Abstract
An 86-year-old female patient from northeast Mexico presented with diffuse lepromatous leprosy (DLL). Sequence analysis of four genes (rrs, rpoB, sigA, and hsp65) from the skin biopsy specimen identified “Mycobacterium lepromatosis.” This is the first independent confirmation of a case of DLL due to M. lepromatosis.
CASE REPORT
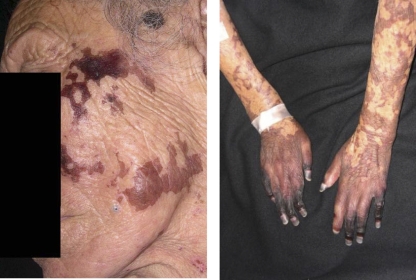
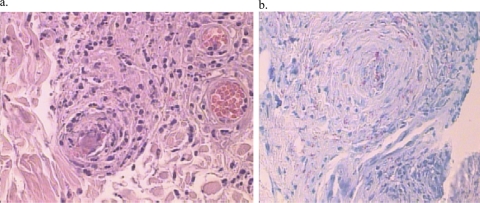
In 2005, an 86-year-old female with no prior medical history of leprosy was admitted to our hospital due to a 10-day bout of distal cyanosis of the extremities (Fig. 1). Physical examination also showed generalized dermatosis characterized by diffuse infiltration of the skin, predominantly in the face, with thickening of the superciliary region and ears plus an accompanying absence of eyebrows and eyelashes. In the rest of the body, the skin had an atrophic “cigarette paper”-like appearance and diminished or absent body hair. The neurological examination revealed extensive areas of dysesthesia. The areas of diffuse lepromatous leprosy (DLL) associated with Lucio's phenomenon were characterized by a purpuric appearance, with necrosis and ulceration in some of the areas, leading to sloughing in acral sites such as fingers and toes. A presumptive diagnosis of cryoglobulinemia versus other vasculitis disorders was considered, and laboratory examination showed microcytic hypochromic anemia, thrombocytosis, leukocytosis, and a rheumatoid factor of 80. A skin biopsy was performed and treatment with pentoxifylline initiated. During the first week, the rheumatologist prescribed cyclophosphamide, but the patient did not improve and developed necrosis in areas that were previously cyanotic (Fig. 1). The skin biopsy specimen showed vasculitis with thrombosis and perivascular and periadnexial lymphocytic infiltrates (Fig. 2) as well as numerous acid-fast bacilli, leading to diagnosis of diffuse lepromatous leprosy (DLL) and Lucio's phenomenon. Treatment with prednisone and multidrug therapy for multibacillary leprosy was initiated. The patient improved after 10 days of treatment, was discharged after 2 weeks in stable condition, but died at home 3 months later of unknown cause.
Fig. 1.
Patient with diffuse lepromatous leprosy, showing the characteristic madarosis and cutaneous lesions (left panel). Initial cyanotic lesions in the hands that evolved to black necrotic lesions, particularly at the fingertips, are shown (right panel).
Fig. 2.
Histopathology. (a) Skin biopsy specimen of the cyanotic lesions, showing vasculitis and thromboembolism. (b) Many acid-fast bacilli were seen upon Fite-Faracco staining.
Leprosy is one of the oldest recorded human afflictions. Depending upon the immune response mounted against the bacilli, the disease presents with a broad clinical spectrum. At one pole are the tuberculoid (TT) patients, with effective T cell-mediated immunity resulting in very low bacterial numbers, while at the other, the lepromatous (LL) patients mount an ineffective humoral response and exhibit a high bacillary load. Other unstable forms, with characteristics between these poles, can also be observed. A particular variation of lepromatous leprosy involving diffuse nonnodular lesions is more frequent in Mexico and the Caribbean than in any other part of the world (2). This variation has been referred to as diffuse lepromatous leprosy (DLL) or Lucio's phenomenon (6, 11).
All these forms of leprosy have been attributed to various host responses to the causative pathogen, Mycobacterium leprae. However, M. leprae exhibits exceptionally high genetic homogeneity within different strains across the world (3, 7, 8, 10, 13); thus, M. leprae may not account for such a diverse range of clinical manifestations. Recently, Han et al. (4, 5) have established the existence of “Mycobacterium lepromatosis,” a closely related but distinct species, as a causative agent of DLL in two patients of Mexican origin in Arizona, who succumbed to the disease. Those authors have further investigated the existence of M. lepromatosis in leprosy patients in Mexico and have claimed that M. lepromatosis, particularly the DLL form, is the predominant cause of leprosy there (5). However, this observation has not been confirmed independently in other studies involving Mexican leprosy patients (8), and the clinical significance (in magnitude and distribution) of M. lepromatosis as the causative agent of DLL remains uncertain and is even questioned, despite the fact that DLL cases require management (diagnosis, care, and therapy) that is more specific due to the higher morbidity and even mortality associated with them. Here, we present a retrospective report of a case of M. lepromatosis from the year 2005.
During our drug resistance surveillance study of recently archived leprosy biopsy specimens from Monterrey, Mexico, we observed that the specimen (Mx1-22A) from the case presented above gave negative test results for PCR targeting the M. leprae folP1 and gyrA loci. As this specimen was positive for acid-fast bacilli in the skin smear and also exhibited clear signs of DLL, it was tested for the M. leprae-specific RLEP repetitive sequence, which is always present in 37 copies in the M. leprae genome. This PCR gave no amplification, confirming the absence of M. leprae DNA, although the sample yielded a product when rpoB primers were used. Upon analysis of the sequences of the rpoB gene flanking the rifampin resistance-determining region (RRDR), there were multiple mismatches with the M. leprae sequence but there was 100% identity with the corresponding sequences of M. lepromatosis FJ924.
To confirm this identification, partial sequences of three other genes (hsp65, rrs, and sigA) were determined using the primers described by Han et al. (4) together with additional sets of primers that could amplify the sigA sequences from both the M. leprae and M. lepromatosis species (primer pair LepMato-F1 [5′-CCAGGTTGCCTTCCTGTATC-3′] and LepMato-R1 [5′-AAGCTTCCACCGATGATGAC-3′] and primer pair LepMato-F2 [5′-CACCACAGATGTGACGCACT-3′] and LepMato-R2 [5′-AACGTCGAGGTCCGGTTC], together covering the initial 900-bp region of sigA in M. leprae TN). All the resulting sequences exhibited 100% identity with the M. lepromatosis FJ924 sequences available in the GenBank database, which led to the unambiguous identification of sample Mx1-22 as M. lepromatosis. To our knowledge, this is the first independent confirmation of the existence of M. lepromatosis, and we believe that M. lepromatosis is indeed associated with DLL in Mexico, at least in some cases. However, the proportion of such cases remains unknown and requires further investigation. Such a study could also explain the clinical and geographic variations in the disease spectrum (4).
Like M. leprae, M. lepromatosis cannot be cultured on artificial media; it also shares other features such as an unusually low G+C content for a mycobacterium (57.8%), the presence of pseudogenes, unique AT-rich insertions in the 16S rRNA gene, and identical six-base tandem repeats in sigA (4, 5). Cases of M. lepromatosis infection have exhibited much higher morbidity and even mortality rates than cases of M. leprae infection (4). Our patient's case should encourage further efforts to detect M. lepromatosis in order to study its association with Lucio's phenomenon (12) and its transmission and to identify potential reservoirs. This is particularly desirable at a time when histological diagnosis of leprosy is disappearing and PCR-based methods are becoming more common, especially for drug susceptibility testing (1, 9). However, with the exception of direct DNA sequencing of the RRDR, none of the current PCR tests for M. leprae detect M. lepromatosis, which means that cases of infection by this newly described leprosy bacillus may go undetected, thus jeopardizing patient recovery.
In many drug resistance surveillance programs, there are some cases where even biopsy specimens from smear-positive lepromatous leprosy patients fail to exhibit PCR amplification using M. leprae-specific primers; such results are assumed to be due to the presence of Taq polymerase inhibitors or of too few bacilli in the specimen analyzed (14). For such PCR-negative leprosy cases, the use of conserved primers capable of amplifying both M. leprae and M. lepromatosis could be a better choice. In the majority of cases, and especially in paucibacillary and early-stage cases, M. leprae DNA is available in limiting amounts (for molecular drug susceptibility testing and genotyping studies). Therefore, it would be ideal to analyze the RRDR of the rpoB gene, especially in the M. leprae PCR-negative DLL cases where suspicion of M. lepromatosis should be considered. The possibility of mixed infections (5) involving both M. leprae and M. lepromatosis also needs further investigation. The recent advances in next-generation sequencing technologies have removed several constraints concerning cost and the requisite amounts of DNA for whole genome resequencing. Hence, application of the much-awaited comparative genomics of M. leprae and M. lepromatosis has the potential to elucidate many issues related to their virulence, evolutionary dynamics, and the endemicity of DLL in Mexico.
Acknowledgments
This work received the financial support of the Fondation Raoul Follereau.
Footnotes
Published ahead of print on 29 October 2011.
REFERENCES
- 1. Cole S. T., et al. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011 [DOI] [PubMed] [Google Scholar]
- 2. Gelber R. H. 2005. Leprosy (Hansen's disease), p. 966–972.In Kasper D. L., et al. (ed.), Harrison's principles of internal medicine, 16th ed. McGraw-Hill, New York, NY. [Google Scholar]
- 3. Groathouse N. A., et al. 2004. Multiple polymorphic loci for molecular typing of strains of Mycobacterium leprae. J. Clin. Microbiol. 42:1666–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han X. Y., et al. 2008. A new Mycobacterium species causing diffuse lepromatous leprosy. Am. J. Clin. Pathol. 130:856–864 [DOI] [PubMed] [Google Scholar]
- 5. Han X. Y., et al. 2009. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J. Bacteriol. 191:6067–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lucio R., Alvarado I. 1852. Opúsculo sobre el mal de San Lázaro o elefantiasis de los Griegos. M. Murguia y Cia, Mexico City, Mexico. [Google Scholar]
- 7. Matsuoka M., Zhang L., Budiawan T., Saeki K., Izumi S. 2004. Genotyping of Mycobacterium leprae on the basis of the polymorphism of TTC repeats for analysis of leprosy transmission. J. Clin. Microbiol. 42:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monot M., et al. 2009. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat. Genet. 41:1282–1289 [DOI] [PubMed] [Google Scholar]
- 9. Singh P., Cole S. T. 2011. Mycobacterium leprae: genes, pseudogenes and genetic diversity. Future Microbiol. 6:57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Truman R., Fontes A. B., de Miranda A. B., Suffys P., Gillis T. 2004. Genotypic variation and stability of four variable-number tandem repeats and their suitability for discriminating strains of Mycobacterium leprae. J. Clin. Microbiol. 42:2558–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vargas-Ocampo F. 2004. Analysis of 6000 skin biopsies of the national leprosy control program in Mexico. Int. J. Lepr. Other Mycobact. Dis. 72:427–436 [DOI] [PubMed] [Google Scholar]
- 12. Vargas-Ocampo F. 2007. Diffuse leprosy of Lucio and Latapi: a histologic study. Lepr. Rev. 78:248–260 [PubMed] [Google Scholar]
- 13. Williams D. L., Gillis T. P., Portaels F. 1990. Geographically distinct isolates of Mycobacterium leprae exhibit no genotypic diversity by restriction fragment-length polymorphism analysis. Mol. Microbiol. 4:1653–1659 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. 2010. Surveillance of drug resistance in leprosy: 2009. Wkly. Epidemiol. Rec. 85:281–284 [PubMed] [Google Scholar]