Abstract
Among Nocardia species causing infections, Nocardia veterana is rarely isolated and is mostly described as causing pulmonary infections. This is the first presentation of a case of brain abscess attributable to an N. veterana infection in a patient with type 2 diabetes. Prolonged antibiotic therapy with trimethoprim-sulfamethoxazole led to successful clinical recovery.
CASE REPORT
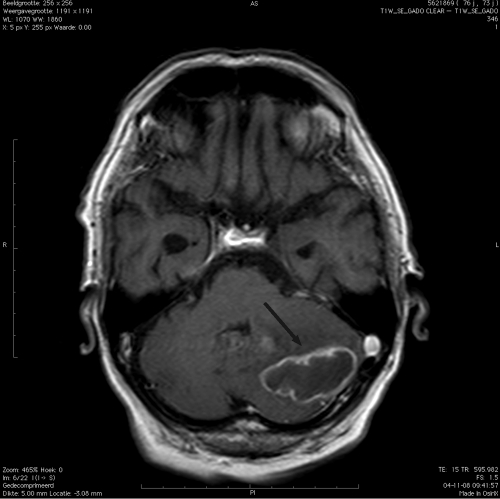
A 73-year-old man was admitted to a regional hospital with complaints of weight loss and a few days of stomachache. His past medical history revealed type 2 diabetes mellitus (DM2) without secondary complications, hypertension, an appendectomy, and a transient ischemic attack. Clinical suspicion of an intestinal obstruction led to a laparotomy, revealing no abnormalities. In the postoperative period, behavioral changes occurred, with passive behavior, slow speech, and an abnormal head positioning to the right side. No vomiting was observed. A computed tomography (CT) scan of the brain showed a large abscess in the left cerebral hemisphere with surrounding edema (Fig. 1). A further radiological examination using CT scans of the thorax and abdomen did not show any other abscesses. Dexamethasone therapy was immediately started, resulting in neurological improvement. The patient was transferred to our academic center, where his physical examination at presentation was unremarkable, while a neurological examination revealed no abnormalities except for disorientation with respect to place and time. Furthermore, brainstem reflexes and lower and upper extremity reflexes were normal. Laboratory analysis at admission showed the following: alkaline phosphatase (AF), 202 U/liter; gamma glutamyltransferase (γ-GT), 277 U/liter; aspartate aminotransferase (ASAT), 46 U/liter; alanine aminotransferase (ALAT), 108 U/liter; lactate dehydrogenase (LDH), 225 U/liter; C-reactive protein (CRP), <2 mg/liter; hemoglobin (Hb), 7.4 mmol/liter; leukocytes, 20 × 10e9/liter; neutrophils, 18.1 × 10e9/liter; thrombocytes, 383 × 10e9/liter; erythrocyte sedimentation rate (ESR), 9 mm in the first hour; creatinine, 83 μmol/liter; glucose, 11.8 mmol/liter.
Fig. 1.
CT scan of the left hemisphere of the cerebellum, showing the abscess (black arrow) caused by Nocardia veterana.
After trepanation and drainage of the abscess was performed, Gram staining revealed branching, bead-staining, Gram-positive rods that were acid fast as shown by a modified Ziehl-Neelsen staining procedure. Cultures on blood agar plates produced a pure growth of Nocardia sp. after 2 days. Using phenotypic tests, the national reference laboratory (Laboratory for Infectious Disease [LIS]-RIVM, Bilthoven, the Netherlands) identified the isolate as Nocardia veterana (aerobic growth at 37°C and 45°C, d-glucose negative, catalase positive, negative for nitrate reductase production and positive for urease production, negative for utilization of Simmons citrate as a sole source of carbon, positive for hydrolysis of esculin, but negative for tyrosine and xanthine). N. veterana was identified using 16S rRNA gene sequence analysis of 500 bp (ABI MicroSeq500 16S rDNA bacterial identification kit) and GenBank and the Ribosomal Database for data analysis. The results showed 100% similarity to the reference strain (similarity to N. nova, 98.76%; similarity to N. otitidiscaviarum, 98.54%, similarity to N. transvalensis, 98.22%, similarity to N. brevicatena, 97.47%). Matrix-assisted laser desorption–ionization time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Bremen, Germany) also revealed N. veterana, with an identification score of 2.078. Using this method, a score of ≥2 has been validated for species identification, and the Bruker database contains 36 Nocardia species, including N. africana, N. elegans, N. nova, and N. kruczakiae. Therefore, three different methods identified the isolate as N. veterana. Since immediate microscopy after drainage led to a high suspicion of a nocardia infection, intravenous (i.v.) administration of meropenem at a high dose was started but was changed to trimethoprim-sulfamethoxazole (TMP-SMX) at three 1,920-mg doses/day (dd) (initially administered i.v. for 6 weeks and then orally for another 10 months) when the results of the antimicrobial susceptibility testing became available. Using broth microdilution according to CLSI methods and breakpoints (3), the TMP-SMX MIC was 0.063/1.2 μg/ml, the amoxicillin MIC was 2 μg/ml, the clarithromycin MIC was ≤0.125 μg/ml, the imipenem MIC was 0.5 μg/ml, the ceftriaaxone MIC was 16 μg/ml, the ciprofloxacin MIC was 16 μg/ml, and the amikacin MIC was ≤0.5 μg/ml.
A thorough analysis to determine the possibility of immunodeficiency showed no abnormalities besides his known DM2, his older age, and a low CD4 cell count of 85/mm3 with a negative HIV serology result. Furthermore, dental, ear, nose, and cardiac evaluations revealed no other abscesses possibly serving as a focus for the brain abscess. Blood cultures were negative. The patient clinically improved and was discharged to the regional hospital for further recovery, which was uneventful. A routine laboratory evaluation 3 months later at the outpatient clinic showed a CD4 count of 619/mm3. A CT scan of the brain, performed 3 months after discontinuation of antibiotic treatment, showed no recurrence of abscess.
Together with our patient, only 13 patients with a Nocardia veterana infection have been reported so far (1, 2, 4, 7–10, 12, 12, 15). A pulmonary infection was diagnosed for most of those patients, whereas 2 patients showed an abdominal localization (7, 15) and another had a mycetoma (9). Here, we present the first description of a brain abscess caused by Nocardia veterana. For 9 of the 13 patients, a clear underlying cause of an immunocompromised condition, such as the use of immunosuppressive drugs (4, 14) or the presence of human immunodeficiency virus (HIV) infection (7, 12) or of an autoimmune disorder such as systemic lupus erythematosus (SLE) (9, 10, 14), could be diagnosed. Four patients had no apparent cause of immunodeficiency (8, 14, 15). However, it has been suggested that both DM2 and older age contribute some degree of impaired immunity (5, 13), leading to a higher susceptibility to opportunistic infections. The median age of the immunocompromised-patient group (73 years) was lower than the median age of the other patients (44 years) (P = 0.004 [Mann-Whitney test]). The initial low CD4 cell count determined for our patient is most likely explained by previous dexamethasone use and the ongoing infection, since his CD4 cell count returned to normal several months after treatment and cessation of the dexamethasone therapy. However, since no earlier CD4 cell count was available, we cannot exclude the possibility that a lymphocytopenia facilitated the development of the nocardiosis.
The initial hospital presentation with acute abdominal pain, deviant liver ultrasound results, and elevated liver enzymes might suggest an abdominal origin of the N. veterana infection. However, neither the abdominal CT scan results nor the operating surgeon suggested abnormalities of the liver. Moreover, the elevated liver enzymes could also be explained by the anticoagulant therapy (enoxaparin) the patient received in hospital.
In the previously described cases, the duration of treatment ranged from a few weeks to a number of years. In our patient, we opted for a treatment duration of 1 year based on expert opinion on the treatment of other Nocardia infections (6, 11). To date, more than 6 months after cessation of antibiotic therapy, the patient is doing well without any signs of recurrence.
Conclusion.
This is the first report of an elderly patient with a brain abscess caused by Nocardia veterana who responded very well to long-term antibiotic therapy and recovered without any signs of recurrence.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Agterof M. J., et al. 2007. Nocardiosis: a case series and a mini review of clinical and microbiological features. Neth. J. Med. 65:199–202 [PubMed] [Google Scholar]
- 2. Ansari S. R., Safdar A., Han X. Y., O'Brien S. 2006. Nocardia veterana bloodstream infection in a patient with cancer and a summary of reported cases. Int. J. Infect. Dis. 10:483–486 [DOI] [PubMed] [Google Scholar]
- 3. CLSI. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes—approved standard, 2nd ed. CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 4. Conville P. S., et al. 2003. Nocardia veterana as a pathogen in North American patients. J. Clin. Microbiol. 41:2560–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geerlings S. E., Hoepelman A. I. 1999. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 26:259–265 [DOI] [PubMed] [Google Scholar]
- 6. Geiseler P. J., Andersen B. R. 1979. Results of therapy in systemic nocardiosis. Am. J. Med. Sci. 278:188–194 [DOI] [PubMed] [Google Scholar]
- 7. Godreuil S., et al. 2003. Nocardia veterana isolated from ascitic fluid of a patient with human immunodeficiency virus infection. J. Clin. Microbiol. 41:2768–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gürtler V., et al. 2001. Nocardia veterana sp. nov., isolated from human bronchial lavage. Int. J. Syst. Evol. Microbiol. 51:933–936 [DOI] [PubMed] [Google Scholar]
- 9. Kano R., et al. 2002. The first isolation of Nocardia veterana from a human mycetoma. Microbiol. Immunol. 46:409–412 [DOI] [PubMed] [Google Scholar]
- 10. Kashima M., et al. 2005. A successfully treated case of mycetoma due to Nocardia veterana. Br. J. Dermatol. 152:1349–1352 [DOI] [PubMed] [Google Scholar]
- 11. Lerner P. I. 1996. Nocardiosis. Clin. Infect. Dis. 22:891–903 [DOI] [PubMed] [Google Scholar]
- 12. Liu W. L., et al. 2011. Bacteremic pneumonia caused by Nocardia veterana in an HIV-infected patient. Int. J. Infect. Dis. 15:e430–e432 [DOI] [PubMed] [Google Scholar]
- 13. Miller R. A. 1996. The aging immune system: primer and prospectus. Science 273:70–74 [DOI] [PubMed] [Google Scholar]
- 14. Pottumarthy S., et al. 2003. Nocardia veterana, a new emerging pathogen. J. Clin. Microbiol. 41:1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlebusch S., Nimmo G., Carter R. 2010. Bowel abscess with Nocardia veterana associated with colon carcinoma. Pathology 42:306–307 [DOI] [PubMed] [Google Scholar]