Abstract
The bacterial loads for gonococcal infections of the pharynx and rectum were determined among men with male sexual partners. The median bacterial load for rectal infections (18,960 copies/swab) was significantly higher than the load for pharyngeal infections (2,100 copies/swab; P = 0.001). Bacterial loads among men with symptomatic proctitis were strikingly high (median, 278,000 copies/swab).
TEXT
Gonorrhea is one of the most common sexually transmitted diseases, and study results suggest that rectal gonorrhea enhances HIV transmission (1–3).
There are limited data on the transmissibility of gonorrhea, particularly from the pharynx and rectum. To examine potential transmissibility from these sites, we measured gonococcal bacterial loads and examined associated clinical and laboratory factors.
All men who have sex with men (MSM) attending the Melbourne Sexual Health Centre between January 2010 and April 2010 who underwent testing for sexually transmitted infections were included. Pharyngeal and rectal specimens were obtained using cotton-tipped swabs. Rectal specimens were obtained either by blind anal swabbing or, in men with symptomatic proctitis, via anoscopy. Swabs were plated onto modified Thayer-Martin medium, placed into phosphate-buffered saline, and then analyzed using real-time quantitative PCR (qPCR) targeting the opa gene of Neisseria gonorrhoeae (4). Positive PCR tests were confirmed using real-time PCR targeting the gonococcal porA pseudogene (7). Gonococcal loads were quantified by comparing the crossing threshold for each sample to the crossing threshold of a standard curve constructed by amplifying different, known copy numbers of N. gonorrhoeae in an opa assay. To determine the adequacy of sample collection, a qPCR targeting the 260-bp region of β-globin was performed.
Inoculated culture plates were incubated at 36°C in 5% CO2 for 48 h. Presumptive N. gonorrhoeae colonies were oxidase tested, and identification of specimens to the species level was confirmed by carbohydrate reaction tests. Specimens that were culture positive and/or PCR positive were regarded as representing true infections. The McNemar test was used to compare detection of gonorrhea by culture versus PCR. The Mann-Whitney test was used to compare bacterial loads. The Human Research Ethics Committee of the Alfred Hospital approved the study.
Specimens were obtained from 1,076 MSM. The median age of the subjects was 32 (range, 18 to 69). For testing using both culture and PCR, 1,011 rectal and 1,076 pharyngeal specimens were obtained. There were 43 (3.9%) confirmed PCR-positive pharyngeal specimens, of which 17 were also culture positive (sensitivity, 39%). There were 47 (4.6%) confirmed PCR-positive rectal specimens, of which 25 were also culture positive (sensitivity, 53%).
Twelve men had concurrent gonorrheal infections of the pharynx and rectum. A further 10 were concurrently infected at the urethra and rectum, while 2 were concurrently infected at the urethra, pharynx, and rectum. Five men were coinfected with rectal chlamydia and gonorrhea. Twenty-nine men had pharyngeal gonorrhea exclusively. HIV infection was more common among men with gonorrhea than men without gonorrhea (15.8% versus 8.8%; P = 0.04). There were no significant differences between HIV-positive and HIV-negative men with respect to the prevalences of pharyngeal and rectal gonorrhea infections.
β-globin was detected in all but one (2,086/2,087; 99.9%) of the specimens. There was no significant difference between the 90 PCR-positive specimens (162,644 copies/swab) and the 1,997 PCR-negative specimens (291,011 copies/swab) in median β-globin levels (P = 0.13). There was no significant difference between the 43 pharyngeal infections (384,502 copies/swab) and the 47 rectal infections (205,311copies/swab) in median β-globin levels (P = 0.24).
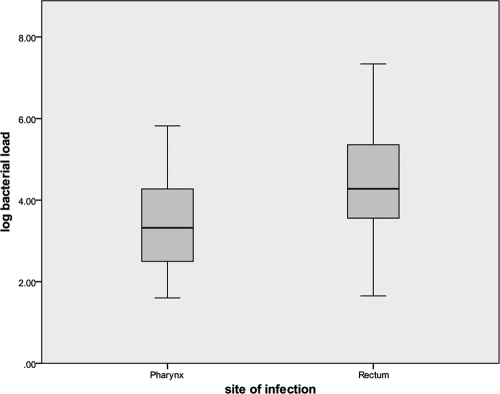
The median bacterial load among PCR-positive rectal infections (18,960; log 4.3) was significantly higher than the load among PCR-positive pharyngeal infections (2,100; log 3.3) (P = 0.001), irrespective of culture results (Fig. 1).
Fig. 1.
Comparison of gonococcal bacterial loads in the rectum and pharynx of men.
None of the men with pharyngeal gonorrhea reported pharyngeal symptoms. Seven men with rectal gonorrhea had symptoms of proctitis: all were positive by both PCR and culture (Table 1). The median bacterial load for those men was 278,800 (log 5.4), significantly higher than the load seen with the remaining 40 men with asymptomatic rectal infections (13,980; log 4.1) (P < 0.001).
Table 1.
Characteristics of men with symptomatic rectal gonorrhoea including gonococcal bacterial loads
| Patient | Age (yr) | Rectal symptom(s) | Duration of symptoms (days) | HIV infection status | Reported contact with gonorrhea | Other rectal infection(s) | Gonococcal bacterial load (no. of copies/swab) |
|---|---|---|---|---|---|---|---|
| 1 | 43 | Rectal discharge | 5 | Negative | No | No | 350,000 |
| 2 | 27 | Rectal pain | 3 | Negative | No | No | 142,000 |
| 3 | 36 | Rectal pain | 2 | Negative | No | No | 2,188,000 |
| 4 | 21 | Rectal pain with anal ulceration | 5 | Negative | No | Herpes simplex virus type 2 | 43,200 |
| 5 | 58 | Rectal discharge | 7 | Negative | No | No | 148,000 |
| 6 | 25 | Rectal pain | 4 | Positive | Yes | Chlamydia trachomatis | 278,800 |
| 7 | 45 | Rectal pain | 10 | Positive | No | No | 1,444,000 |
The median bacterial load among PCR- and culture-positive pharyngeal infections (11,520; log 4.1) was significantly higher than the load for PCR-positive, culture-negative infections (1,150; log 3.1) (P = 0.03). Similarly, the median bacterial load among PCR- and culture-positive rectal infections (150,800; log 5.2) was significantly higher than the load for PCR-positive, culture-negative infections (3,814; log 3.6) (P < 0.001).
To our knowledge, this is the first published report giving data on the bacterial load of gonorrhea infections of the pharynx and rectum. We found bacterial loads to be lower among pharyngeal infections, with strikingly high loads among symptomatic rectal infections.
There have been no reports of studies that have quantified the risk of transmission of gonorrhea from the pharynx or rectum to the male urethra per sexual exposure (8). If the load of N. gonorrhoeae is important in transmission, then these results suggest that transmission may be more likely per sexual exposure from the rectum to the urethra than from the pharynx, particularly for symptomatic rectal infections. The very high bacterial loads seen with symptomatic rectal infections also point to the potentially increased risk of HIV acquisition in men with symptomatic gonococcal proctitis who are exposed to HIV, given the heightened mucosal inflammatory response that would likely be present in such cases.
Previous studies have also underscored the poor sensitivity of culture for detecting pharyngeal and rectal gonococcal infections (5, 6). This report helps to explain the poor performance of gonococcal culture at these sites, with culture missing infections where gonococcal loads are lower. Culture was insensitive compared to nucleic acid amplification tests (NAAT).
There were a number of strengths and limitations of this study. Gonorrheal culture requires stringent transport and laboratory conditions. The on-site location of our laboratory ensured an optimal yield of N. gonorrhoeae from culture. Furthermore, the gonococcal PCRs used are validated assays that had been developed in-house (7). For samples to be considered positive by PCR, both targets had to be positive. There was no significant difference between pharyngeal infections and rectal infections in β-globin levels, suggesting that the differences in bacterial load were not the result of differences in sampling adequacy. A limitation of the study was that men who may have been on antibiotic therapy prior to testing were not excluded; thus, nonviable gonorrhea may have been present at the sampling sites, producing culture-negative, PCR-positive results.
While this report indicates potential differences in the transmissibility of gonorrhea from the pharynx and rectum, with lower bacterial load in the pharynx suggesting a lower probability of transmission per sexual exposure from the pharynx than from the rectum, prospective partner studies that quantify transmission between partners and natural history studies using nucleic acid amplification are needed. These would help to inform mathematical models of gonorrhea transmission and modeling of interventions aimed at improving gonorrhea control.
Acknowledgments
We thank the doctors and nurses who collected specimens for the study. We also thank Jun Kit Sze and Afrizal for data extraction and laboratory staff at the University of Melbourne Microbiology Diagnostic Unit for handling specimens and performing cultures.
This work does not represent a conflict of interest for any of the authors.
No funding has been received for this study.
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Bernstein K. T., Marcus J. L., Nieri G., Philip S. S., Klausner J. D. 2010. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J. Acquir. Immune Defic. Syndr. 53:537–543 [DOI] [PubMed] [Google Scholar]
- 2. Craib K. J., et al. 1995. Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men. Genitourin. Med. 71:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin F., et al. 2010. Anal sexually transmitted infections and risk of HIV infection in homosexual men. J. Acquir. Immune Defic. Syndr. 53:144–149 [DOI] [PubMed] [Google Scholar]
- 4. Lister N. A., Tabrizi S. N., Fairley C. K., Garland S. M. 2004. Validation of Roche COBAS Amplicor assay for detection of Chlamydia trachomatis in rectal and pharyngeal specimens by an omp1 PCR assay. J. Clin. Microbiol. 42:239–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Page-Shafer K. G. 2002. Increased sensitivity of DNA amplification testing for the detection of pharyngeal gonorrhea in men who have sex with men. Clin. Infect. Dis. 34:173–176 [DOI] [PubMed] [Google Scholar]
- 6. Schachter J., Moncada J., Liska S., Shayevich C., Klausner J. D. 2008. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex. Transm. Dis. 35:637–642 [DOI] [PubMed] [Google Scholar]
- 7. Tabrizi S. N., Chen S., Tapsall J., Garland S. M. 2005. Evaluation of opa-based real-time PCR for detection of Neisseria gonorrhoeae. Sex. Transm. Dis. 32:199–202 [DOI] [PubMed] [Google Scholar]
- 8. Templeton D. J., et al. 2010. Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia. Sex. Transm. Infect. 86:90–96 [DOI] [PubMed] [Google Scholar]