Abstract
Preemptive ganciclovir (GCV) therapy is adopted increasingly in hematopoietic stem cell transplant (HCT) recipients, but occasional cases of increasing cytomegalovirus (CMV) antigenemia levels occur during preemptive GCV therapy. This prospective study investigated the incidence, risk factors, and clinical outcomes of paradoxical responses during GCV therapy. Adult patients receiving allogeneic HCTs during a 24-month period were enrolled. Patients were prospectively monitored for CMV antigenemia once a week until 3 months after engraftment. Paradoxical responders were defined as patients exhibiting CMV antigenemia levels elevated from the baseline after the first week of preemptive GCV therapy. Of 252 HCT recipients, 97 (38%) received preemptive GCV therapy due to CMV infection. Of these 97 patients, 23 (24%) were classified as paradoxical responders. Risk factors for paradoxical response were a low white blood cell (WBC) count (P = 0.02) and a prolonged duration of CMV antigenemia (P = 0.04) before preemptive therapy. There were no significant differences in rates of successful viral clearance and secondary episodes of CMV infection between paradoxical responders (87% [20/23] and 26% [6/23]) and nonparadoxical responders (95% [70/74] and 23% [17/74], respectively). However, breakthrough CMV disease during preemptive GCV therapy was significantly more frequent in paradoxical responders (17% [4/23]) than in nonparadoxical responders (3% [2/74], P = 0.03). Paradoxical responses occurred in one-quarter of the HCT recipients receiving preemptive GCV therapy. A low WBC count and a long duration of CMV antigenemia before GCV therapy were associated with paradoxical responses, and breakthrough CMV disease during preemptive GCV therapy occurred more frequently in paradoxical responders.
INTRODUCTION
Cytomegalovirus (CMV) diseases, including pneumonitis, gastroenteritis, retinitis, and encephalitis, are among the most important causes of morbidity and mortality in patients who undergo allogeneic hematopoietic stem cell transplantation (HCT) (3, 17, 18). Recently, the value of noninvasive diagnostic methods such as CMV blood antigenemia assay (1) and CMV DNA assay by PCR (5) in identifying candidates for preemptive therapy after HCT has been demonstrated. While preemptive ganciclovir (GCV) therapy based on the CMV blood antigenemia assay is being adopted increasingly for HCT recipients (2), occasional cases show increasing CMV antigenemia levels during preemptive GCV therapy. However, there are limited data on the clinical characteristics and outcomes of these paradoxical responders to GCV (7–9, 15). We therefore investigated the incidence of and risk factors for paradoxical responses among HCT patients during preemptive GCV therapy and their clinical outcomes.
(This study was presented in part at the 49th Annual Meeting of the Infectious Diseases Society of America, 20 to 23 October 2011 [poster session, abstr. 31020].)
MATERIALS AND METHODS
Patients and data collection.
All adult patients undergoing allogeneic HCT between February 2009 and January 2011 at a 2,700-bed tertiary-care hospital in Seoul, South Korea, were eligible for this study. Patients with CMV antigenemia who were not treated for CMV disease before preemptive GCV therapy were included in the analysis. Patient demographic information, including clinical data, was obtained from the hospital's electronic database. Age, gender, underlying disease, conditioning regimen, donor type, HLA matching, donor CMV serostatus, grade of graft-versus-host disease (GVHD), GVHD prophylaxis, and immunosuppressive status were included in the analysis and were evaluated as risk factors for changes in CMV antigenemia levels on preemptive GCV therapy.
CMV antigenemia monitoring.
HCT recipients were prospectively monitored for CMV antigenemia once weekly from day 21 to day 100 post-HCT. Monitoring could be changed to biweekly between discharge and day 100 post-HCT if the patient's antigenemia had been consistently negative. After 100 days, monthly CMV antigenemia assays were conducted to monitor for CMV reactivation until 1 year after transplantation. For antigenemia monitoring, 10-ml heparinized blood samples were processed and 2 × 105 cells were stained after fixation (Light Diagnostic CMV pp65 Antigenemia; Chemicon International Inc., Temecula, CA) (11). Patients were classified into low and high CMV risk groups. Those receiving antithymoglobulin (ATG) in the preparative regimen, those with grade 3 to 4 acute GVHD, and those receiving more than 0.5 mg/kg methylprednisolone were defined as high-risk patients. CMV antigenemia of ≥5/250,000 cells in high-risk patients or ≥20/250,000 cells in low-risk patients was an indication for preemptive GCV therapy. According to the discretion of each attending hematologist, a conventional dose (5 mg/kg twice daily) or a low dose (5 mg/kg per day) of GCV was administered for at least 2 weeks or until the patients were CMV antigenemia negative.
Definitions.
Paradoxical responders were defined as patients who exhibited a CMV antigenemia level elevated from the baseline, which was defined as the antigenemia level immediately before the initiation of preemptive GCV therapy, after the first week of preemptive GCV therapy. CMV disease was defined as CMV viremia plus clinical or histologic involvement of an organ due to the same infection (6, 13). For example, CMV hepatitis was defined as biochemical and histological evidence of hepatitis with CMV inclusions, positive immunohistochemical staining, or in situ hybridization for CMV. CMV gastrointestinal disease was defined as symptoms and signs of upper or lower gastrointestinal dysfunction and a tissue biopsy sample showing CMV inclusions or positive immunohistochemical staining. CMV pneumonitis was defined as symptoms like dyspnea and interstitial infiltrations on a chest radiograph confirmed by bronchoalveolar lavage sample cytology or culture. CMV syndrome was defined as meeting all of the following requirements: fever (>38°C) for at least 2 days within a 4-day period, the presence of neutropenia or thrombocytopenia, and CMV viremia (6, 13). CMV disease occurring during preemptive antiviral therapy was defined as breakthrough disease. CMV disease was stratified into two groups: early, occurring before day 100 post-HCT, and late, occurring after day 100 post-HCT (18).
Statistical analysis.
Categorical variables were compared using Fisher's exact test or the Pearson χ2 test, as appropriate, and continuous variables were compared using the Mann-Whitney U test or Student's t test. All tests of significance were two tailed, and a P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS (version 18.0; SPSS, Chicago, IL) and GraphPad Prism (version 5.0; GraphPad Software, San Diego, CA).
RESULTS
Patient demographic data.
During the study period, 252 adult patients underwent allogeneic HCT. Of these, six patients who received GCV therapy due to CMV diseases before preemptive CMV therapy were excluded from the analysis. Finally, 97 (38%) HCT recipients who showed CMV antigenemia and received preemptive GCV therapy were analyzed. Of these, 23 (24%; 95% confidence interval, 16 to 34%) were classified as paradoxical responders whose antigenemia level was increased after 1 week of antiviral therapy. The remaining 74 (76%) patients were classified as nonparadoxical responders. Patient baseline characteristics are shown in Table 1. Both groups of donors and recipients were similar in terms of median age, initial diagnosis, underlying disease, hematopoietic progenitor cell source, and CMV serostatus. There was no significant difference in the incidence of acute GVHD between the two groups (P = 0.66). The overall proportion of patients with each grade of acute GVHD or without GVHD did not differ between the patients who showed paradoxical responses and those who showed nonparadoxical responses.
Table 1.
Baseline clinical characteristics and risk factors for increasing antigenemia of HCT recipients by response
| Patient characteristic | Paradoxical respondersa | Nonparadoxical respondersb | P value |
|---|---|---|---|
| Median age [yr (range)] | 47 (20–63) | 42 (16–70) | 0.70 |
| No. (%) of males | 11 (48) | 46 (62) | 0.22 |
| No. (%) with diagnosis of: | 0.73 | ||
| Acute myeloid leukemia | 11 (48) | 36 (49) | |
| Acute lymphoblastic leukemia | 4 (17) | 16 (22) | |
| Chronic myeloid leukemia | 0 | 1 (1) | |
| Aplastic anemia | 2 (9) | 9 (12) | |
| Myelodysplastic syndrome | 5 (22) | 6 (8) | |
| Non-Hodgkin lymphoma | 1 (4) | 5 (7) | |
| Paroxysmal nocturnal hemoglobinuria | 0 | 1 (1) | |
| No. (%) whose underlying disease was: | |||
| Hypertension | 22 (96) | 60 (81) | 0.11 |
| Diabetes mellitus | 1 (4) | 6 (8) | >0.99 |
| Hemodialysis | 0 | 1 (1) | >0.99 |
| Heart failure | 0 | 3 (4) | >0.99 |
| Solid tumor | 1 (4) | 1 (1) | 0.42 |
| No. (%) with following transplant type: | 0.08 | ||
| Allogeneic, sibling | 9 (39) | 24 (32) | |
| Allogeneic, family donor other than sibling | 11 (48) | 22 (30) | |
| Allogeneic, unrelated | 3 (13) | 28 (38) | |
| No. (%) whose stem cell source was: | 0.95 | ||
| Bone marrow | 3 (13) | 10 (13.5) | |
| Peripheral blood | 20 (87) | 64 (87.5) | |
| No. (%) whose conditioning regimen was: | 0.15 | ||
| Cyclophosphamide + ATG | 0 | 4 (5) | |
| Busulfan + cyclophosphamide | 2 (9) | 7 (10) | |
| Cyclophosphamide + fludarabine + ATG | 1 (4) | 2 (3) | |
| Busulfan + fludarabine + ATG | 19 (83) | 52 (70) | |
| Other | 1 (4) | 9 (12) | |
| No. (%) of donors CMV serology positive | 23 (100) | 73 (99) | >0.99 |
| No. (%) of recipients CMV serology positive | 23 (100) | 74 (100) | >0.99 |
| Median WBC count/mm3 before GCV treatment (IQR) | 2,700 (1,500–3,700) | 3,900 (2,175–5,800) | 0.02 |
| Median hemoglobin level (g/dl) before GCV treatment (IQR) | 9.9 (8.9–11.3) | 10.1 (9.1–11.2) | 0.89 |
| Median platelet count (103/mm3) before GCV treatment (IQR) | 108 (44–166) | 114 (31–183) | 0.79 |
| Median baseline antigenemia level (IQR) | 14 (6–77) | 23 (6–86) | 0.96 |
| Median duration of antigenemia positivity prior to antiviral therapy (IQR) | 7.0 (0–14) | 0 (0–7) | 0.04 |
| No. (%) who received preemptive GCV therapy | 0.63 | ||
| Low dose | 14 (61) | 39 (53) | |
| Conventional dose | 9 (39) | 35 (47) | |
| Immunosuppressed status | |||
| Median change in cyclosporine at first antigenemia (IQR) | 69 (53–72) | 69 (49–89) | 0.84 |
| Median no. of mg of ATG used (IQR) | 175 (150–199) | 180 (160–220) | 0.83 |
| No. (%) using steroids at: | |||
| 0 mg/kg | 14 (60) | 37 (50) | 0.85 |
| <1 mg/kg | 5 (22) | 20 (27) | |
| 1-2 mg/kg | 2 (9) | 9 (12) | |
| ≥2 mg/kg | 2 (9) | 8 (11) | |
| No. (%) who experienced GVHD in first 100 days | 10 (44) | 36 (49) | 0.66 |
| No. (%) with GVHD | 0.41 | ||
| Grade 1 | 1 (10) | 3 (8) | |
| Grade 2 | 5 (50) | 12 (33) | |
| Grade 3 | 2 (20) | 11 (31) | |
| Grade 4 | 2 (20) | 10 (28) |
Total n = 23.
Total n = 74.
Risk factors for a paradoxical response.
Baseline CMV antigenemia levels before the initiation of preemptive GCV therapy in patients with paradoxical responses were not different from those observed in nonparadoxical responders (median, 14/250,000 cells [interquartile range [IQR], 6 to 77] versus 23/250,000 cells [IQR, 6 to 86]; P = 0.96) (Table 1). However, the initial median white blood cell (WBC) count before preemptive therapy was significantly lower in paradoxical responders than in nonparadoxical responders (2,700 cells/mm3 versus 3,900 cells/mm3; P = 0 0.02). The median number of days of CMV antigenemia positivity before preemptive therapy was also significantly longer in paradoxical responders than in nonparadoxical responders (7 days versus 0 days; P = 0.04). However, there was no evidence that immunosuppressive status affected the paradoxical response group. There were no significant interactions between response, increasing antigenemia level, and immunosuppressive status, such as the use of systemic steroids, the steroid dose (exceeding or not exceeding 1 mg/kg), the percent change in the cyclosporine dosage after the initiation of antiviral therapy, and the use of ATG in the conditioning regimen (Table 1). In addition, low-dose GCV therapy was not associated with a paradoxical response (Table 1).
Paradoxical responses and clinical outcomes.
There were no significant differences in successful viral clearance or in secondary episodes of CMV infection between paradoxical responders and nonparadoxical responders (P = 0.35 and P = 0.78, respectively; Table 2). Patients with paradoxical responses received preemptive GCV for a median of 17 days, and the nonparadoxical response group received preemptive GCV for 14 days (P = 0.36). The total duration of antigenemia clearance was delayed in the paradoxical response group compared with that in the nonparadoxical response group (median, 21 days versus 14 days; P < 0.001).
Table 2.
Comparison of outcomes of paradoxical and nonparadoxical responders who received preemptive GCV therapy
| Parameter | Paradoxical respondersa | Nonparadoxical respondersb | P value |
|---|---|---|---|
| Median total duration [days (IQR)] of: | |||
| GCV use | 17 (13–20) | 13.5 (7–19) | 0.36 |
| Antigenemia clearance | 21 (21–21) | 14 (7–21) | <0.001 |
| Median posttreatment antigenemia level (IQR)c | 85 (23–268) | 1 (0–6) | <0.001 |
| No. (%) with: | |||
| Successful viral clearance | 20 (87) | 70 (95) | 0.35 |
| Secondary episode of CMV infection | 6 (26) | 17 (23) | 0.78 |
| Mean no. of days to onset of secondary episode after transplantation ± SD | 30.3 ± 33.9 | 52.2 ± 37.0 | 0.13 |
| CMV disease | |||
| Breakthrough disease | 4 (17) | 2 (3) | 0.03 |
| Late infection | 1 (4) | 3 (4) | >0.99 |
| Overall no. (%) of deaths | 8 (35) | 29 (39) | 0.70 |
| No. of CMV-related deaths | 0 | 0 |
Total n = 23.
Total n = 74.
Posttreatment levels are antigenemia levels measured after 7 days of GCV treatment.
The incidence of breakthrough CMV diseases during preemptive GCV therapy was significantly higher in paradoxical responders (17% [4/23], two patients with gastroenteritis, one with pneumonia, and one with CMV syndrome) than in nonparadoxical responders (3% [2/74], one patient pneumonia and one with gastroenteritis; P = 0.03). After 100 days following HCT, the two groups did not differ significantly in the number of patients who developed confirmed late CMV disease (4% in both groups). By 1 year after transplantation, 8 (35%) of 23 patients had died in the paradoxical response group, compared with 29 (39%) in the nonparadoxical response group (P = 0.70). There were no deaths due to CMV disease in either group.
Detailed kinetics of CMV antigenemia during preemptive GCV therapy.
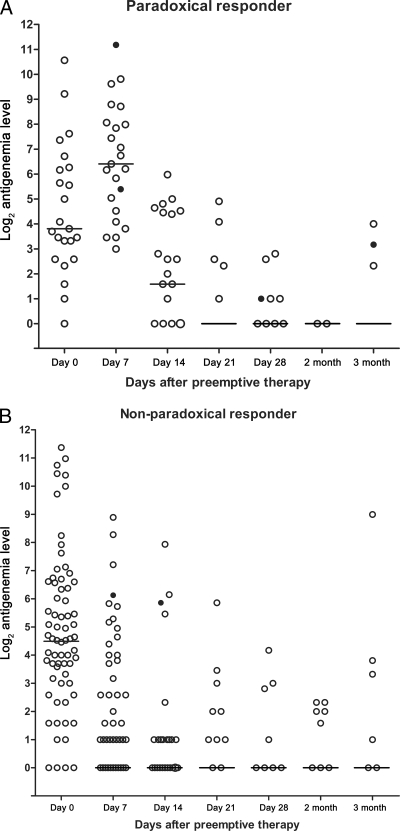
Median values of CMV antigenemia increased during the first week after preemptive GCV therapy in patients with paradoxical responses (median, 14 [IQR, 6 to 77] before preemptive therapy and 85 [IQR, 23 to 268] at 1 week after preemptive therapy) and then steadily decreased over the next 3 to 4 weeks after GCV therapy (median, 3 [IQR, 1 to 22] at 2 weeks, 0 [IQR, 0 to 1] at 3 weeks, and 0 [IQR, 0 to 1] at 4 weeks after preemptive therapy) (Fig. 1A). In nonparadoxical responders, the median CMV antigenemia values decreased over 4 weeks after preemptive GCV therapy (median, 23 [IQR, 6 to 86] before preemptive therapy and 1 [IQR, 0 to 6] at 1 week, 0 [IQR, 0 to 1] at 2 weeks, 0 [IQR, 0] at 3 weeks, and 0 [IQR, 0] at 4 weeks after preemptive therapy) (Fig. 1B). Breakthrough CMV disease cases that occurred at various times during preemptive GCV therapy are shown in Fig. 1.
Fig. 1.
Detailed kinetic data of CMV antigenemia titers of paradoxical responders (A) and nonparadoxical responders (B) after preemptive GCV therapy. Patients who developed breakthrough CMV diseases are represented by closed circles. The bars indicate median CMV antigenemia values.
DISCUSSION
This study demonstrated that paradoxical responses after the first week of preemptive GCV therapy occurred in about one-quarter of the HCT recipients receiving preemptive GCV therapy. A low baseline WBC count and a longer duration of CMV antigenemia before GCV therapy were associated with paradoxical responses. Breakthrough CMV disease during preemptive GCV therapy occurred more frequently in these paradoxical responders, but there was no significant association of paradoxical responses with late CMV disease or CMV-related death during preemptive therapy.
There are limited data on risk factors for paradoxical rising CMV antigenemia in patients who receive preemptive GCV therapy. Gerna et al. reported that the duration of antigenemia positivity prior to antiviral therapy was shorter, and the pretreatment level of antigenemia was lower, in paradoxical responders than in nonparadoxical responders (7). However, our study showed that a low baseline WBC count and a longer duration of CMV antigenemia before GCV therapy were associated with paradoxical response. To our knowledge, there are only two studies that have addressed the possibility that these clinical variables are positively or negatively associated with a paradoxical response. However, the previous study (7) included a small number of patients (n = 18) and in our study, the WBC ranges of the two groups overlapped. In addition, the previous study was specific to solid organ transplantation (7) while ours was specific to HCT. Further studies on initial laboratory parameters for predicting paradoxical response are needed.
We did not find that the use of immunosuppressive agents such as steroids, ATG, and cyclosporine, including percent changes in the cyclosporine dosage, had any association with paradoxical responses during preemptive GCV therapy. In addition, we found no dose-response relationship between steroid use and paradoxical responses. However, the previous studies showed that the immune status of the patients, such as the use of corticosteroids, was significantly associated with increasing CMV antigenemia during preemptive GCV therapy (9, 15). In contrast to this finding, a recent study reported that steroid use was not associated with paradoxical rising CMV PCR values during therapy (4), which is in line with our finding. The reason for this discrepancy is not clear. The use of immunosuppressive agents, including steroids, is affected by the presence or absence of GVHD, donor human leukocyte antigen matching, or the presence or absence of infectious complications. It is therefore difficult to demonstrate a true association of immunosuppressive agents with paradoxical responses. However, it is known that the clearance of CMV is affected by host immune status (i.e., CMV-seronegative recipients in solid organ transplantation) (16). So, we assume that cautious modification of immunosuppressive agents is needed in patients with paradoxical rising CMV antigenemia during GCV therapy until further larger studies reveal the true association of immunosuppressive agents with paradoxical responses.
It is worth mentioning the relationships between paradoxical rising CMV antigenemia and CMV DNA PCR or blood CMV culture results during preemptive GCV therapy. Nichols et al. showed that 33 (28%) of 119 patients who received GCV had at least a 5-fold increase in CMV antigenemia from the baseline and that this rising CMV antigenemia was well correlated with quantitative CMV PCR results in 10 consecutive patients of those with a 5-fold CMV antigenemia increase from the baseline (15). In contrast, Gerna et al. reported that one-quarter of their patients with rising CMV antigenemia during GCV therapy revealed rising CMV PCR values and increasing viral titers (so-called “dissociate increase in CMV antigenemia”) while three-quarters of those with CMV antigenemia during GCV therapy showed decreasing CMV PCR values and viral titers (9). They also hypothesized that the pathogenetic basis for such a “dissociate increase in CMV antigenemia” may be the partial synthesis of CMV phosphoprotein 65 caused by the use of GCV concentrations in the rage of 90 to 99% of the inhibitory dose (8). In this context, they suggested that rising CMV antigenemia with decreasing CMV PCR values do not warrant the modification of GCV therapy. However, the previous studies did not demonstrate that the patients with initial paradoxical rising CMV PCR values or CMV antigenemia were associated with breakthrough CMV disease (9, 15). So, it is an interesting issue whether rising CMV PCR values during 1 week of GCV therapy are truly associated with breakthrough CMV disease. Unfortunately, our hospital did not routinely perform quantitative CMV PCR assays and blood CMV cultures. Further studies on this issue are needed.
There are some reasons for the failure of preemptive GCV, including a failure to detect CMV before the onset of disease (six [2%] of 256 HCT recipients in this study), CMV progression during preemptive therapy (breakthrough CMV disease), and relapse after the discontinuation of preemptive therapy (late CMV disease) (2). Gerna et al. recommended that treatment modifications, such as increasing the GCV dose or switching to alternative antiviral agents, are not needed for patients with rising CMV antigen titers during the early phase of preemptive GCV therapy (7). This recommendation is also supported by the previous report (15) that 8 (17%) of 47 patients with CMV antigenemia increased at least 2 times above the baseline developed CMV disease while 8 (12%) of 68 patients with no rising CMV antigenemia developed CMV disease (P > 0.05). However, we clearly showed that breakthrough CMV disease during preemptive GCV therapy occurred more frequently in paradoxical responders than in nonparadoxical responders. So, we assume that, until further studies are available, induction preemptive therapy may be extended or reinduction may be initiated if the patient with a rise in CMV antigenemia (or viremia or DNAemia) has already transitioned to maintenance therapy. In addition, careful monitoring of breakthrough CMV disease during GCV therapy is needed in paradoxical responders, because paradoxical rising CMV antigenemia might herald breakthrough CMV disease. Interestingly, there were no differences in successful viral clearance and late CMV diseases between paradoxical responders and nonparadoxical responders; however, this is consistent with previous studies (7, 15). Therefore, a switch from routine treatment to alternative regimens for paradoxical responders during preemptive GCV therapy may not be warranted.
This study has a few limitations. First, the question of whether a paradoxical response during preemptive therapy is a kind of missed CMV disease arises. This is not likely, considering the strict exclusion criteria that were used. In addition, attending physicians performed thorough studies to distinguish preemptive GCV therapy for CMV infection from GCV treatment for CMV disease prior to GCV use. Second, our study permitted two dosing strategies, low-dose GCV and conventional-dose preemptive GCV therapy, which may confound paradoxical rising CMV antigenemia during GCV therapy. However, low-dose GCV therapy was not associated with paradoxical responses (P = 0.63). Furthermore, additional analysis demonstrated that low-dose GCV may be safe and at least as effective as preemptive therapy for CMV viremia in allogeneic HCT recipients, compared with conventional-dose GCV (data not shown). Third, we did not perform quantitative real-time CMV PCR prior to and during preemptive GCV therapy, so we could not evaluate the emergence of GCV-resistant infection during preemptive GCV therapy. However, we assume that it may be rare that 1 week of GCV use results in the emergence of resistance, because prolonged GCV exposure with ongoing high viral replication and impaired host defenses is usually associated with the emergence of GCV-resistant strains (10, 12, 14). Indeed, the previous studies showed that no GCV-resistant strain was detected in patients with rising CMV PCR levels during 1 week of GCV therapy (4, 9, 10).
In conclusion, paradoxical responses occurred in one-quarter of our HCT recipients receiving preemptive GCV therapy. Low WBC counts and long durations of CMV antigenemia before GCV therapy were associated with paradoxical responses, and breakthrough CMV disease during preemptive GCV therapy more frequently occurred in paradoxical responders. We identified the impact of paradoxical response on the outcomes of patients receiving preemptive GCV therapy after allogeneic HCT.
ACKNOWLEDGMENT
There are no potential conflicts of interest for any of us.
Footnotes
Published ahead of print on 26 October 2011.
REFERENCES
- 1. Boeckh M., et al. 1996. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 88:4063–4071 [PubMed] [Google Scholar]
- 2. Boeckh M., Ljungman P. 2003. Cytomegalovirus infection after hemopoietic stem cell transplantation, p. 286.In Bowden R. A., Ljungman P., Paya C. V. (ed.), Transplant infections, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3. Boeckh M., Nichols W. G. 2004. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103:2003–2008 [DOI] [PubMed] [Google Scholar]
- 4. Buyck H. C., Griffiths P. D., Emery V. C. 2010. Human cytomegalovirus (HCMV) replication kinetics in stem cell transplant recipients following anti-HCMV therapy. J. Clin. Virol. 49:32–36 [DOI] [PubMed] [Google Scholar]
- 5. Einsele H., et al. 1995. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood 86:2815–2820 [PubMed] [Google Scholar]
- 6. Gane E., et al. 1997. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. Lancet 350:1729–1733 [DOI] [PubMed] [Google Scholar]
- 7. Gerna G., et al. 1998. Rising levels of human cytomegalovirus (HCMV) antigenemia during initial antiviral treatment of solid-organ transplant recipients with primary HCMV infection. J. Clin. Microbiol. 36:1113–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerna G., et al. 2003. In vitro model for the study of the dissociation of increasing antigenemia and decreasing DNAemia and viremia during treatment of human cytomegalovirus infection with ganciclovir in transplant recipients. J. Infect. Dis. 188:1639–1647 [DOI] [PubMed] [Google Scholar]
- 9. Gerna G., et al. 2005. Rising antigenemia levels may be misleading in pre-emptive therapy of human cytomegalovirus infection in allogeneic hematopoietic stem cell transplant recipients. Haematologica 90:526–533 [PubMed] [Google Scholar]
- 10. Gilbert C., et al. 2001. Lack of emergence of cytomegalovirus UL97 mutations conferring ganciclovir (GCV) resistance following preemptive GCV therapy in allogeneic stem cell transplant recipients. Antimicrob. Agents Chemother. 45:3669–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jang E. Y., et al. 2009. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. . Clin. Infect. Dis. 48:e121–e124 [DOI] [PubMed] [Google Scholar]
- 12. Limaye A. P. 2002. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. . Clin. Infect. Dis. 35:866–872 [DOI] [PubMed] [Google Scholar]
- 13. Ljungman P., Griffiths P., Paya C. 2002. Definitions of cytomegalovirus infection and disease in transplant recipients. . Clin. Infect. Dis. 34:1094–1097 [DOI] [PubMed] [Google Scholar]
- 14. Lurain N. S., Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 23:689–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nichols W. G., et al. 2001. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 97:867–874 [DOI] [PubMed] [Google Scholar]
- 16. Paya C. V. 2001. Prevention of cytomegalovirus disease in recipients of solid-organ transplants.. Clin. Infect. Dis. 32:596–603 [DOI] [PubMed] [Google Scholar]
- 17. Reusser P., Fisher L. D., Buckner C. D., Thomas E. D., Meyers J. D. 1990. Cytomegalovirus infection after autologous bone marrow transplantation: occurrence of cytomegalovirus disease and effect on engraftment. Blood 75:1888–1894 [PubMed] [Google Scholar]
- 18. Zaia J. A., et al. 1997. Late cytomegalovirus disease in marrow transplantation is predicted by virus load in plasma. J. Infect. Dis. 176:782–785 [DOI] [PubMed] [Google Scholar]