Abstract
A 2.5-year-old girl died suddenly during the course of rotavirus gastroenteritis. The autopsy showed encephalopathy with rotavirus systemic infection. Here, we provide evidence of rotavirus replication in multiple organs. Our findings clarify that rotavirus infection in children can extend beyond the intestinal tract through viremia.
CASE REPORT
A previously healthy 2.5-year-old girl presented to our hospital following two febrile seizures. She had a 2-day history of diarrhea due to rotavirus gastroenteritis, which had been diagnosed before admission by a positive rotavirus antigen test of her stool. The seizures were generalized tonic-clonic seizures lasting 1 to 5 min each and occurred within 5 h of each other.
On examination, she was alert with a temperature of 39.3°C. Examination showed normal pupillary light reflexes, normal muscle tone, and normal patellar and Achilles tendon reflexes. The heart and lung sounds were also normal.
Laboratory analysis of blood obtained while in the emergency room showed the following: white blood cell count, 23,800/μl (97% neutrophils); hemoglobin, 11.9 g/dl; platelet count, 366,000/μl; erythrocyte sedimentation rate, 11 mm/h; C-reactive protein, 0.79 mg/dl; blood sugar, 121 mg/dl; serum ammonia, 22 mg/dl; sodium, 131 meq/liter; potassium, 4.1 meq/liter; chloride, 99 meq/liter; aspartate aminotransferase (AST), 50 IU/liter; alanine aminotransferase (ALT), 32 IU/liter; lactate dehydrogenase, 317 IU/liter; and creatinine phosphokinase, 420 IU/liter. Immune function testing revealed a low IgG level, at 384 mg/dl (normal range for age 2 years, 649 to 1,306 mg/dl) but normal IgG subclass analysis. the IgA level was 31 mg/dl, and the IgM level was 74 mg/dl. Coagulation tests were not performed. A blood culture yielded no bacterial growth.
An hour after arrival at the hospital, the patient experienced two more seizures. The fourth and final seizure developed into status epilepticus and was treated with intravenous diazepam. As the seizure gradually ended, the electrocardiogram monitor showed a brief period of ventricular fibrillation followed by asystole. Immediate resuscitation attempts were unsuccessful.
An autopsy was undertaken 12 h after the patient died. Cardiac tissues were submitted to the National Cardiovascular Center (Japan) for pathological analysis.
Analysis of the cerebrospinal fluid (CSF) obtained at autopsy revealed a leukocyte level of 240/mm3 and a protein level of 288 mg/dl. Rotavirus RNA was detected in the CSF by reverse transcription-PCR (RT-PCR). Serum diluted 1:16 showed a positive enzyme-linked immunosorbent assay (ELISA) reaction using an anti-VP6 mouse monoclonal antibody (YO-156) to detect antigenemia (12). The optical density at 492 nm obtained with the patient's serum was 0.932, compared with 0.035 for the negative control.
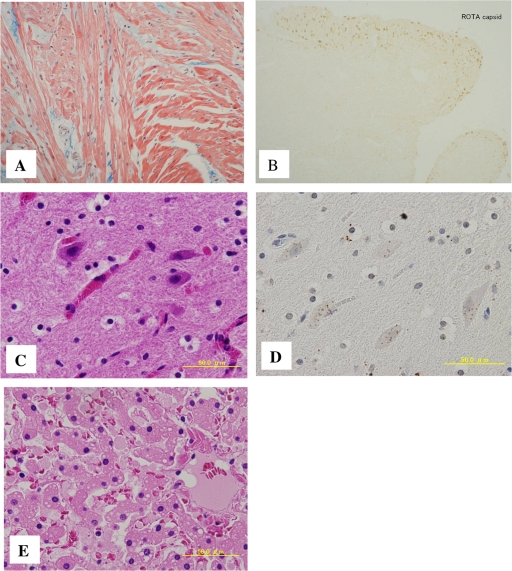
The autopsy revealed brain edema but no cerebral herniation. Although there was focal inflammatory cell infiltration in parts of the subarachnoid space, this was not indicative of encephalitis or meningitis. There was concentric cardiac hypertrophy, with left ventricular wall thickness of 9 mm. Histopathological review confirmed hypertrophic cardiomyopathy with focal disorganized myocardial architecture. Rotavirus capsid protein was observed in the endocardium and myocardium. Histological findings did not suggest acute viral myocarditis, as no inflammatory changes were seen in the myocardium. The liver showed fatty degeneration (Fig. 1). The bone marrow showed blood cells engulfed by macrophages.
Fig. 1.
Pathological findings in heart, brain, and liver tissue. (A) Hypertrophic cardiomyopathy with focal disorganized myocardial architecture. (B) Endocardium showing immunohistochemical detection of rotavirus capsid protein with a mouse monoclonal antibody. (C) Brain tissue with hematoxylin-and-eosin staining. (D) Brain tissue stained with anti-VP6 antibody. The brown color indicates a positive reaction. (E) Liver section with hematoxylin-and-eosin staining showing a multitude of small lipid droplets.
Selected tissue samples were also tested for rotavirus by immunohistochemistry (IHC) and RT-PCR. For IHC, paraffin-embedded tissue sections were subjected to immunostaining using Histofine simple stain MAX-PO kits (Nichirei, Tokyo, Japan). The primary antibodies used in these studies included an anti-VP6 mouse monoclonal antibody (YO-5) and anti-NSP1, anti-NSP3, and anti-NSP4 rabbit polyclonal antibodies. Normal mouse ascitic fluid and preimmune rabbit sera were used as negative controls.
YO-5 is broadly reactive with VP6 of human rotaviruses and has specificity for subgroup II, which is found in most human rotaviruses. The reactivity pattern of YO-5 was published previously (7). The antibodies against NSP1, NSP3, and NSP4 were prepared using the peptide CLTEEFELLISNSEDDNE, glutathione S-transferase (GST)-full-length NSP3 expressed in E. coli, and a peptide cocktail of CLKLAGYKEQITTKDEIE and CEWESGKNPYEPREVTA as an immunogen. The antibodies against these nonstructural proteins are broadly reactive with human and simian rotaviruses (7). Rotavirus antigens VP6, NSP1, NSP3, and NSP4 were detected in the brain, heart, liver, and small intestine by IHC using an anti-VP6 mouse monoclonal antibody and anti-NSP1, anti-NSP3, and anti-NSP4 rabbit polyclonal antibodies (Fig. 2). The VP6 and NSP4 genes were detected in the liver, intestine, and brain by RT-PCR. These data are summarized in Table 1.
Fig. 2.
Immunohistochemical detection of rotavirus nonstructural proteins. (A) Hepatocyte cytoplasm stained with anti-NSP1 polyclonal antibody. (B) Hepatocyte cytoplasm stained with preimmune rabbit serum. (C) Cardiomyocytes stained with anti-NSP3 polyclonal antibody. (D) Cardiomyocytes stained with preimmune rabbit serum. The reddish-brown color (diaminobenzidine) denotes a positive reaction.
Table 1.
Results of immunohistochemistry and RT-PCR for the detection of rotavirus antigens and RNA in postmortem tissue samples
| Organ | Presence or absence of antigen or RNA detected by: |
|||||
|---|---|---|---|---|---|---|
| Immunohistochemistry |
RT-PCR |
|||||
| VP6 | NSP4 | NSP3 | NSP1 | VP6 | NSP4 | |
| Heart | + | + | + | + | − | − |
| Liver | + | + | + | + | + | + |
| Intestine | + | + | + | + | + | + |
| Kidney | − | − | − | − | − | − |
| Brain | + | + | + | + | + | + |
Rotavirus is a member of the family Reoviridae. It is a triple-layered virus particle enclosing a genome of 11 double-stranded RNA segments that encode six structural proteins, VP1 to VP4, VP6, and VP7, and six nonstructural proteins, NSP1 to NSP6. Rotavirus is a common cause of gastroenteritis in infants and children. Recent studies have shown that rotavirus infection is commonly associated with antigenemia and can occasionally spread beyond the gastrointestinal tract, causing systemic infection (1, 4, 12). This report highlights encephalopathy due to systemic rotavirus infection and reports novel findings relating to the characteristics of this disease. In this case, rotavirus antigen was detected in the serum of a patient who presented with a high fever. Blutt et al. reported that antigenemia is predictive of viremia and can be used as a marker for the presence of extraintestinal rotavirus (1). Fever has been correlated with the severity of rotavirus antigenemia (12). The presence of fever supports a diagnosis of systemic rotavirus infection in our patient. The detection of nonstructural rotavirus proteins in the extraintestinal organs implies that rotavirus actively replicated in these organs. There have been only a few reports of autopsy cases of lethal rotavirus infection. In cases of myocarditis (2) and unexpected death (10), rotavirus antigen and RNA have been detected in infiltrating mononuclear cells or endothelial cells. To our knowledge, there is only one report documenting systemic rotavirus infection and replication in children, which used a nonstructural protein as an indicator of viral replication (4). In our case, rotavirus antigen was detected in the serum and in multiple organs, including the intestine, brain, heart, and liver. Rotavirus RNA was not detected in the heart even though the heart tissue was antigen positive.
Examination of the CSF revealed rotavirus RNA, pleocytosis, and an increased protein level, but rotavirus antigen testing was not undertaken. Rotavirus RNA detection has been reported to be very sensitive, with frequent contamination of samples (11). The significance of pleocytosis and increased CSF protein level is uncertain because of postmortem changes such as cell lysis and breakdown of the blood-brain barrier (3). Our rotavirus encephalopathy case showed various findings at autopsy, including fatty degeneration of the liver and left ventricular hypertrophy. Reye's syndrome is characterized by encephalopathy and fatty degeneration in the liver. The pathogenesis of Reye's syndrome is thought to be a profound failure of mitochondrial function, but the precise cause of the disorder remains uncertain. Young children should be carefully screened for metabolic diseases if Reye's syndrome is suspected. Reye's-like syndrome sometimes mimics Reye's syndrome in children with genetic defects of fatty acid oxidation. There are three reports of systemic rotavirus infection involving the central nervous system, all of which included elevated transaminase levels and lipid droplets in the liver (5, 6, 9). In these cases, analysis of amino acids and/or organic acids was normal. In two of the three cases, the patients had a history of chickenpox 2 weeks before admission (6, 9). Mitochondrial functional analysis was undertaken in another two cases and was normal (5, 6). Fever has been correlated with the severity of rotavirus antigenemia (12). In a case of acute necrotizing encephalopathy reported by Kirton et al., there was no fever, and autopsy revealed biventricular focal myocardial necrosis and mild microdroplet steatosis of the liver (6). In that case, detection of rotavirus antigen in the serum, liver, and heart tissue was not undertaken. In our case, although the liver pathology was microscopically compatible with Reye's syndrome, other aspects were not indicative of Reye's syndrome, such as a normal transaminase level, concentric myocardial hypertrophy, and very rapid death from seizure with a high fever.
Bacteremia secondary to rotavirus gastroenteritis has been reported (8). An increased blood white cell count was seen in our case, but there was no growth on blood culture, and histopathological findings did not indicate bacterial infection. Our patient's pre-existing hypertrophic cardiomyopathy and the direct viral damage to her vital organs might have resulted in her sudden death. It is possible that this patient had an underlying disorder such as mitochondrial disease or an immune disorder, but we were not able to fully explore these possibilities. Few fatal cases of extraintestinal rotavirus dissemination developing from viremia have been reported to date, and the pathogenic process is therefore largely uncharacterized. Our findings have important implications for determining the pathology of fatal rotavirus infection. The possible causes and clinical consequences of systemic rotavirus infections are not yet fully understood. Our findings may help to elucidate the pathology of fatal rotavirus infection.
Acknowledgments
We thank Yutaka Tomita, Kyoto Min-iren Chuo Hospital, Kyoto, Japan, for helpful discussions.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Blutt S. E., et al. 2007. Rotavirus antigenemia in children is associated with viremia. PLoS Med. 4: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciok A. M., Nuovo G. J. 2002. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod. Pathol. 15: 914–922 [DOI] [PubMed] [Google Scholar]
- 3. Finehout E. J., Franck Z., Relkin N., Lee K. H. 2006. Proteomic analysis of cerebrospinal fluid changes related to postmortem interval. Clin. Chem. 52: 1906–1913 [DOI] [PubMed] [Google Scholar]
- 4. Gilger M. A., et al. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120: 912–917 [DOI] [PubMed] [Google Scholar]
- 5. Ioi H., et al. 2005. A case of Reye syndrome with rotavirus infection accompanied with high cytokines. J. Infect. 52: e124–128 [DOI] [PubMed] [Google Scholar]
- 6. Kirton A., Busche K., Ross C., Wirrell E. 2005. Acute necrotizing encephalopathy in Caucasian children: two cases and review of the literature. J. Child Neurol. 20: 527–532 [DOI] [PubMed] [Google Scholar]
- 7. Koki T., Tomoko U., Shozo U., Toshio Y. 1984. Production of subgroup-specific monoclonal antibodies against human rotaviruses and their application to an enzyme-linked immunosorbent assay for subgroup determination. J. Med. Virol. 14: 115–125 [DOI] [PubMed] [Google Scholar]
- 8. Lowenthal A., Livni G., Amir J., Samra Z., Ashkenazi S. 2006. Secondary bacteremia after rotavirus gastroenteritis in infancy. Pediatrics 117: 224–226 [DOI] [PubMed] [Google Scholar]
- 9. Lynch M., et al. 2003. The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin. Infect. Dis. 37: 1327–1333 [DOI] [PubMed] [Google Scholar]
- 10. Morrison C., Gilson T., Nuovo G. J. 2001. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum. Pathol. 32: 216–221 [DOI] [PubMed] [Google Scholar]
- 11. Nakagomi T., Nakagomi O. 2005. Rotavirus antigenemia in children with encephalopathy accompanied by rotavirus gastroenteritis. Arch. Virol. 150: 1927–1931 [DOI] [PubMed] [Google Scholar]
- 12. Sugata K., et al. 2008. Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics 122: 392–397 [DOI] [PubMed] [Google Scholar]