, both of whom were previously healthy; the mother died and the young girl recovered. One cluster of two invasive infections and one noninvasive infection due to an emm89 strain (cluster 2) occurred in three chronically disabled residents of a nursing home. Four postpartum clusters involved two women each who shared the same emm4, emm11, emm28, or emm89 strain. Investigation reports were available for 3 clusters; the suspected route of contamination was direct transmission in the members of the family in cluster 1, indirect transmission from one resident to another during skin wound care in cluster 2, and intrahospital patient-to-patient contamination during the postdelivery period in cluster 5 (Table 5).
Table 5.
Confirmed clustersa of invasive GAS infections
| Cluster no. | Setting | No. of cases (clinical presentation) |
Duration (days) | emm type(s) (no. of strains/total no. of strains) | Suspected mode of transmissionb | |
|---|---|---|---|---|---|---|
| Invasive | Noninvasive | |||||
| 1 | Household | 2 (1 pneumonia with empyema, 1 pneumonia and TSS) | 1 (pharyngitis) | 7 | emm1 (3/3) | Intrafamilial |
| 2 | Nursing home | 2 (2 bacteremic skin infections) | 1 (lower genital tract infection) | 77 | emm89 (3/3) | Indirect transmission from patient to patient during the care of skin wounds of the 3 patients by hand-borne transmission or by transmission from a carrier among health care staff (not screened) |
| 3 | Postnatal ward A | 3 (1 wound infection from cesarean delivery and endometritis, 2 endometritis) | 20 | emm11 (2/3), emm1 (1/3) | Unknown | |
| 4 | Postnatal ward B | 2 (1 bacteremic endometritis, 1 endometritis) | 3 | emm89 (2/2) | Unknown | |
| 5 | Postnatal ward C | 2 (1 bacteremic endometritis, 1 endometritis) | 2 | emm4 (2/2) | Patient to patient in the postdelivery period, where the 2nd case shared the same room as the index case | |
| 6 | Postnatal ward D | 2 (1 bacteremic endometritis, 1 endometritis) | 3 | emm28 (2/2) | Unknown | |
A confirmed cluster was defined as the occurrence in 30 days (in the community) or 6 months (in institutional settings) of ≥2 invasive GAS cases due to GAS strains sharing identical emm sequence types.
The mode of transmission was suspected on the basis of the results of investigations of the infection control staff.
DISCUSSION
This nationwide prospective survey enables the determination of the incidence of invasive GAS infections in France. It indicates that cases not captured by routine surveillance of bacteremia and meningitis account for a substantial part (29%) of invasive GAS infections. Given this result, the estimated rate in 2007 was 3.1 cases per 100,000 population, a rate comparable to the 2.2 to 3.8 recently reported from surveys in Europe, the United States, and Canada (11, 22, 27).
The highest risks of invasive GAS infections were identified in young children and elderly patients, as previously reported (11, 27). This study also highlights the high severity of invasive GAS infections, with a case-fatality ratio of 14%, which is also in agreement with the ratios reported in the United States (14%) (22), Sweden (14.5%) (8), and Denmark (16%) (16).
This survey allowed a comprehensive assessment of the epidemiology of invasive GAS infections in France, since predisposing factors, outcomes, and GAS isolates were obtained from more than 90% of the cases.
Regarding the representativeness of the survey, although the survey was conducted on a voluntary basis, the participating hospitals accounted for 51% of French acute care admissions in 2007 and were distributed in all French regions. Regarding case characteristics, the age and sex distribution of bacteremic invasive GAS cases included did not differ from those of bacteremic cases identified in Epibac surveillance in 2007, suggesting no significant bias in the recruitment of cases. Regarding the estimation of invasive GAS infection incidence, as Epibac results are regularly validated through 3 sources of capture-recapture analysis, we consider that in our survey our estimation of global invasive GAS infection incidence based on the Epibac rates for bacteremia or meningitis, corrected by the proportion of invasive GAS infections where the GAS has not been isolated from blood or CSF, provides the best incidence estimate.
Inclusion of cases with GAS isolates obtained from a nonsterile site (i.e., 23 cases of endometritis, 2 cases of salpingitis, and 2 cases of pneumonia), accounting for 4% of the total cases, may hamper comparison of the results with those of surveys which include only cases with GAS isolates from normally sterile sites (8, 16, 22). However, the design of our survey led to a more comprehensive ascertainment of the burden of invasive GAS infections in our country.
The results emphasize the severity of invasive GAS infections in adults over 50 years of age, who accounted for the majority (82%) of fatal invasive GAS infections. This may in part be explained by the high frequency of TSS and preexisting chronic medical conditions in this age group. On the contrary, children and women with postpartum endometritis were less likely to present severe clinical manifestations, such as necrotizing fasciitis, TSS, or a fatal outcome. The highest risk of fatal outcome was associated with pneumonia, necrotizing fasciitis, and bacteremia without an identified focus.
emm1 was the main emm type associated with a higher risk of a fatal outcome, after adjusting for other predisposing factors, as previously reported in Europe and the United States (15, 22). Most of the emm1 strains carried the speA gene, which was also associated with severe infections and outcomes. However, as the presence of the speA gene was highly correlated with emm1 type, the role of the speA gene in the occurrence of TSS or a fatal outcome could not be distinguished from that of emm1 type. Moreover, emm1 strains were more frequent in patients without chronic medical conditions (36% versus 27%, P < 0.02) and in children. These results confirm the high virulence potential of emm1 strains circulating in France and other countries. The second most frequent emm types included emm89 and emm28, which were also frequent in the United States, Denmark, and Sweden (8, 16, 22). On the contrary, the emm3 type, which was identified to be preponderant and highly virulent in the United States and other European countries (15, 21, 22), accounted for only 3% of the strains isolated from invasive infections in our survey.
Skin diseases or cutaneous lesions were the most common predisposing factor, especially in elderly and younger patients. Skin conditions were reported in more than half of the elderly (52%), and varicella was reported in one-fifth of children under the age of 15 years. This observation highlights the importance of skin breaches as portals of entry for GAS infection, as also noticed in previous reports (12, 29, 30).
Six clusters of 12 GAS invasive infections with identical emm types were identified; 4 of them involved 8 postpartum cases accounting for 25% (8/32) of postpartum cases. This suggests that a substantial proportion of postpartum GAS infections is preventable. In addition, 10% (64/632) of nonpostpartum invasive GAS infections were suspected to have been acquired in hospital. The significant number of postpartum cases reported in the survey could partially be related to the mandatory reporting of nosocomial infections since 2001 and the incentive to investigate postpartum and postsurgery GAS infections, as recommended in guidelines issued in 2006 (5). Sequencing of the emm gene was very important in the investigation of clusters of cases to confirm the links between epidemiologically related cases.
The prescription of antibiotic prophylaxis in household members of GAS-infected patients when one of them presents with a predisposing factor has been recommended since 2005 (5, 6). This survey suggests that these recommendations were inconsistently applied and strengthens the need to reinforce their implementation in community settings.
In France, as in other developed countries, invasive GAS infections are among the most severe bacterial infections. Overall, we estimated that 1,900 cases of these infections occurred in France in 2007. Assuming that the cases included in the survey were representative of invasive GAS infections in France, we estimated that 270 patients died from such infections in 2007.
Current strategies to prevent GAS invasive diseases still rely on early detection of GAS infections, rapid and effective medical care, and reinforcement of outbreak investigation and control, particularly in health care settings. However, identification of factors associated with mortality can guide disease prevention efforts. It was estimated that a 26-valent vaccine candidate, which has reached phase II trials in adults, could potentially prevent 40% to 50% of cases and 50% to 60% of deaths due to invasive GAS infections among children and the elderly (4, 19). In this context, the follow-up of GAS strains involved in severe GAS infections is important to adapt current vaccine development strategies.
ACKNOWLEDGMENTS
We thank the members of the survey's scientific committee, Catherine Chirouze, Julien Loubinoux, Alain Lortat Jacob, Jean Pierre Bedos, Frédéric Laurent, Marc Lecuit, and Claude Bernet, for their assistance in the preparation of the survey's protocol and questionnaire and their advice for the implementation of the survey; Jean Claude Desenclos for his helpful reading and comments on the manuscript; all the clinicians and microbiologists from the participating hospitals who collected the data; and Gislène Collobert and Gérald Touak for excellent technical assistance.
Footnotes
A list of the microbiologists of the Epibac network is available at http://www.invs.sante.fr/.
Published ahead of print on 5 October 2011.
REFERENCES
- 1. Beall B., Facklam R., Thompson T. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger F., Bernillon P., Gallay A. 2010. Surveillance des infections invasives à méningocoque en France métropolitaine en 2005. Évaluation quantitative par la méthode de capture-recapture à trois sources. Institut de Veille Sanitaire, Saint-Maurice. France: http://www.invs.sante.fr/surveillance/iim/default.htm [Google Scholar]
- 3. Berger F., et al. 2007. Three-sources capture-recapture analysis to evaluate the comprehensiveness of reporting invasive meningococcal infections in France, poster 3. Abstr. 9th Meet. Eur. Monitoring Group Meningococci http://www.invs.sante.fr/surveillance/iim/poster_emgm_rome.pdf [Google Scholar]
- 4. Bisno A. L., Rubin F. A., Cleary P. P., Dale J. B. 2005. Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles—report of a National Institute of Allergy and Infectious Diseases workshop. Clin. Infect. Dis. 41:1150–1156 [DOI] [PubMed] [Google Scholar]
- 5. Conseil Supérieur d'Hygiène Publique de France, Comité Technique des Infections Nosocomiales et des Infections Liées aux Soins 2006. Guide pour la prévention et l'investigation des infections hospitalières à Streptococcus pyogenes. Ministère de la Santé, Paris, France [Google Scholar]
- 6. Conseil Supérieur d'Hygiène Publique de France, Section des Maladies Transmissibles 2005. Avis relatif à la conduite à tenir autour d'un ou de plusieurs cas, d'origine communautaire, d'infections invasives à Streptococcus pyogenes (ou streptocoques du groupe A) séance du 18 Novembre 2005. Ministère de la Santé, Paris, France [Google Scholar]
- 7. Daneman N., et al. 2005. Hospital-acquired invasive group A streptococcal infections in Ontario, Canada, 1992-2000. Clin. Infect. Dis. 41:334–342 [DOI] [PubMed] [Google Scholar]
- 8. Darenberg J., et al. 2007. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin. Infect. Dis. 45:450–458 [DOI] [PubMed] [Google Scholar]
- 9. Davies H. D., et al. 1996. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N. Engl. J. Med. 335:547–554 [DOI] [PubMed] [Google Scholar]
- 10. Goulet V., et al. 2001. Effect of prevention measures on incidence of human listeriosis, France, 1987-1997. Emerg. Infect. Dis. 7:983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamagni T. L., et al. 2008. Epidemiology of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 46:2359–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamagni T. L., et al. 2008. Severe Streptococcus pyogenes infections, United Kingdom, 2003-2004. Emerg. Infect. Dis. 14:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamagni T. L., et al. 2008. Epidemic of severe Streptococcus pyogenes infections in injecting drug users in the UK, 2003-2004. Clin. Microbiol. Infect. 14:1002–1009 [DOI] [PubMed] [Google Scholar]
- 14. Lepoutre A., Varon E., Georges S., Gutmann L., Levy-Bruhl D. 2008. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill. 13(35):pii=18962 [DOI] [PubMed] [Google Scholar]
- 15. Luca-Harari B., et al. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 47:1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luca-Harari B., et al. 2008. Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J. Clin. Microbiol. 46:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manning S. E., et al. 2005. Invasive group A streptococcal infection in high school football players, New York City, 2003. Emerg. Infect. Dis. 11:146–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinaud C., et al. 2009. A family outbreak due to an emm-type 11 multiresistant strain of Streptococcus pyogenes. Clin. Microbiol. Infect. 16:292–295 [DOI] [PubMed] [Google Scholar]
- 19. McNeil S. A., et al. 2005. Safety and immunogenicity of 26-valent group a Streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114–1122 [DOI] [PubMed] [Google Scholar]
- 20. Mihaila-Amrouche L., Bouvet A., Loubinoux J. 2004. Clonal spread of emm type 28 isolates of Streptococcus pyogenes that are multiresistant to antibiotics. J. Clin. Microbiol. 42:3844–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Brien K. L., et al. 2002. Epidemiology of invasive group a Streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35:268–276 [DOI] [PubMed] [Google Scholar]
- 22. O'Loughlin R. E., et al. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45:853–862 [DOI] [PubMed] [Google Scholar]
- 23. Perrocheau A., et al. 2006. Estimation du nombre total de méningites à pneumocoque de l'enfant par la méthode capture-recapture à 3 sources, France, 2001-2002, p. 16-19 Bulletin epidémiologique hebdomadaire 2-3. Institut de Veille Sanitaire, Saint-Maurice, France: http://www.invs.sante.fr/beh/2006/02_03/beh_02_03_2006.pdf [Google Scholar]
- 24. Raymond J., Schlegel L., Garnier F., Bouvet A. 2005. Molecular characterization of Streptococcus pyogenes isolates to investigate an outbreak of puerperal sepsis. Infect. Control Hosp. Epidemiol. 26:455–461 [DOI] [PubMed] [Google Scholar]
- 25. Robinson K. A., et al. 2003. Risk for severe group A streptococcal disease among patients' household contacts. Emerg. Infect. Dis. 9:443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thigpen M. C., et al. 2007. Nursing home outbreak of invasive group A streptococcal infections caused by 2 distinct strains. Infect. Control Hosp. Epidemiol. 28:68–74 [DOI] [PubMed] [Google Scholar]
- 27. Tyrrell G. J., Lovgren M., Kress B., Grimsrud K. 2005. Invasive group A streptococcal disease in Alberta, Canada (2000 to 2002). J. Clin. Microbiol. 43:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varon E., et al. 1997. Comparison of invasive (septicemic) and non invasive strains of group A streptococci isolated during a one-year national survey in France. The Groupe d'Enquete 1995 sur les Infections Streptococciques. Adv. Exp. Med. Biol. 418:83–85 [DOI] [PubMed] [Google Scholar]
- 29. Vlaminckx B., et al. 2004. Epidemiological features of invasive and noninvasive group A streptococcal disease in The Netherlands, 1992-1996. Eur. J. Clin. Microbiol. Infect. Dis. 23:434–444 [DOI] [PubMed] [Google Scholar]
- 30. Wahl R. U., Lutticken R., Stanzel S., van der Linden M., Reinert R. R. 2007. Epidemiology of invasive Streptococcus pyogenes infections in Germany, 1996-2002: results from a voluntary laboratory surveillance system. Clin. Microbiol. Infect. 13:1173–1178 [DOI] [PubMed] [Google Scholar]
- 31. Working Group on Severe Streptococcal Infections 1993. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA 269:390–391 [PubMed] [Google Scholar]