Abstract
Mycobacterium porcinum is a rarely encountered rapidly growing Mycobacterium (RGM). We identified M. porcinum from 24 patients at a Galveston university hospital (University of Texas Medical Branch) over a 5-year period. M. porcinum was considered a pathogen in 11 (46%) of 24 infected patients, including 4 patients with community-acquired disease. Retrospective patient data were collected, and water samples were cultured. Molecular analysis of water isolates, clustered clinical isolates, and 15 unrelated control strains of M. porcinum was performed. Among samples of hospital ice and tap water, 63% were positive for RGM, 50% of which were M. porcinum. Among samples of water from the city of Galveston, four of five households (80%) were positive for M. porcinum. By pulsed-field gel electrophoresis (PFGE), 8 of 10 environmental M. porcinum were determined to belong to two closely related clones. A total of 26 of 29 clinical isolates subjected to PFGE (including isolates from all positive patients) were clonal with the water patterns, including patients with community-acquired disease. Fifteen control strains of M. porcinum had unique profiles. Sequencing of hsp65, recA, and rpoB revealed the PFGE outbreak clones to have identical sequences, while unrelated strains exhibited multiple sequence variants. M. porcinum from 22 (92%) of 24 patients were clonal, matched hospital- and household water-acquired isolates, and differed from epidemiologically unrelated strains. M. porcinum can be a drinking water contaminant, serve as a long-term reservoir (years) for patient contamination (especially sputum), and be a source of clinical disease. This study expands concern about public health issues regarding nontuberculous mycobacteria. Multilocus gene sequencing helped define clonal populations.
INTRODUCTION
Mycobacterium porcinum, a rapidly growing mycobacterium (RGM), was first isolated in 1973 from lymph nodes of swine with tuberculous-like infection (33). In 1983, it was further characterized and deposited in the American Type Culture Collection (ATCC) as type strain ATCC 33776 (33). The organism was first recognized as a human pathogen in 2004 when it was distinguished from other members of the Mycobacterium fortuitum sorbitol negative third biovariant complex using molecular techniques (29, 35). Clinical infections include wound infections, intravascular catheter-related infections, and osteomyelitis. The species has also been recovered from tap water but, to our knowledge, has not been associated with an outbreak or pseudo-outbreak (29). Most case series of RGM published since 2004 only report a small proportion of M. porcinum isolates (14, 24, 28).
A sharp increase in the number of M. porcinum isolates at the University of Texas Medical Branch (UTMB) Clinical Microbiology Laboratory in Galveston, TX, initiated a retrospective evaluation of RGM isolation rates and confirmed a high proportion of these isolates to be M. porcinum (Table 1). The aim of the present study is to characterize the clinical patients and sources of this increasingly recognized pathogen and to study the molecular relatedness of clinical isolates to control isolates and to water isolates recovered within the hospital and from the community.
Table 1.
Rapidly growing mycobacterial species recovered from 60 patients at UTMB from September 2005 through September 2010
| RGM species | No. of patients | No. of isolates |
|
|---|---|---|---|
| Confirmed | Presumptiveb | ||
| M. porcinum | 24 | 29 | 26 |
| M. abscessus | 15 | 22 | 12 |
| M. fortuitum | 8 | 9 | 3 |
| M. senegalense | 4 | 4 | 2 |
| M. houstonense | 2 | 2 | 0 |
| M. immunogenum | 2 | 2 | 0 |
| M. mucogenicum | 2 | 2 | 1 |
| M. fortuitum complexa | 1 | 1 | 0 |
| M. cosmeticum | 1 | 1 | 0 |
| M. chelonae | 1 | 1 | 0 |
| Total | 60 | 73 | 44 |
M. fortuitum complex includes isolates of the M. fortuitum group that could not be identified to species level using 500-bp 16SrRNA gene sequencing alone.
Multiple isolates from the same patient of the same colony type as isolates already identified.
(A portion of this study was presented at the 110th General Meeting of the American Society for Microbiology, San Diego, CA, 23 to 27 May 2010 [abstr. U-470].)
MATERIALS AND METHODS
Isolates.
A relative increase in the isolation of RGM (especially M. porcinum) from clinical samples was noted by the clinical microbiology laboratory at UTMB in 2008. This prompted a retrospective and prospective analysis of all RGM isolates recovered between 1 January 2003 and 31 August 2010 in this institution. Initial isolates from RGM culture-positive patients were sent to The University of Texas Health Science Center, Tyler (UTHSCT) Mycobacteria/Nocardia Research Laboratory for species identification and antimicrobial susceptibility testing. Patients with multiple positive cultures of the same colony type and morphology recovered within 30 days of each other were reported as presumptive M. porcinum. (These isolates had been saved but were destroyed with the power loss due to Hurricane Ike in September 2008.)
Patients.
Patients with positive cultures of M. porcinum were identified using the microbiology laboratory database. Charts were reviewed retrospectively after obtaining institutional review board approval. Patients were studied related to their city of residence and subsequent location within the hospital.
Source of patient water supplies.
The sources of the municipal water supply for the hospital (e.g., surface water, wells, etc.) were investigated for the period of time of the outbreak.
Environmental sampling.
RGM isolates from hospital water and ice machines were collected between 28 October 2008 and 7 January 2009. RGM isolates from household tap water on Galveston Island were sampled in February 2011. Molecular analysis was performed several years after hospital water/ice isolates were originally identified. Sampling of household water was performed after the molecular analysis indicated a clonal strain.
Specimen collection and isolate recovery.
The hospital procedures for sputum collection and specimen processing were reviewed for potential exposure to hospital water. The number of isolates per patient and smear and culture results, including the sources of the isolates, were obtained.
Control strains.
Four clinical isolates of M. porcinum representative of the four recognized human genotypes of the hsp65 gene (MF114, ATCC 49939, MF205, and ATCC BAA-328) (34), 10 recent random clinical M. porcinum submitted for susceptibility testing and/or identification to the UTHSCT laboratory, isolates identified during the outbreak study that had unique PFGE profiles, and the M. porcinum type strain (ATCC 33776T) obtained from the ATCC were used as controls.
Molecular identification.
Isolates were identified using PCR restriction enzyme analysis (PRA) of the 441-bp Telenti fragment of the hsp65 gene (30, 31).
DNA strain typing.
Typing was performed by pulsed-field gel electrophoresis (PFGE) using XbaI and AseI (38). Interpretations of “indistinguishable,” “closely related,” “possibly related,” and “different” used previously described criteria for evaluation of (bacterial) outbreaks (32).
Dendrogram of AseI restriction patterns of M. porcinum isolates was constructed used Bionumerics software v.4.0 (Applied Maths). This comparison was performed using the Ward algorithm and calculated using the Pearson correlation coefficient with 0.8% optimization and a 1.0% position tolerance. The dendrogram included all eight environmental outbreak isolates and patient outbreak isolates. Two isolates were tested in duplicate using different plugs, with the AseI digests and PFGE performed at different times. One unrelated isolate (MF-3176) was included as a control.
Partial gene sequencing.
Partial gene sequencing of the hsp65 gene (441 bp) and region V of the rpoB gene (723 bp) was performed as previously described (1, 26). M. porcinum hsp65 sequences of the 441-bp Telenti fragment have previously been deposited in GenBank for MF114 (AF496139), ATCC 49939 (AY496140), MF205 (AY496141), ATCC BAA-328 (AY496138), and the M. porcinum ATCC 33776T (AY496137) (34). rpoB gene sequences of region V have previously been deposited in GenBank for the type strain M. porcinum (CIP105392) (AY262737). Partial gene sequencing of the recA gene (bp position 43 to 491) was performed using the primers GGA-CAT-YCT-GGT-GAT-CGA-CT (forward) and GCC-GAG-CTT-TTC-CTT-GAT-CT (reverse).
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed using the Clinical and Laboratory Standards Institute (CLSI) recommended broth microdilution method for RGM (37).
Susceptibility to clarithromycin was read after incubation for 3 and 14 days to ascertain isolates that had inducible macrolide resistance (5, 21).
RESULTS
Isolates.
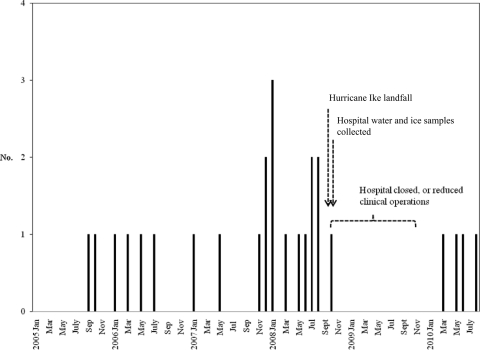
A total of 29 confirmed and 26 presumptive isolates of M. porcinum from 24 patients were identified during the 89-month study period (see Table 1). The first patient was identified in September 2005, and the last patient was identified in August 2010, 5 years later (see Fig. 1). The hospital was closed from 11 September 2008 to 5 January 2009 (16 weeks) due to damage from Hurricane Ike.
Fig. 1.
Positive cases of M. porcinum at the UTMB from January 2005 to August 2010, charted by date of collection of first positive specimen (n = 26 [24 patients; 2 patients each had two separate episodes]).
During the 5-year study period, M. porcinum was recovered from 24 of 60 (40%) of patients culture positive for RGM and represented 55 of 117 (47%) of all cultures found to be positive for RGM (Table 1).
Patients.
Of the 24 patients, 17 had respiratory isolates of M. porcinum. All but two pulmonary cases underwent chest computerized tomography (CT) at the time the positive RGM specimens were first recovered. Of the 15 patients with positive respiratory cultures for M. porcinum who had chest CT scans performed, 2 had bronchiectasis, 5 had multiple pulmonary small nodules, and 1 had both. The number of AFB culture-positive specimens divided by the total number of AFB cultures collected were listed (see Table 2).
Table 2.
Patient demographics including mycobacterial therapy and environmental isolates of M. porcinum from UTMB (September 2005 through September 2010)a
| Patient | Isolate, MF no. | Age (yr) | Sex | Source (no. of positive cultures/no. tested) | Date of collectionb | PFGE pattern |
Underlying disease or condition | Antimycobacterial treatment | |
|---|---|---|---|---|---|---|---|---|---|
| XbaI | AseI | ||||||||
| Pulmonary | |||||||||
| 1 | 2817 | 58 | M | Sputum (2/3) | 1/1/07 | B3 | B2 | COPD (no CT) | None |
| 2 | 2763 | 42 | F | Sputum (3/3) | 7/4/06 | A | A | COPD, HIV infection, hemoptysis, multiple lung nodules (CT, 7/14/10) | DOX + CLA × 4 mo, CIP × 2 mo |
| 3 | 3112 | 56 | F | Sputum (1/1) | 10/22/08 | A | A2 | Perihilar infiltrate (no CT) | None |
| 4 | 2987 | 47 | M | Sputum (2/3) | 1/24/08 | B3 | B | Necrotizing MRSA pneumonia, porphyria cutanea tarda, no bronchiectasis, no nodules (CT, 1/21/08) | Levo × 2 wk, SXT × 2 wk |
| 5 | 3054 | 70 | M | Sputum (2/3 | 6/23/08 | B3 | B | COPD, lung cancer (CT, 6/20/08 | None |
| 6 | 2983 | 32 | M | Bronchoalveolar lavage (2/4) | 12/14/07 | B4 | B2 | Hypogammaglobulinemia, necrotizing pneumonia, multiple lung nodules (CT, 12/13/07) | None |
| 7 | 3075 | 64 | F | Sputum (2/3) | 7/7/08 | B3 | B | Multiple lung nodules, AIDS, disseminated MAC (CT, 7/21/08) | None |
| 8 | 2629 | 75 | F | Sputum (2/2) | 10/17/05 | A | A3 | COPD, necrotizing pneumonia; bilateral upper lobe cavities; multiple small nodules (stable) (CT, 9/6/05) | CIP + SXT × 2 wk, CIP × 9 mo |
| 9 | 3084 | 67 | F | Sputum (2/2) | 8/6/08 | B3 | B2 | Lung cancer, postobstruction pneumonia, no nodules or bronchiectasis (CT, 7/17/08) | AMI + FOX + DOX × 14 days/DOX + CLA |
| 10 | 2672, #1 | 58 | M | Sputum | 1/31/06 | A | A4 | AIDS, hazy infiltrates, mediastinal lymphadenitis, no nodules or bronchiectasis (CT, 3/03/06) | CIP + CLA × 6 mo |
| 10 | 2672, #2 | Sputum (4/6) | 3/15/06 | A2 | A2 | ||||
| 11 | 2989 | 60 | F | Sputum (1/4) | 1/11/08 | A3 | A2 | Rheumatoid arthritis, bronchiectasis, pneumonia (CT, 1/12/08) | SXT + MIN × 10 days |
| 12 | 2602, #2 | 81 | F | Sputum (2/2) | 7/18/08 | A5 | A5 | Bronchiectasis, multiple lung nodules, chronic Pseudomonas (CT, 8/08/08) | CIP × 9 mo, CLA + CIP × 2 mo |
| 13 | 2977 | 53 | M | Sputum (1/1) | 11/27/07 | B3 | B | AIDS, pneumonia, no nodules or bronchiectasis (CT, 11/27/07) | Levo × 7 days, SXT × 21 days |
| 14 | 2978 | 55 | M | Sputum (6/7) | 1/1/08 | B3 | B | Pulmonary embolus; LLL pneumonia, bronchiectasis, bullous emphysema (CT, 12/31/07) | CIP + CLA + SXT (unknown duration) |
| 15 | 3449 | 79 | M | Sputum (1/3) | 5/24/10 | A | B3 | Hemoptysis, metastatic adenocarcinoma of lung, no nodules or bronchiectasis (CT, 5/22/10) | None |
| 16 | 3452 | 50 | M | Sputum (1/4) | 6/12/10 | A | B3 | Staphylococcal pneumonia, bilateral pleura effusions, diabetes (CT, 6/11/10) | Vancomycin (5 days), oral DOX (4 wk) |
| 17 | 3460 | 41 | M | Sputum (4/4) | 8/4/10 | A | B3 | Pneumococcal, necrotizing pneumonia, loculated lung abscess, empyema (CT, 8/4/10) | Levo (2 wk), metronidazole (2 wk), vancomycin (4 days) |
| 17 | 3469 | 8/5/10 | A | B3 | |||||
| 17 | 3470 | 8/6/10 | A | B3 | |||||
| 17 | 3471 | 8/8/10 | A5 | A2 | |||||
| Extrapulmonary | |||||||||
| 18 | 2612, #1 | 17 | F | Spinal wound | 10/28/05 | C | C | Thoracolumbar scoliosis, Harrington rods inserted (UTMB 7/05); postoperative spinal epidural abscesses | Removal of hardware, SXT (unknown duration) |
| 18 | 2612, #2 | 20 | F | Paraspinal tissue | 12/16/07 | C | C | Same as above | Surgical debridement (unknown duration) |
| 19 | 2875 | 38 | F | Port exit site | 5/9/07 | A | A3 | Port infection; cancer, chemotherapy, UTMB hospitalization 1 mo earlier | Port removal |
| 20 | 3082 | 33 | M | Neck fluid | 8/1/08 | D | D | HIV infection, neck abscess | Abscess drainage |
| 21 | 3036 | 35 | F | Blood, catheter tip | 5/24/08 | B4 | B3 | Sickle cell disease, port infection; UTMB hospitalization, 2 mo earlier | Port removal, CIP + SXT + CLA × 1 mo |
| 22 | 2721 | 36 | F | Breast fluid | 5/25/06 | A6 | A | Breast abscess | Drainage |
| 23 | 3019 | 58 | F | Peritoneal fluid | 3/21/08 | B3 | B | Hepatocellular cancer, gastric perforation with polymicrobial peritonitis | Peritoneal fluid drainage |
| 24 | 3451 | 63 | M | Finger nodule | 3/18/10 | A | A | Biopsy had granulomatous inflammation, AFB smear positive | Excision |
| Environmental | |||||||||
| 3130 | Hospital water | 10/30/08 | A | A | |||||
| 3131 | Hospital water | 10/30/08 | A | A2 | |||||
| 3132 | Hospital ice machine | 10/30/08 | A3 | B | |||||
| 3128 | Hospital water | 10/31/08 | A2 | A2 | |||||
| 3129 | Hospital water | 10/28/08 | A2 | A | |||||
| 3546 | Household 1 | 02/11 | A | A | |||||
| 3547 | Household 1 | 02/11 | U | U | |||||
| 3548 | Household 2 | 02/11 | A | A | |||||
| 3549 | Household 3 | 02/11 | A | A | |||||
| 3550 | Household 5 | 02/11 | U | U | |||||
CIP, ciprofloxacin; SXT, trimethoprim-sulfamethoxazole; MAC, M. avium complex; MIN, minocycline; Levo, levofloxacin; DOX, doxycycline; FOX, cefoxitin; CLA, clarithromycin; AMI, amikacin; LLL, lower left lobe; MRSA, methicillin-resistant Staphylococcus aureus; COPD, chronic obstructive pulmonary disease.
Dates are expressed as month/day/year. See also the dates for CT analyses in column 9.
Patients considered to have clinical disease (11/24 [46%]) included four with lung involvement, four with soft tissue or skin abscesses (neck, breast, finger, and spinal epidural space), two infected ports, and one patient with peritonitis. Four of these patients had community acquired disease (peritonitis, neck abscess, breast abscess, and inflammatory skin nodule). One patient was from the city of Galveston, while the remaining three were from other cities on the mainland within Galveston County. Three patients (cases 18, 19, and 21 in Table 2) did not live in Galveston County, but had undergone a recent surgical procedure at UTMB with prolonged hospitalization.
The remaining patients with positive respiratory cultures (13/24 [54%]) were not considered clinically significant. A total of 14 patients were treated with drugs active against M. porcinum, while eight patients received prolonged drug therapy (≥1 month) because of suspected mycobacterial disease. Five were treated with local abscess drainage or removal of an infected port, and four patients received no therapy.
Specimen collection and processing.
The standard procedure for spontaneous or induced sputum collection did not have the patient brush their teeth or rinse their mouth with tap water before sputum collection. However, there were no restrictions on drinking ice water prior to specimen collection. The percentage of positive sputum samples among culture-positive patients was similar for induced (9/14 [64.3%]) and spontaneous (25/34 [73.5%]) sputum.
There was no evidence for specimen contamination after receipt in the laboratory, as commercial sterile water was used during AFB culture processing. The samples were processed according to standard procedures (37).
Source of patient water supplies.
The hospital water supply was provided directly from city water. The latter was derived from surface water treatment facilities that provide water to the city of Galveston and other areas and cities within Galveston County. Surface water was derived from two canal systems and the Brazos river (www.gulfcoastwaterauthority.com).
Environmental sampling.
Ice from five of six patient floor ice machines were cultured, of which five grew nontuberculous mycobacteria (NTM). One culture was identified as M. abscessus, one was identified as M. porcinum, and the remaining three cultures were not identified.
A total of 139 tap water samples were taken from all patient areas, with 112 (81%) growing one or more species of NTM. Among the 112 positive samples, 86 (77%) contained RGM and 26 (23%) contained slow growers. Five of ten samples of RGM identified to the species level were M. porcinum.
Overall, 88/139 (63%) of all samples of hospital ice and water contained one or more species of RGM, with 6/12 (50%) of samples identified to species containing M. porcinum.
Tap water from five residences on Galveston Island were sampled. M. porcinum was recovered from four of the five residences (80%), with one residence having two colony types.
Isolate recovery.
A total of 117 RGM isolates were recovered from 60 patients during the study period (Table 1). Twenty-nine patient isolates and five environmental isolates were confirmed as M. porcinum, while 26 were presumed to be M. porcinum (see Table 1). The first isolate of M. porcinum was identified in September 2005. All positive cultures were collected in the hospital or related clinics. Of the 55 M. porcinum clinical isolates, 38 (69%) were from respiratory samples; 3 from a spinal abscess (same patient) following Harrington rod insertion, 10 from blood and/or port-a-cath sites (from two patients), and one each from the peritoneal fluid, finger, neck, and breast (Table 2). All cultures were acid-fact bacillus (AFB) smear negative. Specimens were collected in the emergency room (cutaneous abscesses, blood cultures), medicine clinic, multiple hospital floors and wards (sputa), and operating rooms and bronchoscopy suites. During the study period, M. porcinum was the predominant RGM species recovered.
Molecular identification.
Twenty-nine clinical isolates (including at least one isolate from all 24 patients), the five hospital water isolates, the five residential water isolates, and the 15 control strains were identified by PRA of the hsp65 gene as M. porcinum (30, 31, 34). Three isolates were confirmed also by partial gene sequencing (500 bp) of the 16S rRNA gene (13, 24), although this technique does not separate M. porcinum from M. neworleansense (25, 34). The remaining 24 isolates were recovered within 30 days from a patient with a confirmed isolate of M. porcinum, exhibited similar growth and colony morphology, and were identified as a presumptive M. porcinum.
DNA strain typing.
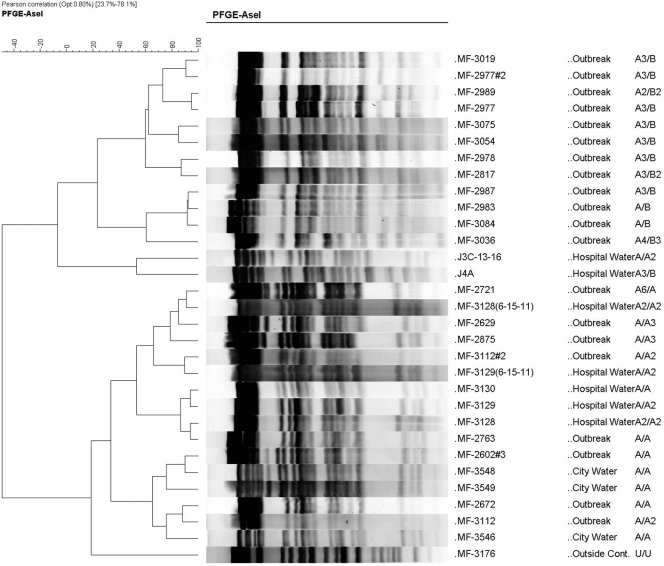
Five hospital water isolates and three isolates from different households on Galveston Island belonged to two closely related clones with XbaI and AseI (seven- to nine-band difference) that were designated as “A” and “B” (Fig. 2 and see Fig. S1 in the supplemental material). There were minor restriction fragment length polymorphism differences among all five hospital environmental water and ice isolates.
Fig. 2.
Dendrogram of AseI digests of the two PFGE M. porcinum outbreak clones (A/A and A/B) grouped by visual reading using the criteria of Tenover et al. (32). The dendrogram prepared using the Pearson correlation method also produced two groups of closely related isolates that included patient isolates, hospital water isolates, and city water isolates (outbreak = clinical isolate). The first column includes the isolate number, the second column the isolate source, and the third column, the PFGE patterns for AseI/XbaI obtained by visual reading. Two isolates (MF-3128 and MF-3129) were run in duplicate. MF-3176, which was unrelated to the two clones by visual reading, was included as a control.
Of 29 clinical isolates, 26 exhibited the same PFGE grouping. Three wound isolates from two patients were unique (one wound infection related to Harrington rod spinal insertion had two isolates with indistinguishable patterns 26 months apart, and a second was from a community-acquired neck abscess). Thirteen (44.8%) of the patient isolates exhibited “indistinguishable” PFGE patterns from one of the environmental isolates. The remaining 13 UTMB clinical isolates gave nine different but “closely related” patterns designated as XbaI (A1 to A6); AseI (A1 to A5, B1 to B3). The two environmental PFGE clones were seen at different time periods. From October 2005 to May 2007 six of seven environmentally related patient isolates had the pattern of the A/A clone and its variants, from November 2007 to October 2008 ten of twelve environmentally related isolates belonged to the A/B clone, and five of seven isolates from 2010 belonged to the A/B3 clone.
A dendrogram of the two closely related PFGE clones is shown in Fig. 2. This resulted in the same grouping of outbreak isolates, hospital water, and household city water isolates (with the exception of one isolate per group) as was obtained with the visual strain comparison method (32). The two duplicate controls (MF-3128 and MF-3129) were closely aligned with the first result.
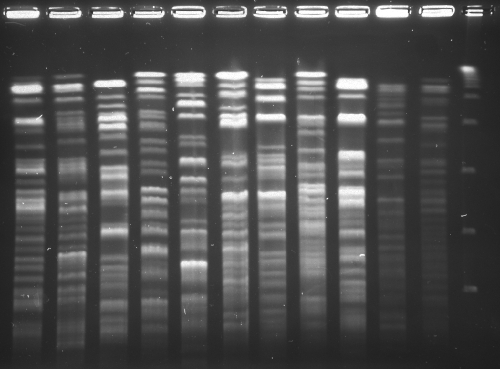
The four isolates with different hsp65 sequence genotypes previously submitted to GenBank (34), the M. porcinum ATCC 33776T isolates from two patients studied as part of the current outbreak investigation (cases 2 and 8, Table 2), two household water isolates, and the ten epidemiologically unrelated control isolates had “unique” PFGE profiles (Fig. 3).
Fig. 3.
PFGE of XbaI digests of the two outbreak clones and random clinical isolates of M. porcinum. Lane 1, hospital water isolate with pattern A with AseI digests; lane 2, hospital ice machine water isolate with AseI digest pattern B; lane 3, ATCC 33776T with a unique pattern; lanes 4 to 11, random patient isolates of M. porcinum, all of which have unique (unrelated) PFGE patterns; lane 12, lambda ladder. The letters “A” and “B” represent the two (closely related) outbreak clones; the letter “U” means unique.
Partial gene sequencing.
Partial sequencing of the hsp65, recA, and rpoB genes of selected environmental and outbreak isolates showed isolates clustered by PFGE to have 100% sequence identity with each other (including both PFGE clones) (Table 3). The ATCC type strain, the examples of the four previously described hsp65 sequence genotypes, two UTMB patient isolates with unrelated PFGE profiles recovered during the outbreak investigation (cases 2 and 8, Table 2), and 10 random control clinical isolates (18 total strains) exhibited 10 sequence variants with concatenated hsp65, recA, and rpoB sequences (Table 3).
Table 3.
M. porcinum alleles for recA, rpoB, and hsp65 partial gene sequences
| Isolate | PFGE profilea (Xba/Ase) | No. of alleles |
||
|---|---|---|---|---|
| recA | hsp65 | rpoB | ||
| Type strain (ATCC 33776T) | U/U | 1 | 1 | 1 |
| Previously described hsp65 clinical sequevarsb | ||||
| MF-0114 | U/U | 1 | 3 | 1 |
| ATCC 49939 | U/U | 1 | 4 | 2 |
| ATCC BAA-328 | U/U | 2 | 2 | 3 |
| MF-205 | U/U | 3 | 5 | 4 |
| Random clinical isolates | ||||
| MF-3362, 3082, 3266, 3240, 2854, 3051 | U/U | 1 | 3 | 1 |
| MF-3176 | U/U | 6 | 4 | 7 |
| MF-3260 | U/U | 4 | 6 | 5 |
| MF-3213 | U/U | 6 | 3 | 1 |
| Environmental water isolates | ||||
| MF-3128 (hospital water) | A2/A2 | 1 | 3 | 1 |
| MF-3129 (hospital water) | A2/A | 1 | 3 | 1 |
| MF-3130 (hospital water) | A/A | 1 | 3 | 1 |
| MF-3131 (hospital water) | A/A2 | 1 | 3 | 1 |
| MF-3132 (hospital ice machine) | B3/B | 1 | 3 | 1 |
| MF-3546 (household 1) | A/A | 1 | 3 | 1 |
| MF-3547 (household 1) | U/U | 1 | 3 | 1 |
| MF-3548 (household 2) | A/A | 1 | 3 | 1 |
| MF-3549 (household 3) | A/A | 1 | 3 | 1 |
| MF-3550 (household 5) | U/U | 1 | 3 | 1 |
| Clinical outbreak isolates (clonal) | ||||
| MF-2763, 2451 | A/A | 1 | 3 | 1 |
| MF-3112 | A/A2 | 1 | 3 | 1 |
| MF-2987, 3075, 2977, 2978, 3054 | B3/B | 1 | 3 | 1 |
| MF-2989, 2817 | B3/B2 | 1 | 3 | 1 |
| MF-2875 | A/A3 | 1 | 3 | 1 |
| MF-3084 | B3/B3 | 1 | 3 | 1 |
| MF-3471 | A5/A2 | 1 | 3 | 1 |
| MF-3452, 3449, 3460, 3469, 3470 | A/B3 | 1 | 3 | 1 |
| Clinical outbreak isolates (nonclonal) | ||||
| MF-3082 | U/U | 1 | 3 | 1 |
| MF-2612 | U/U | 5 | 7 | 6 |
U, unique. The first PFGE profile in an outbreak is designated “A”, the second is designated “B”, etc.
Includes the hsp65 sequence only (28).
Antimicrobial susceptibility testing.
Susceptibilities to 13 antimicrobials were typical of those previously described for M. porcinum (see Table S1 in the supplemental material) and showed few differences between each outbreak isolate.
GenBank accession numbers.
For the rpoB gene, MF114 sequevar 1 (GenBank accession no. JN682043), ATCC 49939 sequevar 2 (JN682044), ATCC BAA-328 sequevar 3 (JN682045), MF-205 sequevar 4 (JN682046), MF-3260 sequevar 5 (JN682047), MF-2612 sequevar 6 (JN682048), and MF-3176 sequevar 7 (JN682049) were submitted. For the hsp65 gene, MF-2612 sequevar 7 (JN682051) and MF-3260 sequevar 6 (JN682050) were submitted. For the recA gene, ATCC 49939 sequevar 1 (JN682052), ATCC BAA-328 sequevar 2 (JN682053), MF-205 sequevar 3 (JN682054), MF-3260 sequevar 4 (JN682055), MF-2612 sequevar 5 (JN682056), and MF-3176 sequevar 6 (JN682057) were submitted.
DISCUSSION
This study demonstrates that potentially 50 of 55 isolates (including all 38 respiratory isolates) of the relatively rare RGM species M. porcinum recovered from a single medical center (UTMB) and the surrounding communities were indistinguishable or closely related to hospital and residential water isolates by PFGE. A retrospective chart review suggests that some isolates were respiratory contaminants, while others were associated with clinical disease that included infected central catheters, localized abscesses, and pulmonary infections.
Prior to the present study, M. porcinum has not been reported as a respiratory pathogen. In a previous study of 52 cases of M. porcinum from our laboratory published in 2003, there were only four respiratory isolates (8%) (34). Clinical information was available on only one respiratory patient, who had underlying bronchiectasis and Mycobacterium avium complex lung disease (34). Since that time, we have seen two additional respiratory isolates, both in patients with underlying bronchiectasis, none of whom had apparent disease and none of whom were treated (R. J. Wallace, unpublished data). The presence of multiple positive sputum cultures, the clinical setting of bronchiectasis, and radiographic findings of multiple small pulmonary nodules seen with some patients with respiratory M. porcinum who underwent chest CT is typical of the prior noted cases and of NTM lung infections in general, especially M. avium complex and M. abscessus (11). Four of the current pulmonary cases met the ATS definition for lung disease and clinically were considered to have disease (11).
There have been a number of other hospital-based outbreaks or pseudo-outbreaks related to NTM contamination from hospital water or ice (4, 9, 10, 15). RGM species related to hospital water systems, including M. fortuitum (3, 17, 22), M. immunogenum (9, 36), and M. abscessus (38), have been described, as well as several outbreaks related to ice machines due to M. peregrinum (18) and M. fortuitum (15). Some episodes of RGM water contamination have included cases of disease, as well as sputum contamination (16), as was noted in the current outbreak. Slowly growing mycobacteria associated with hospital water contamination have been described due to M. xenopi (12), M. kansasii (19), M. lentiflavum (20), and M. simiae (6, 7), as well as outbreaks related to ice machines (2, 23). We were unable to identify any prior outbreak or pseudo-outbreak that involved M. porcinum.
The minor diversity of PFGE profiles among clinical isolates, as well as water isolates, makes a single environmental source other than water highly unlikely. City water inside the hospital and from multiple residences located elsewhere on Galveston Island were positive for M. porcinum, as were patients who were never hospitalized but who lived on the mainland part of Galveston County and who received water from the same surface water treatment facilities. The two patients with central line sepsis and the one patient with postoperative wound infection (cases 18, 19, and 21 inTable 2) lived outside Galveston County but had been hospitalized at UTMB in the recent past.
The high frequency of minor differences in PFGE profiles noted with both restriction enzymes among the clonal outbreak strains was unexpected. This suggests that changes in the DNA have taken place, either by mutation, loss of DNA, or (most likely) positional changes in the restriction sites. Such differences previously have been noted in water isolates of M. avium, following restriction enzyme digestion and hybridization with IS1245 (8). Previous PFGE studies of M. simiae recovered from hospital water also showed the high frequency of minor diversities, with recovery of similar divergent strains from city water outside the hospital (6, 7, 27). M. porcinum clearly behaves in a similar fashion. This suggests that NTM outbreaks or pseudo-outbreaks related to water sources may not be identical (“indistinguishable”) using PFGE (32) but rather only clonal.
In the present study, we used DNA sequencing to try to validate the concept of clonality, assuming that the “time clock” to sequence changes (insertions, mutations, deletions, or recombination) within a single gene would be slower than genomic rearranging that is thought to be responsible for most minor changes in PFGE large restriction fragment patterns. The identity of studied sequences in the outbreak and environmental isolates in the present study supports their clonal nature. More sequences with more polymorphic regions are needed if this approach is to be expanded, since many unrelated control strains had the same concatenated sequences demonstrating linkage disequilibrium. Its potential is good, however, given the time and complexity of PFGE and the increasing availability of routine DNA sequencing.
The present study expands on the importance of drinking water in the United States as a source of NTM colonization and disease. Greater attention should be paid to these species as a source of water contamination. Clinical microbiology laboratories should be alert to increases in isolates of both common and unique NTM as a sign of tap water contamination. Many RGM are naturally occurring in tap water and present minimal risk. An unusually large number of reports of a species should be investigated by infection control committees to determine whether the increase in isolation of the species is a result of contamination or possible outbreak.
The reason for the appearance of M. porcinum in patient isolates in Galveston County in September 2005 is unknown. Routine NTM water cultures were not performed, and no obvious change in the water system was noted at that time. Because this species can only be recognized by molecular testing, not routinely performed in clinical laboratories, it is doubtful that the other hospitals would be aware of similar increase. One author (M. Loeffelholz) contacted the closest hospital, Mainland Medical Center, Texas City, which is on the same water system. They also report an increase in RGM but with no details on species identification. All other hospitals in Galveston County are on different water systems (unpublished data).
M. porcinum remains problematic, with additional hospital- and community-acquired cases of M. porcinum due to the epidemic clone continuing to the present (M. Loeffelholz and R. J. Wallace, Jr., unpublished observations).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by institutional funding and a grant from the Amon Carter Foundation.
We thank the members of the Mycobacteria/Nocardia Laboratory at the University of Texas Health Science Center at Tyler and the hospital epidemiology laboratory at the University of Texas Medical Branch. We also thank Joanne Woodring for preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Adékambi T., Drancourt M. 2004. Dissection of phylogenetic relationships among nineteen rapidly growing Mycobacterium species by 16S r-RNA, hps65, sodA, recA, and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 54:2095–2105 [DOI] [PubMed] [Google Scholar]
- 2. Brust R. A., Ayers L. W. 1974. “Epidemic” contamination of patients' sputa by an environmental Mycobacterium kansasii contaminating ingested ice, abstr. M-245, p. 107. Abstr. 74th Annu. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 3. Burns D. N., et al. 1991. Nosocomial outbreak of respiratory tract colonization with Mycobacterium fortuitum: demonstration of the usefulness of pulsed-field gel electrophoresis in an epidemiologic investigation. Am. Rev. Respir. Dis. 144:1153–1159 [DOI] [PubMed] [Google Scholar]
- 4. Chang C. T., Wang L. Y., Liao C. Y., Huang S. P. 2002. Identification of nontuberculous mycobacteria existing in tap water by PCR-restriction fragment length polymorphism. App. Environ. Microbiol. 68:3159–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. Document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Conger N. A., et al. 2004. Mycobacterium simiae outbreak associated with a hospital water supply. Infect. Control Hosp. Epidemiol. 25:1050–1055 [DOI] [PubMed] [Google Scholar]
- 7. El Sahly H. M., et al. 2002. Mycobacterium simiae pseudo-outbreak resulting from a contaminated hospital water supply in Houston, Texas. Clin. Infect. Dis. 35:802–807 [DOI] [PubMed] [Google Scholar]
- 8. Falkinham J. O., III, Iseman M. D., de Haas P., van Soolingen D. 2008. Mycobacterium avium in a shower linked to pulmonary disease. J. Water Health 6:209–213 [DOI] [PubMed] [Google Scholar]
- 9. Fraser V. J., et al. 1992. Contamination of flexible fiberoptic bronchoscopes with Mycobacterium chelonae linked to an automated bronchoscope disinfection machine. Am. Rev. Respir. Dis. 145:853–855 [DOI] [PubMed] [Google Scholar]
- 10. Fraser V., Wallace R. J., Jr. 1996. Nontuberculous mycobacteria, p. 1224–1237. In Mayhall C. G. (ed.), Hospital epidemiology and infection control. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 11. Griffith D. E., et al. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 12. Gross W. N., Hawkins J. E., Murphy D. B. 1976. Origin and significance of Mycobacterium xenopi in clinical specimens. Bull. Int. Union Tuberc. 51:267–269 [PubMed] [Google Scholar]
- 13. Hall L., Doerr K. A., Wohlfiel S. L., Roberts G. D. 2003. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J. Clin. Microbiol. 41:1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han X. Y., De I., Jacobson K. L. 2007. Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am. J. Clin. Pathol. 128:612–621 [DOI] [PubMed] [Google Scholar]
- 15. Hoy J., Rolston K., Hopfer R. L. 1987. Pseudoepidemic of Mycobacterium fortuitum in bone marrow cultures. Am. J. Infect. Control 15:268–271 [DOI] [PubMed] [Google Scholar]
- 16. Kuritsky J. N., et al. 1983. Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann. Intern. Med. 98:938–939 [DOI] [PubMed] [Google Scholar]
- 17. LaBombardi V. J., O'Brien A. M., Kislak J. W. 2002. Pseudo-outbreak of Myocbacterium fortuitum due to contaminated ice machines. Am. J. Infect. Control. 30:184–186 [DOI] [PubMed] [Google Scholar]
- 18. Laussucq S., et al. 1988. Nosocomial Mycobacterium fortuitum colonization from a contaminated ice machine. Am. Rev. Respir. Dis. 138:891–894 [DOI] [PubMed] [Google Scholar]
- 19. Lévy-Frébault V., David H. L. 1983. Mycobacterium kansasii: drinking water contaminant of a hospital. Rev. Epidemiol. Sante Publique 31:11–20 [PubMed] [Google Scholar]
- 20. Marshall H. M., et al. 2011. Mycobacterium lentiflavum in drinking water supplies, Australia. Emerg. Infect. Dis. 17:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash K. A., Andini N., Zhang Y., Brown-Elliott B. A., Wallace R. J., Jr. 2006. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob. Agents Chemother. 50:3476–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortiz A., Esteban J., Zamora N. 2007. Molecular identification by random amplified polymorphic DNA analysis of a pseudo-outbreak of Mycobacterium fortuitum due to cross-contamination of clinical samples. J. Med. Microbiol. 56:871–872 [DOI] [PubMed] [Google Scholar]
- 23. Panwalker A. P., Fuhse E. 1986. Nosocomial Mycobacterium gordonae pseudoinfection from contaminated ice machines. Infect. Control 7:67–70 [DOI] [PubMed] [Google Scholar]
- 24. Patel J. B., et al. 2000. Sequence-based identification of Mycobacterium species using the MicroSeq 500 16S rDNA bacterial identification system. J. Clin. Microbiol. 38:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petti C. A., et al. 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI document 29:MM18-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 26. Ringuet H., et al. 1999. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 37:852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rynkiewicz D. L., Cage G. D., Butler W. R., Ampel N. M. 1998. Clinical and microbiological assessment of Mycobacterium simiae isolates from a single laboratory in southern Arizona. Clin. Infect. Dis. 26:625–630 [DOI] [PubMed] [Google Scholar]
- 28. Sampaio J. L., et al. 2006. Application of four molecular typing methods for analysis of Mycobacterium fortuitum group strains causing post-mammaplasty infections. Clin. Microbiol. Infect. 12:142–149 [DOI] [PubMed] [Google Scholar]
- 29. Schinsky M. F., et al. 2004. Taxonomic variation in the Mycobacterium fortuitum third-biovariant complex: description of Mycobacterium boenickei sp. nov., Mycobacterium houstonense sp. nov., Mycobacterium neworleansense sp. nov., Mycobacterium brisbanense sp. nov., and recognition of Mycobacterium porcinum from human clinical isolates. Int. J. Syst. Evol. Microbiol. 54:1653–1667 [DOI] [PubMed] [Google Scholar]
- 30. Steingrube V. A., et al. 1995. PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J. Clin. Microbiol. 33:149–153 (Erratum, 33:1686.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Telenti A., et al. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsukamura M., Nemoto H., Yugi H. 1983. Mycobacterium porcinum sp. nov., a porcine pathogen.Int. J. Syst. Bacteriol. 33:162–165 [Google Scholar]
- 34. Wallace R. J., Jr., et al. 2004. Clinical and laboratory features of Mycobacterium porcinum. J. Clin. Microbiol. 42:5689–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wallace R. J., Jr., et al. 1991. Clinical disease, drug susceptibility, and biochemical patterns of the unnamed third biovariant complex of Mycobacterium fortuitum. J. Infect. Dis. 163:598–603 [DOI] [PubMed] [Google Scholar]
- 36. Wilson R. W., et al. 2001. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks, and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int. J. Syst. Evol. Microbiol. 51:1751–1764 [DOI] [PubMed] [Google Scholar]
- 37. Woods G. L., et al. 2011. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes; approved standard, 2nd ed CLSI document 31:M24–A2. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 38. Zhang Y., et al. 2004. Pulsed-field gel electrophoresis study of Mycobacterium abscessus isolates previously affected by DNA degradation. J. Clin. Microbiol. 42:5582–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.